Amino Acids during Pregnancy and Offspring Cardiovascular–Kidney–Metabolic Health
Abstract
1. Introduction
2. Materials and Methods
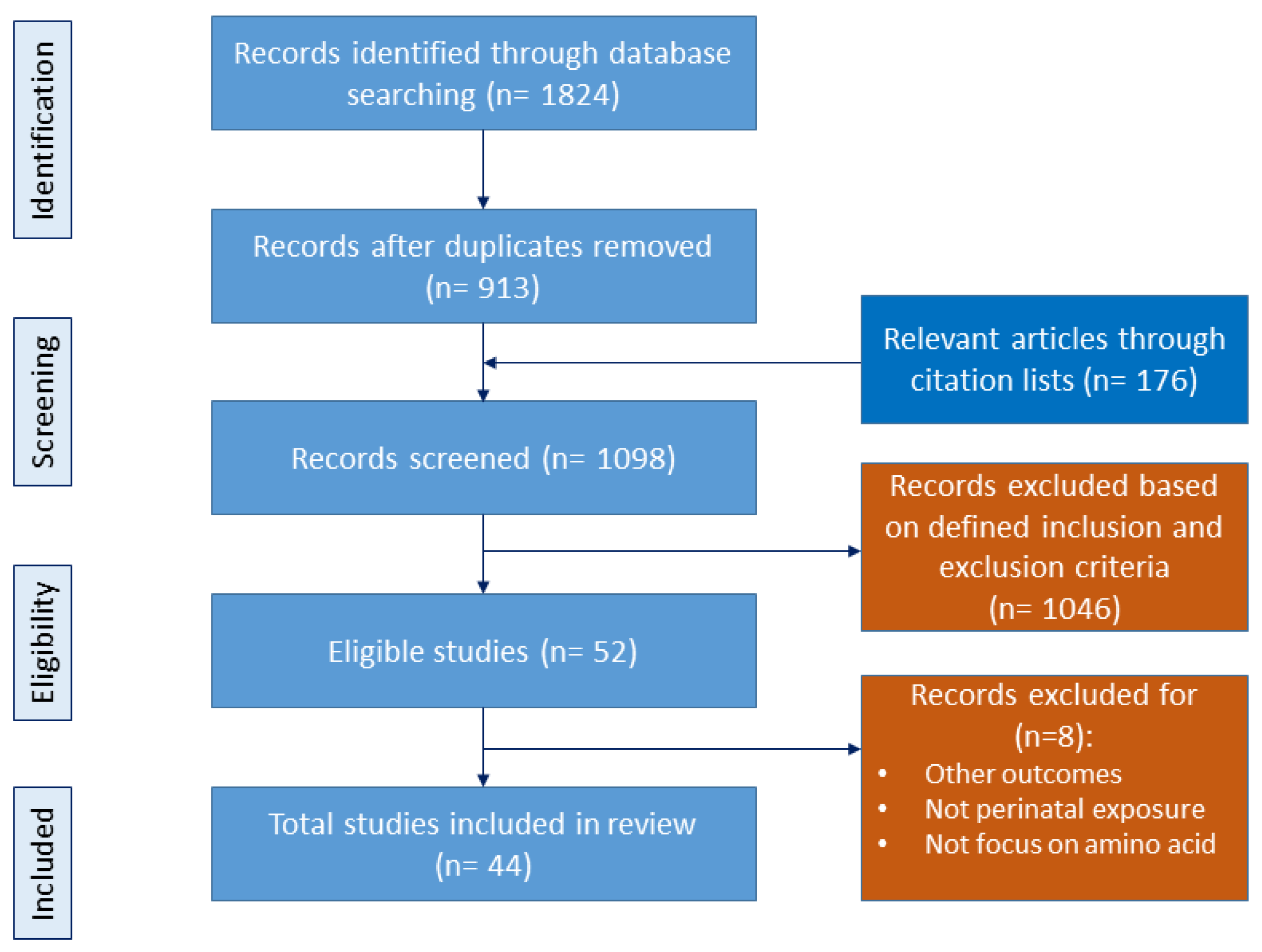
2.1. Data Sources and Search Strategy
2.2. Eligibility Criteria
2.3. Data Extraction and Synthesis
3. Impact of Amino Acids on Pregnancy and Fetal Development
3.1. The Impact of Amino Acids on Pregnancy
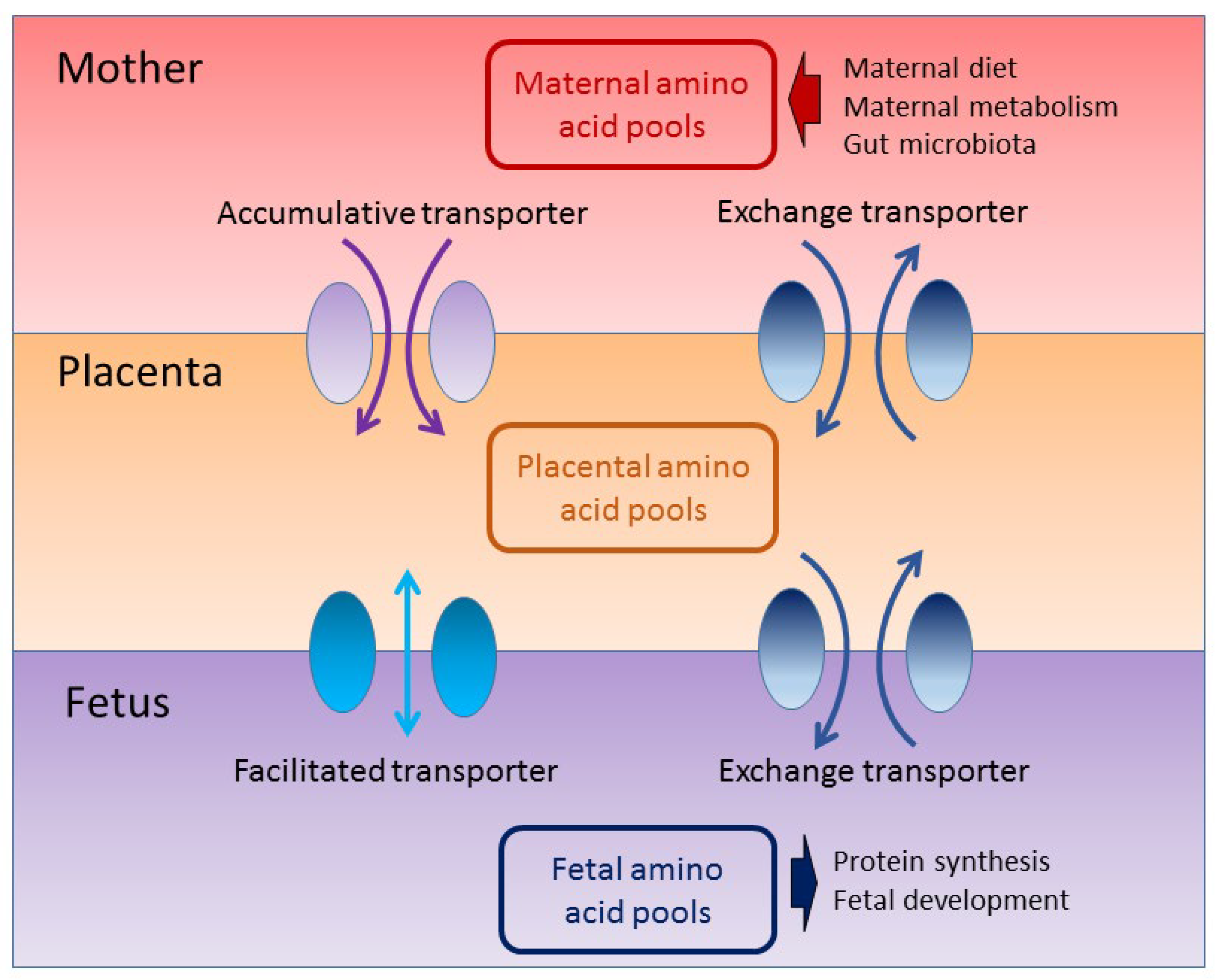
3.2. The Impact of Amino Acids on Fetal Development
4. The Connection between Dietary Amino Acids and Gut Microbiota
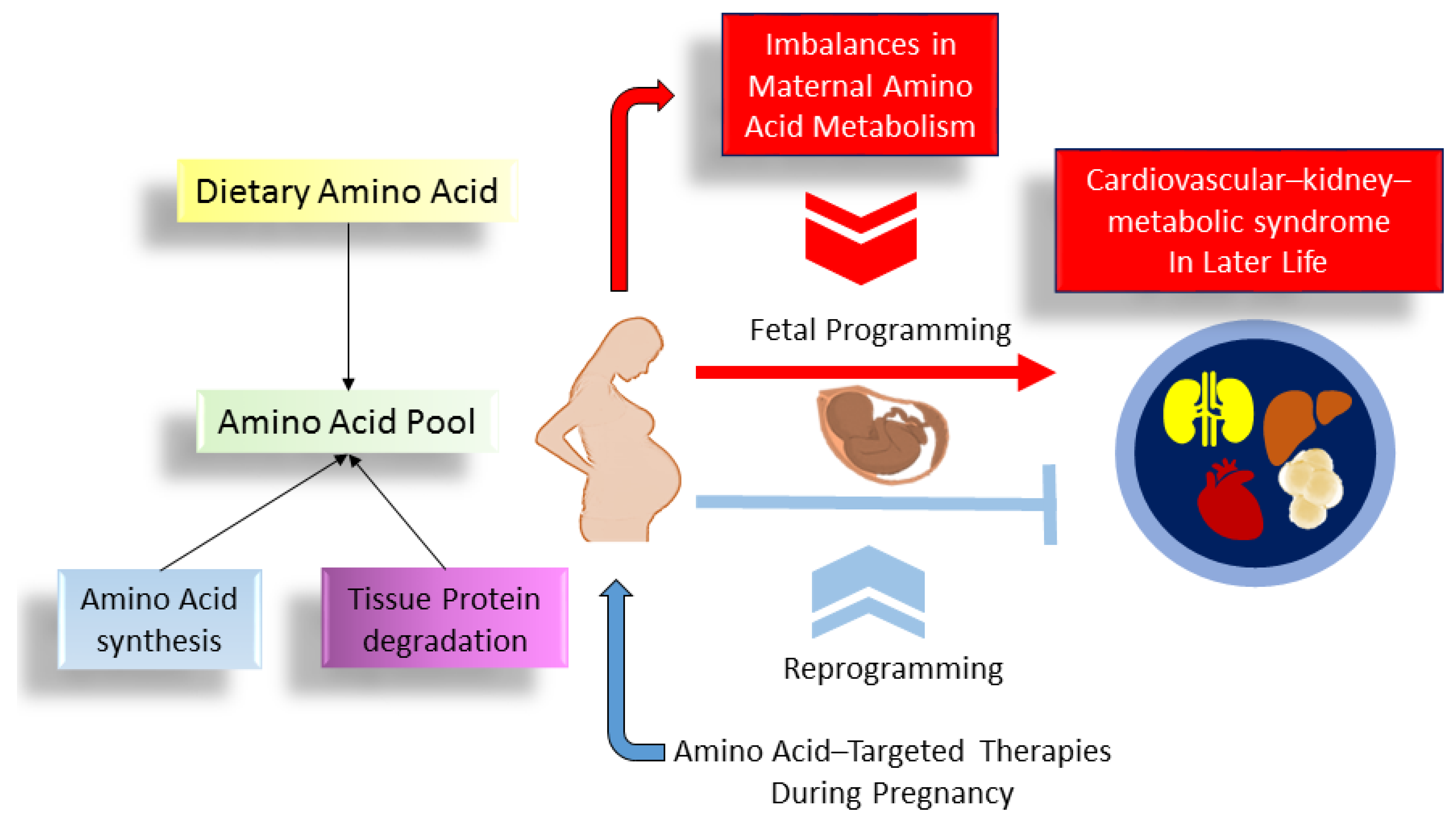
5. Amino Acids and CKM Syndrome
5.1. Hypertension and Cardiovascular Disease
5.2. Obesity
5.3. Diabetes
5.4. NAFLD and Dyslipidemia
5.5. CKD
6. Effects of Perinatal Amino Acid Supplementation on Offspring CKM Syndrome
6.1. Arginine
6.2. Citrulline
6.3. Taurine
6.4. Cysteine
6.5. Others
7. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Godfrey, K.M.; Barker, D.J. Fetal programming and adult health. Public Health Nutr. 2001, 4, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Langley-Evans, S.C. Nutrition in early life and the programming of adult disease: A review. J. Hum. Nutr. Diet. 2015, 28 (Suppl. S1), 1–14. [Google Scholar] [CrossRef] [PubMed]
- King, J.C. Physiology of pregnancy and nutrient metabolism. Am. J. Clin. Nutr. 2000, 71, 1218S–1225S. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J.P. Fetal nutrition and cardiovascular disease in later life. Br. Med. Bull. 1997, 53, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Tain, Y.L. Early-Life Programming and Reprogramming of Adult Kidney Disease and Hypertension: The Interplay between Maternal Nutrition and Oxidative Stress. Int. J. Mol. Sci. 2020, 21, 3572. [Google Scholar] [CrossRef] [PubMed]
- Armitage, J.A.; Khan, I.Y.; Taylor, P.D.; Nathanielsz, P.W.; Poston, L. Developmental programming of the metabolic syndrome by maternal nutritional imbalance: How strong is the evidence from experimental models in mammals? J. Physiol. 2004, 561, 355–377. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Tain, Y.L. The Good, the Bad, and the Ugly of Pregnancy Nutrients and Developmental Programming of Adult Disease. Nutrients 2019, 11, 894. [Google Scholar] [CrossRef] [PubMed]
- Hanson, M. The birth and future health of DOHaD. J. Dev. Orig. Health Dis. 2015, 6, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J.; Eriksson, J.G.; Forsen, T.; Osmond, C. Fetal origins of adult disease: Strength of effects and biological basis. Int. J. Epidemiol. 2002, 31, 1235–1239. [Google Scholar] [CrossRef]
- Tain, Y.L.; Joles, J.A. Reprogramming: A Preventive Strategy in Hypertension Focusing on the Kidney. Int. J. Mol. Sci. 2015, 17, 23. [Google Scholar] [CrossRef]
- Paauw, N.D.; Van Rijn, B.B.; Lely, A.T.; Joles, J.A. Pregnancy as a critical window for blood pressure regulation in mother and child: Programming and reprogramming. Acta Physiol. 2016, 219, 241–259. [Google Scholar] [CrossRef] [PubMed]
- Noyan-Ashraf, M.H.; Wu, L.; Wang, R.; Juurlink, B.H. Dietary approaches to positively influence fetal determinants of adult health. FASEB J. 2006, 20, 371–373. [Google Scholar] [CrossRef] [PubMed]
- Nüsken, E.; Dötsch, J.; Weber, L.T.; Nüsken, K.D. Developmental Programming of Renal Function and Re-Programming Approaches. Front. Pediatr. 2018, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Ndumele, C.E.; Rangaswami, J.; Chow, S.L.; Neeland, I.J.; Tuttle, K.R.; Khan, S.S.; Coresh, J.; Mathew, R.O.; Baker-Smith, C.M.; Carnethon, M.R.; et al. Cardiovascular-Kidney-Metabolic Health: A Presidential Advisory from the American Heart Association. Circulation 2023, 148, 1606–1635. [Google Scholar] [CrossRef] [PubMed]
- Jaradat, J.H.; Nashwan, A.J. Cardiovascular-kidney-metabolic syndrome: Understanding the interconnections and the need for holistic intervention. J. Med. Surg. Public Health 2023, 1, 100028. [Google Scholar] [CrossRef]
- Elango, R.; Ball, R.O. Protein and amino acid requirements during pregnancy. Adv. Nutr. 2016, 7, 839S–844S. [Google Scholar] [CrossRef] [PubMed]
- Manta-Vogli, P.D.; Schulpis, K.H.; Dotsikas, Y.; Loukas, Y.L. The significant role of amino acids during pregnancy: Nutritional support. J. Matern. Neonatal Med. 2020, 33, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes: Energy, carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids; National Academies Press: Washington, DC, USA, 2005; p. 19. [Google Scholar]
- Hsu, C.N.; Tain, Y.L. Amino Acids and Developmental Origins of Hypertension. Nutrients 2020, 12, 1763. [Google Scholar] [CrossRef] [PubMed]
- Duca, F.A.; Lam, T.K. Gut microbiota, nutrient sensing and energy balance. Diabetes Obes. Metab. 2014, 16, 68–76. [Google Scholar] [CrossRef]
- Hills, R.D., Jr.; Pontefract, B.A.; Mishcon, H.R.; Black, C.A.; Sutton, S.C.; Theberge, C.R. Gut Microbiome: Profound Implications for Diet and Disease. Nutrients 2019, 11, 1613. [Google Scholar] [CrossRef]
- Yang, T.; Richards, E.M.; Pepine, C.J.; Raizada, M.K. The gut microbiota and the brain-gut-kidney axis in hypertension and chronic kidney disease. Nat. Rev. Nephrol. 2018, 14, 442–456. [Google Scholar] [CrossRef]
- Witkowski, M.; Weeks, T.L.; Hazen, S.L. Gut Microbiota and Cardiovascular Disease. Circ. Res. 2020, 127, 553–570. [Google Scholar] [CrossRef]
- Al Khodor, S.; Shatat, I.F. Gut microbiome and kidney disease: A bidirectional relationship. Pediatr. Nephrol. 2017, 32, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Pluznick, J.L. Microbial short-chain fatty acids and blood pressure regulation. Curr. Hypertens. Rep. 2017, 19, 25. [Google Scholar] [CrossRef]
- Zixin, Y.; Lulu, C.; Xiangchang, Z.; Qing, F.; Binjie, Z.; Chunyang, L.; Tai, R.; Dongsheng, O. TMAO as a potential biomarker and therapeutic target for chronic kidney disease: A review. Front. Pharmacol. 2022, 13, 929262. [Google Scholar] [CrossRef]
- Chu, D.M.; Meyer, K.M.; Prince, A.L.; Aagaard, K.M. Impact of maternal nutrition in pregnancy and lactation on offspring gut microbial composition and function. Gut Microbes 2016, 7, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Felig, P.; Kim, Y.J.; Lynch, V.; Hendler, R. Amino acid metabolism during starvation in human pregnancy. J. Clin. Investig. 1972, 51, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Schoengold, D.M.; DeFiore, R.H.; Parlett, R.C. Free amino acids in plasma throughout pregnancy. Am. J. Obstet. Gynecol. 1978, 131, 490–499. [Google Scholar] [CrossRef]
- Payne, M.; Stephens, T.; Lim, K.; Ball, R.O.; Pencharz, P.B.; Elango, R. Lysine Requirements of Healthy Pregnant Women are Higher During Late Stages of Gestation Compared to Early Gestation. J. Nutr. 2018, 148, 94–99. [Google Scholar] [CrossRef]
- Ennis, M.A.; Rasmussen, B.F.; Lim, K.; Ball, R.O.; Pencharz, P.B.; Courtney-Martin, G.; Elango, R. Dietary phenylalanine requirements during early and late gestation in healthy pregnant women. Am. J. Clin. Nutr. 2020, 111, 351–359. [Google Scholar] [CrossRef]
- Soltesz, G.; Harris, D.; Mackenzie, I.Z.; Aynsley-Green, A. The metabolic and endocrine milieu of the human fetus and mother at 18–21 weeks of gestation. I. Plasma amino acid concentrations. Pediatr. Res. 1985, 19, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Cleal, J.K.; Lewis, R.M. The mechanisms and regulation of placental amino acid transport to the human foetus. J. Neuroendocrinol. 2008, 20, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Cleal, J.K.; Lofthouse, E.M.; Sengers, B.G.; Lewis, R.M. A systems perspective on placental amino acid transport. J. Physiol. 2018, 596, 5511–5522. [Google Scholar] [CrossRef] [PubMed]
- Goberdhan, D.C.; Wilson, C.; Harris, A.L. Amino acid sensing by mTORC1: Intracellular transporters mark the spot. Cell Metab. 2016, 23, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Cramer, S.; Beveridge, M.; Kilberg, M.; Novak, D. Physiological importance of system A-mediated amino acid transport to rat fetal development. Am. J. Physiol. Cell Physiol. 2002, 282, C153–C160. [Google Scholar] [CrossRef] [PubMed]
- Roos, S.; Kanai, Y.; Prasad, P.D.; Powell, T.L.; Jansson, T. Regulation of placental amino acid transporter activity by mammalian target of rapamycin. Am. J. Physiol. Cell Physiol. 2009, 296, C142–C150. [Google Scholar] [CrossRef] [PubMed]
- Avagliano, L.; Garò, C.; Marconi, A.M. Placental amino acids transport in intrauterine growth restriction. J. Pregnancy 2012, 2012, 972562. [Google Scholar] [CrossRef]
- Kalkhoff, R.K.; Kandaraki, E.; Morrow, P.G.; Mitchell, T.H.; Kelber, S.; Borkowf, H.I. Relationship between neonatal birth weight and maternal plasma amino acid profiles in lean and obese nondiabetic women and in type I diabetic pregnant women. Metabolism 1988, 37, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Bazer, F.W.; Satterfield, M.C.; Li, X.; Wang, X.; Johnson, G.A.; Burghardt, R.C.; Dai, Z.; Wang, J.; Wu, Z. Impacts of arginine nutrition on embryonic and fetal development in mammals. Amino Acids 2013, 45, 241–256. [Google Scholar] [CrossRef]
- Moores, R.R., Jr.; Rietberg, C.C.; Battaglia, F.C.; Fennessey, P.V.; Meschia, G. Metabolism and transport of maternal serine by the ovine placenta: Glycine production and absence of serine transport into the fetus. Pediatr. Res. 1993, 33, 590–594. [Google Scholar] [CrossRef]
- Jahan-Mihan, A.; Rodriguez, J.; Christie, C.; Sadeghi, M.; Zerbe, T. The Role of Maternal Dietary Proteins in Development of Metabolic Syndrome in Offspring. Nutrients 2015, 7, 9185–9217. [Google Scholar] [CrossRef] [PubMed]
- Langley-Evans, S.C. Nutritional programming of disease: Unravelling the mechanism. J. Anat. 2009, 215, 36–51. [Google Scholar] [CrossRef] [PubMed]
- Remacle, C.; Bieswal, F.; Bol, V.; Reusens, B. Developmental programming of adult obesity and cardiovascular disease in rodents by maternal nutrition imbalance. Am. J. Clin. Nutr. 2011, 94, 1846S–1852S. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Hou, C.Y.; Hsu, W.H.; Tain, Y.L. Cardiovascular Diseases of Developmental Origins: Preventive Aspects of Gut Microbiota-Targeted Therapy. Nutrients 2021, 13, 2290. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.V.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef]
- Mardinoglu, A.; Shoaie, S.; Bergentall, M.; Ghafari, P.; Zhang, C.; Larsson, E.; Backhed, F.; Nielsen, J. The gut microbiota modulates host amino acid and glutathione metabolism in mice. Mol. Syst. Biol. 2015, 11, 834. [Google Scholar] [CrossRef]
- Dai, Z.; Wu, Z.; Hang, S.; Zhu, W.; Wu, G. Amino acid metabolism in intestinal bacteria and its potential implications for mammalian reproduction. Mol. Hum. Reprod. 2015, 21, 389–409. [Google Scholar] [CrossRef] [PubMed]
- Hullar, M.A.J.; Fu, B.C. Diet, the gut microbiome, and epigenetics. Cancer J. 2014, 20, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Mu, C.L.; Yang, Y.X.; Yu, K.F.; Yu, M.; Zhang, C.J.; Su, Y.; Zhu, W.Y. Alteration of metabolomic markers of amino-acid metabolism in piglets with in-feed antibiotics. Amino Acids 2017, 49, 771–781. [Google Scholar] [CrossRef]
- Neis, E.P.; Dejong, C.H.; Rensen, S.S. The role of microbial amino acid metabolism in host metabolism. Nutrients 2015, 7, 2930–2946. [Google Scholar] [CrossRef]
- Davila, A.M.; Blachier, F.; Gotteland, M.; Andriamihaja, M.; Benetti, P.H.; Sanz, Y.; Tome, D. Re-print of “Intestinal luminal nitrogen metabolism: Role of the gut microbiota and consequences for the host”. Pharmacol. Res. 2013, 69, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Liu, W.; Piao, M.; Zhu, H. A review of the relationship between the gut microbiota and amino acid metabolism. Amino Acids 2017, 49, 2083–2090. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.N.; Jiang, Y.F.; Ru, J.N.; Lu, J.H.; Ding, B.; Wu, J. Amino acid metabolism in health and disease. Signal Transduct. Target. Ther. 2023, 8, 345. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, Y. Amino acids that centrally influence blood pressure and regional blood flow in conscious rats. J. Amino Acids 2012, 2012, 831759. [Google Scholar] [CrossRef] [PubMed]
- Nitz, K.; Lacy, M.; Atzler, D. Amino Acids and Their Metabolism in Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, S.; Subramani, E.; Mitra, I.; Bhattacharya, A.; Sherpa, D.D.; Joshi, M.; Chakraborty, P.; Ray, C.D.; Chaudhury, K. Discovery of novel metabolic signatures for early identification of women at risk of developing gestational hypertension. Metabolomics 2023, 19, 50. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Morris, S.M., Jr. Arginine metabolism: Nitric oxide and beyond. Biochem. J. 1998, 336, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Leiper, J.; Vallance, P. Biological significance of endogenous methylarginines that inhibit nitric oxide synthases. Cardiovasc. Res. 1999, 43, 542–548. [Google Scholar] [CrossRef]
- Böger, R.H.; Bode-Böger, S.M.; Szuba, A.; Tsao, P.S.; Chan, J.R.; Tangphao, O.; Blaschke, T.F.; Cooke, J.P. Asymmetric dimethylarginine (ADMA): A novel risk factor for endothelial dysfunction: Its role in hypercholesterolemia. Circulation 1998, 98, 1842–1847. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hsu, C.N. Toxic Dimethylarginines: Asymmetric Dimethylarginine (ADMA) and Symmetric Dimethylarginine (SDMA). Toxins 2017, 9, 92. [Google Scholar] [CrossRef]
- Koeners, M.P.; van Faassen, E.E.; Wesseling, S.; de Sain-van der Velden, M.; Koomans, H.A.; Braam, B.; Joles, J.A. Maternal Supplementation with Citrulline Increases Renal Nitric Oxide in Young Spontaneously Hypertensive Rats and Has Long-Term Antihypertensive Effects. Hypertension 2007, 50, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Moali, C.; Boucher, J.L.; Sari, M.A.; Stuehr, D.J.; Mansuy, D. Substrate specificity of NO synthases: Detailed comparison of L-arginine, homo-L-arginine, their N omega-hydroxy derivatives, and N omega-hydroxynor-L-arginine. Biochemistry 1998, 37, 10453–10460. [Google Scholar] [CrossRef] [PubMed]
- Koch, V.; Gruenewald, L.D.; Gruber-Rouh, T.; Martin, S.; Eichler, K.; Booz, C.; Yel, I.; Vogl, T.J.; Buchner, K.; Hagenmueller, M.; et al. Homoarginine treatment of rats improves cardiac function and remodeling in response to pressure overload. Fundam. Clin. Pharmacol. 2022, 36, 992–1004. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.L.; Jacobsen, D.W.; Robinson, K. Homocysteine and coronary atherosclerosis. J. Am. Coll. Cardiol. 1996, 27, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Van Guldener, C.; Nanayakkara, P.W.; Stehouwer, C.D. Homocysteine and blood pressure. Curr. Hypertens. Rep. 2003, 5, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Robaczewska, J.; Kedziora-Kornatowska, K.; Kozakiewicz, M.; Zary-Sikorska, E.; Pawluk, H.; Pawliszak, W.; Kedziora, J. Role of glutathione metabolism and glutathione-related antioxidant defense systems in hypertension. J. Physiol. Pharmacol. 2016, 67, 331–337. [Google Scholar] [PubMed]
- Hsu, C.N.; Tain, Y.L. Hydrogen Sulfide in Hypertension and Kidney Disease of Developmental Origins. Int. J. Mol. Sci. 2018, 19, 1438. [Google Scholar] [CrossRef] [PubMed]
- Vasdev, S.; Singal, P.; Gill, V. The antihypertensive effect of cysteine. Int. J. Angiol. 2009, 18, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Abebe, W.; Mozaffari, M.S. Role of taurine in the vasculature: An overview of experimental and human studies. Am. J. Cardiovasc. Dis. 2011, 1, 293–311. [Google Scholar]
- Militante, J.D.; Lombardini, J.B. Treatment of hypertension with oral taurine: Experimental and clinical studies. Amino Acids 2002, 23, 381–393. [Google Scholar] [CrossRef]
- McGarrah, R.W.; White, P.J. Branched-chain amino acids in cardiovascular disease. Nat. Rev. Cardiol. 2023, 20, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, K.; Liu, F.; Lu, X.; Huang, J.; Gu, D. Association of circulating branched-chain amino acids with risk of cardiovascular disease: A systematic review and meta-analysis. Atherosclerosis 2022, 350, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Sudar-Milovanovic, E.; Gluvic, Z.; Obradovic, M.; Zaric, B.; Isenovic, E.R. Tryptophan Metabolism in Atherosclerosis and Diabetes. Curr. Med. Chem. 2022, 29, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, H.; McKenzie, G.; Witting, P.K.; Stasch, J.P.; Hahn, M.; Changsirivathanathamrong, D.; Wu, B.J.; Ball, H.J.; Thomas, S.R.; et al. Kynurenine is an endothelium-derived relaxing factor produced during inflammation. Nat. Med. 2010, 16, 279–285. [Google Scholar] [CrossRef]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Fang, J.; Yin, Y.L.; Feng, Z.M.; Tang, Z.R.; Wu, G. Tryptophan metabolism in animals: Important roles in nutrition and health. Front. Biosci. Schol. Ed. 2011, 3, 286–297. [Google Scholar]
- Stone, T.W.; Darlington, L.G. Endogenous kynurenines as targets for drug discovery and development. Nat. Rev. Drug Discov. 2002, 1, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Fregly, M.J.; Lockley, O.E.; Cade, J.R. Effect of chronic dietary treatment with L-tryptophan on the development of renal hypertension in rats. Pharmacology 1988, 36, 91–100. [Google Scholar] [CrossRef]
- Bartosiewicz, J.; Kaminski, T.; Pawlak, K.; Karbowska, M.; Tankiewicz-Kwedlo, A.; Pawlak, D. The activation of the kynurenine pathway in a rat model with renovascular hypertension. Exp. Biol. Med. 2017, 242, 750–761. [Google Scholar] [CrossRef]
- Addi, T.; Dou, L.; Burtey, S. Tryptophan-Derived Uremic Toxins and Thrombosis in Chronic Kidney Disease. Toxins 2018, 10, 412. [Google Scholar] [CrossRef]
- Pawlak, D.; Pawlak, K.; Malyszko, J.; Mysliwiec, M.; Buczko, W. Accumulation of toxic products degradation of kynurenine in hemodialyzed patients. Int. Urol. Nephrol. 2001, 33, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Watts, S.W.; Morrison, S.F.; Davis, R.P.; Barman, S.M. Serotonin and blood pressure regulation. Pharmacol. Rev. 2012, 64, 59–88. [Google Scholar] [CrossRef] [PubMed]
- Baron, A.; Riesselmann, A.; Fregly, M.J. Reduction in the elevated blood pressure of Dahl salt-sensitive rats treated chronically with L-5-hydroxytryptophan. Pharmacology 1991, 42, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Simonson, M.; Boirie, Y.; Guillet, C. Protein, amino acids and obesity treatment. Rev. Endocr. Metab. Disord. 2020, 21, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Duan, Y.; Li, F.; Tan, B.; Hou, Y.; Wu, G.; Yin, Y. Leucine in Obesity: Therapeutic Prospects. Trends Pharmacol. Sci. 2016, 37, 714–727. [Google Scholar] [CrossRef] [PubMed]
- Spring, S.; Singh, A.; Zapata, R.; Chelikani, P.; Pezeshki, A. Methionine restriction partly recapitulates the sympathetically mediated enhanced energy expenditure induced by Total amino acid restriction in rats. Nutrients 2019, 11, 707. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yang, Y.; Sun, J.; Zhang, Y.; Luo, T.; Li, B.; Jiang, Y.; Shi, Y.; Le, G. Dietary methionine restriction ameliorates the impairment of learning and memory function induced by obesity in mice. Food Funct. 2019, 10, 1411–1425. [Google Scholar] [CrossRef]
- De Spiegeleer, M.; De Paepe, E.; Van Meulebroek, L.; Gies, I.; De Schepper, J.; Vanhaecke, L. Paediatric obesity: A systematic review and pathway mapping of metabolic alterations underlying early disease processes. Mol. Med. 2021, 27, 145. [Google Scholar] [CrossRef]
- Zapata, R.C.; Singh, A.; Ajdari, N.M.; Chelikani, P.K. Dietary Tryptophan Restriction Dose-Dependently Modulates Energy Balance, Gut Hormones, and Microbiota in Obesity-Prone Rats. Obesity 2018, 26, 730–739. [Google Scholar] [CrossRef]
- Zapata, R.C.; Singh, A.; Pezeshki, A.; Chelikani, P.K. Tryptophan restriction partially recapitulates the age-dependent effects of total amino acid restriction on energy balance in diet-induced obese rats. J. Nutr. Biochem. 2019, 65, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Chaouche, L.; Marcotte, F.; Maltais-Payette, I.; Tchernof, A. Glutamate and obesity—What is the link? Curr. Opin. Clin. Nutr. Metab. Care 2024, 27, 70–76. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Hruby, A.; Toledo, E.; Clish, C.B.; Martínez-González, M.A.; Salas-Salvadó, J.; Hu, F.B. Metabolomics in Prediabetes and Diabetes: A Systematic Review and Meta-analysis. Diabetes Care 2016, 39, 833–846. [Google Scholar] [CrossRef] [PubMed]
- Holeček, M. Role of Impaired Glycolysis in Perturbations of Amino Acid Metabolism in Diabetes Mellitus. Int. J. Mol. Sci. 2023, 24, 1724. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; McGarrah, R.W.; Herman, M.A.; Bain, J.R.; Shah, S.H.; Newgard, C.B. Insulin action, type 2 diabetes, and branched-chain amino acids: A two-way street. Mol. Metab. 2021, 52, 101261. [Google Scholar] [CrossRef] [PubMed]
- Dollet, L.; Kuefner, M.; Caria, E.; Rizo-Roca, D.; Pendergrast, L.; Abdelmoez, A.M.; Karlsson, H.K.R.; Björnholm, M.; Dalbram, E.; Treebak, J.T.; et al. Glutamine Regulates Skeletal Muscle Immunometabolism in Type 2 Diabetes. Diabetes 2022, 71, 624–636. [Google Scholar] [CrossRef] [PubMed]
- Yan-Do, R.; MacDonald, P.E. Impaired “Glycine”-mia in Type 2 Diabetes and Potential Mechanisms Contributing to Glucose Homeostasis. Endocrinology 2017, 158, 1064–1073. [Google Scholar] [CrossRef] [PubMed]
- Putnam, K.; Shoemaker, R.; Yiannikouris, F.; Cassis, L.A. The renin-Angiotensin system: A target of and contributor to dyslipidemias, altered glucose homeostasis, and hypertension of the metabolic syndrome. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H1219–H1230. [Google Scholar] [CrossRef] [PubMed]
- Gaggini, M.; Carli, F.; Rosso, C.; Buzzigoli, E.; Marietti, M.; Della Latta, V.; Ciociaro, D.; Abate, M.L.; Gambino, R.; Cassader, M.; et al. Altered amino acid concentrations in NAFLD: Impact of obesity and insulin resistance. Hepatology 2018, 67, 145–158. [Google Scholar] [CrossRef]
- Kim, H.Y. Recent advances in nonalcoholic fatty liver disease metabolomics. Clin. Mol. Hepatol. 2021, 27, 553–559. [Google Scholar] [CrossRef]
- Rom, O.; Liu, Y.; Liu, Z.; Zhao, Y.; Wu, J.; Ghrayeb, A.; Villacorta, L.; Fan, Y.; Chang, L.; Wang, L.; et al. Glycine-based treatment ameliorates NAFLD by modulating fatty acid oxidation, glutathione synthesis, and the gut microbiome. Sci. Transl. Med. 2020, 12, eaaz2841. [Google Scholar] [CrossRef]
- Lin, Z.; Cai, F.; Lin, N.; Ye, J.; Zheng, Q.; Ding, G. Effects of glutamine on oxidative stress and nuclear factor-κB expression in the livers of rats with nonalcoholic fatty liver disease. Exp. Ther. Med. 2014, 7, 365–370. [Google Scholar] [CrossRef]
- Fang, T.; Wang, H.; Pan, X.; Little, P.J.; Xu, S.; Weng, J. Mouse models of nonalcoholic fatty liver disease (NAFLD): Pathomechanisms and pharmacotherapies. Int. J. Biol. Sci. 2022, 18, 5681–5697. [Google Scholar] [CrossRef] [PubMed]
- Garibotto, G.; Pastorino, N.; Dertenois, L. Nutritional Management of renal diseases. In Protein and Amino Acid Metabolism in Renal Disease and in Renal Failure; Kopple, J.D., Massry, S., Eds.; William and Wilkins: Philadelphia, PA, USA, 2003; pp. 20–32. [Google Scholar]
- Kopple, J.D. Phenylalanine and tyrosine metabolism in chronic kidney failure. J. Nutr. 2007, 137, 1586S–1590S. [Google Scholar] [CrossRef]
- Vallance, P.; Leone, A.; Calver, A.; Collier, J.; Moncada, S. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet 1992, 339, 572–575. [Google Scholar]
- Hasegawa, S.; Jao, T.M.; Inagi, R. Dietary Metabolites and Chronic Kidney Disease. Nutrients 2017, 9, 358. [Google Scholar] [CrossRef] [PubMed]
- Scott, S.A.; Fu, J.; Chang, P.V. Microbial tryptophan metabolites regulate gut barrier function via the aryl hydrocarbon receptor. Proc. Natl. Acad. Sci. USA 2020, 117, 19376–19387. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.R.; Miao, H.; Deng, D.Q.; Vaziri, N.D.; Li, P.; Zhao, Y.Y. Gut microbiota-derived tryptophan metabolism mediates renal fibrosis by aryl hydrocarbon receptor signaling activation. Cell Mol. Life Sci. 2021, 78, 909–922. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.L.; Regnault, T.R. Nutrition in Pregnancy: Optimising Maternal Diet and Fetal Adaptations to Altered Nutrient Supply. Nutrients 2016, 8, 342. [Google Scholar] [CrossRef]
- Gingras, V.; Hivert, M.F.; Oken, E. Early-Life Exposures and Risk of Diabetes Mellitus and Obesity. Curr. Diab. Rep. 2018, 18, 89. [Google Scholar] [CrossRef]
- Yadav, A.; Beilin, L.J.; Huang, R.C.; Vlaskovsky, P.; Newnham, J.P.; White, S.W.; Mori, T.A. The relationship between intrauterine foetal growth trajectories and blood pressure in young adults. J. Hypertens. 2022, 40, 478–489. [Google Scholar] [CrossRef]
- Zohdi, V.; Pearson, J.T.; Kett, M.M.; Lombardo, P.; Schneider, M.; Black, M.J. When early life growth restriction in rats is followed by attenuated postnatal growth: Effects on cardiac function in adulthood. Eur. J. Nutr. 2015, 54, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Terstappen, F.; Tol, A.J.C.; Gremmels, H.; Wever, K.E.; Paauw, N.D.; Joles, J.A.; Beek, E.M.V.; Lely, A.T. Prenatal Amino Acid Supplementation to Improve Fetal Growth: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 2535. [Google Scholar] [CrossRef]
- Luo, K.; Chen, P.; Li, S.; Li, W.; He, M.; Wang, T.; Chen, J. Effect of L-arginine supplementation on the hepatic phosphatidylinositol 3-kinase signaling pathway and gluconeogenic enzymes in early intrauterine growth-restricted rats. Exp. Ther. Med. 2017, 14, 2355–2360. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Hsieh, C.S.; Lin, I.C.; Chen, C.C.; Sheen, J.M.; Huang, L.T. Effects of maternal L-citrulline supplementation on renal function and blood pressure in offspring exposed to maternal caloric restriction: The impact of nitric oxide pathway. Nitric Oxide 2010, 23, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Sheen, J.M.; Chen, C.C.; Yu, H.R.; Tiao, M.M.; Kuo, H.C.; Huang, L.T. Maternal citrulline supplementation prevents prenatal dexamethasone-induced programmed hypertension. Free Radic. Res. 2014, 48, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Lee, W.C.; Hsu, C.N.; Lee, W.C.; Huang, L.T.; Lee, C.T.; Lin, C.Y. Asymmetric dimethylarginine is associated with developmental programming of adult kidney disease and hypertension in offspring of streptozotocin-treated mothers. PLoS ONE 2013, 8, e55420. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Huang, L.T.; Lee, C.T.; Chan, J.Y.; Hsu, C.N. Maternal citrulline supplementation prevents prenatal NG-nitro-l-arginine-methyl ester (L-NAME)-induced programmed hypertension in rats. Biol. Reprod. 2015, 92, 7. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Hsu, C.N. Perinatal Use of Citrulline Rescues Hypertension in Adult Male Offspring Born to Pregnant Uremic Rats. Int. J. Mol. Sci. 2024, 25, 1612. [Google Scholar] [CrossRef]
- Li, M.; Reynolds, C.M.; Gray, C.; Patel, R.; Sloboda, D.M.; Vickers, M.H. Long-term effects of a maternal high-fat: High-fructose diet on offspring growth and metabolism and impact of maternal taurine supplementation. J. Dev. Orig. Health Dis. 2020, 11, 419–426. [Google Scholar] [CrossRef]
- Arany, E.; Strutt, B.; Romanus, P.; Remacle, C.; Reusens, B.; Hill, D.J. Taurine supplement in early life altered islet morphology, decreased insulitis and delayed the onset of diabetes in non-obese diabetic mice. Diabetologia 2004, 47, 1831–1837. [Google Scholar] [CrossRef]
- Roysommuti, S.; Lerdweeraphon, W.; Malila, P.; Jirakulsomchok, D.; Wyss, J.M. Perinatal taurine alters arterial pressure control and renal function in adult offspring. Adv. Exp. Med. Biol. 2009, 643, 145–156. [Google Scholar]
- Tain, Y.L.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Hsu, C.N. Protective Role of Taurine on Rat Offspring Hypertension in the Setting of Maternal Chronic Kidney Disease. Antioxidants 2023, 12, 2059. [Google Scholar] [CrossRef] [PubMed]
- Thaeomor, A.; Teangphuck, P.; Chaisakul, J.; Seanthaweesuk, S.; Somparn, N.; Roysommuti, S. Perinatal Taurine Supplementation Prevents Metabolic and Cardiovascular Effects of Maternal Diabetes in Adult Rat Offspring. Adv. Exp. Med. Biol. 2017, 975, 295–305. [Google Scholar] [PubMed]
- Thaeomor, A.; Tangnoi, C.; Seanthaweesuk, S.; Somparn, N.; Roysommuti, S. Perinatal Taurine Supplementation Prevents the Adverse Effects of Maternal Dyslipidemia on Growth and Cardiovascular Control in Adult Rat Offspring. Adv. Exp. Med. Biol. 2019, 1155, 415–427. [Google Scholar] [PubMed]
- Mensegue, M.F.; Burgueño, A.L.; Tellechea, M.L. Perinatal taurine exerts a hypotensive effect in male spontaneously hypertensive rats and down-regulates endothelial oxide nitric synthase in the aortic arch. Clin. Exp. Pharmacol. Physiol. 2020, 47, 780–789. [Google Scholar] [CrossRef]
- Horie, R.; Yamori, Y.; Nara, Y.; Sawamura, M.; Mano, M. Effects of sulphur amino acids on the development of hypertension and atherosclerosis in stroke-prone spontaneously hypertensive rats. J. Hypertens. Suppl. 1987, 5, S223–S225. [Google Scholar]
- Hsu, C.N.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Tain, Y.L. Dietary Supplementation with Cysteine during Pregnancy Rescues Maternal Chronic Kidney Disease-Induced Hypertension in Male Rat Offspring: The Impact of Hydrogen Sulfide and Microbiota-Derived Tryptophan Metabolites. Antioxidants 2022, 11, 483. [Google Scholar] [CrossRef]
- Tai, I.H.; Sheen, J.M.; Lin, Y.J.; Yu, H.R.; Tiao, M.M.; Chen, C.C.; Huang, L.T.; Tain, Y.L. Maternal N-acetylcysteine therapy regulates hydrogen sulfide-generating pathway and prevents programmed hypertension in male offspring exposed to prenatal dexamethasone and postnatal high-fat diet. Nitric Oxide 2016, 53, 6–12. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hsu, C.N.; Lee, C.Y.; Lin, Y.J.; Tsai, C.C. N-Acetylcysteine prevents programmed hypertension in male rat offspring born to suramin-treated mothers. Biol. Reprod. 2016, 95, 8. [Google Scholar] [CrossRef]
- Tain, Y.L.; Lee, C.T.; Chan, J.Y.; Hsu, C.N. Maternal melatonin or N-acetylcysteine therapy regulates hydrogen sulfide-generating pathway and renal transcriptome to prevent prenatal N(G)-Nitro-L-arginine-methyl ester (L-NAME)-induced fetal programming of hypertension in adult male offspring. Am. J. Obstet. Gynecol. 2016, 215, 636.e1–636.e72. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Huang, D.X.; Li, Y.; Dasgupta, C.; Wang, L.; Zhang, L. Antenatal antioxidant prevents nicotine-mediated hypertensive response in rat adult offspring. Biol. Reprod. 2015, 93, 66. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.A.; Dunn, R.L.; Marchand, M.C.; Langley-Evans, S.C. Increased systolic blood pressure in rats induced by a maternal low-protein diet is reversed by dietary supplementation with glycine. Clin. Sci. 2002, 103, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Yura, S.; Tatsumi, K.; Kondoh, E.; Mogami, H.; Fujita, K.; Kakui, K.; Aoe, S.; Itoh, H.; Sagawa, N.; et al. Branched-chain amino acid supplemented diet during maternal food restriction prevents developmental hypertension in adult rat offspring. J. Dev. Orig. Health Dis. 2011, 2, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, J.; Kwon, Y.H. Leucine supplementation in maternal high-fat diet alleviated adiposity and glucose intolerance of adult mice offspring fed a postweaning high-fat diet. Lipids Health Dis. 2023, 22, 50. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Lin, I.C.; Yu, H.R.; Huang, L.T.; Tiao, M.M.; Tain, Y.L. Maternal Tryptophan Supplementation Protects Adult Rat Offspring against Hypertension Programmed by Maternal Chronic Kidney Disease: Implication of Tryptophan-Metabolizing Microbiome and Aryl Hydrocarbon Receptor. Int. J. Mol. Sci. 2020, 21, 4552. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P. The Laboratory Rat: Relating Its Age with Human’s. Int. J. Prev. Med. 2013, 4, 624–630. [Google Scholar] [PubMed]
- Luiking, Y.C.; Ten Have, G.A.M.; Wolfe, R.R.; Deutz, N.E.P. Arginine de novo and nitric oxide production in disease states. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E1177–E1189. [Google Scholar] [CrossRef]
- Grimble, G.K. Adverse gastrointestinal effects of arginine and related amino acids. J. Nutr. 2007, 137, 1693S–1701S. [Google Scholar] [CrossRef]
- Rodrigues-Krause, J.; Krause, M.; Rocha, I.M.G.D.; Umpierre, D.; Fayh, A.P.T. Association of l-Arginine Supplementation with Markers of Endothelial Function in Patients with Cardiovascular or Metabolic Disorders: A Systematic Review and Meta-Analysis. Nutrients 2018, 11, 15. [Google Scholar] [CrossRef]
- Racasan, S.; Braam, B.; van der Giezen, D.M.; Goldschmeding, R.; Boer, P.; Koomans, H.A.; Joles, J.A. Perinatal L-arginine and antioxidant supplements reduce adult blood pressure in spontaneously hypertensive rats. Hypertension 2004, 44, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Koeners, M.P.; Racasan, S.; Koomans, H.A.; Joles, J.A.; Braam, B. Nitric oxide, superoxide and renal blood flow autoregulation in SHR after perinatal L-arginine and antioxidants. Acta Physiol. 2007, 190, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Koeners, M.P.; Braam, B.; van der Giezen, D.M.; Goldschmeding, R.; Joles, J.A. Perinatal micronutrient supplements ameliorate hypertension and proteinuria in adult fawn-hooded hypertensive rats. Am. J. Hypertens. 2010, 23, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Alves, G.M.; Barão, M.A.; Odo, L.N.; Nascimento Gomes, G.; Franco Md Mdo, C.; Nigro, D.; Lucas, S.R.; Laurindo, F.R.; Brandizzi, L.I.; Zaladek Gil, F. L-Arginine effects on blood pressure and renal function of intrauterine restricted rats. Pediatr. Nephrol. 2002, 17, 856–862. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, D.S.; Diniz, M.M.; Haidar, A.A.; Cavanal, M.F.; da Silva Alves, E.; Carpinelli, A.R.; Gil, F.Z.; Hirata, A.E. L-Arginine supplementation improves insulin sensitivity and beta cell function in the offspring of diabetic rats through AKT and PDX-1 activation. Eur. J. Pharmacol. 2016, 791, 780–787. [Google Scholar] [CrossRef]
- Lassala, A.; Bazer, F.W.; Cudd, T.A.; Datta, S.; Keisler, D.H.; Satterfield, M.C.; Spencer, T.E.; Wu, G. Parenteral administration of L-arginine prevents fetal growth restriction in undernourished ewes. J. Nutr. 2010, 140, 1242–1248. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.D.; Wu, X.; Yin, Y.L.; Liu, Y.Q.; Geng, M.M.; Yang, H.S.; Blachier, F.; Wu, G.Y. Effects of dietary L-arginine or N-car-bamylglutamate supplementation during late gestation of sows on the miR-15b/16, miR-221/222, VEGFA and eNOS expression in umbilical vein. Amino Acids 2012, 42, 2111–2119. [Google Scholar] [CrossRef]
- Cynober, L.; Moinard, C.; De Bandt, J.P. The 2009 ESPEN Sir David Cuthbertson. Citrulline: A new major signaling molecule or just another player in the pharmaconutrition game? Clin. Nutr. 2010, 29, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Schwedhelm, E.; Maas, R.; Freese, R.; Jung, D.; Lukacs, Z.; Jambrecina, A.; Spickler, W.; Schulze, F.; Boger, R.H. Pharmacokinetic and pharmacodynamic properties of oral L-citrulline and L-arginine: Impact on nitric oxide metabolism. Br. J. Clin. Pharmacol. 2008, 65, 51–59. [Google Scholar] [CrossRef]
- Boucknooghe, T.; Remacle, C.; Reusens, B. Is taurine a functional nutrient? Curr. Opin. Clin. Nutr. Metab. Care 2006, 9, 728–733. [Google Scholar] [CrossRef]
- Duszka, K. Versatile Triad Alliance: Bile Acid, Taurine and Microbiota. Cells 2022, 11, 2337. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Cheng, M.C.; Hsu, C.N.; Tain, Y.L. Sulfur-Containing Amino Acids, Hydrogen Sulfide, and Sulfur Compounds on Kidney Health and Disease. Metabolites 2023, 13, 688. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Lin, Y.J.; Lu, P.C.; Tain, Y.L. Early supplementation of D-cysteine or L-cysteine prevents hypertension and kidney damage in spontaneously hypertensive rats exposed to high-salt intake. Mol. Nutr. Food Res. 2018, 62, 2. [Google Scholar] [CrossRef]
- Blachier, F.; Beaumont, M.; Kim, E. Cysteine-derived hydrogen sulfide and gut health: A matter of endogenous or bacterial origin. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, M.; Roura, E.; Lambert, W.; Turni, C.; Michiels, J.; Chalvon-Demersay, T. Selective nourishing of gut microbiota with amino acids: A novel prebiotic approach? Front. Nutr. 2022, 9, 1066898. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.F.; O’Keefe, J.H.; DiNicolantonio, J.J. Dietary Glycine Is Rate-Limiting for Glutathione Synthesis and May Have Broad Potential for Health Protection. Ochsner. J. 2018, 18, 81–87. [Google Scholar] [PubMed]
- Siomkajło, M.; Rybka, J.; Mierzchała-Pasierb, M.; Gamian, A.; Stankiewicz-Olczyk, J.; Bolanowski, M.; Daroszewski, J. Specific plasma amino acid disturbances associated with metabolic syndrome. Endocrine 2017, 58, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Sun, L.; Gong, Y.; Zhou, Y.; Yang, P.; Ye, Z.; Fu, J.; Huang, A.; Fu, Z.; Yu, W.; et al. Relationship between Branched-Chain Amino Acids, Metabolic Syndrome, and Cardiovascular Risk Profile in a Chinese Population: A Cross-Sectional Study. Int. J. Endocrinol. 2016, 2016, 8173905. [Google Scholar] [CrossRef] [PubMed]
- Jennings, A.; MacGregor, A.; Pallister, T.; Spector, T.; Cassidy, A. Associations between branched chain amino acid intake and biomarkers of adiposity and cardiometabolic health independent of genetic factors: A twin study. Int. J. Cardiol. 2016, 223, 992–998. [Google Scholar] [CrossRef]
- Bokor, S.; Vass, R.A.; Funke, S.; Ertl, T.; Molnár, D. Epigenetic Effect of Maternal Methyl-Group Donor Intake on Offspring’s Health and Disease. Life 2022, 12, 609. [Google Scholar] [CrossRef]
| Amino acid Supplementation Dose | Period | Experimental Model | Species | Age at Evaluation (Weeks) | Protective Effects | Ref. |
|---|---|---|---|---|---|---|
| Arginine | ||||||
| 200 mg/kg/day | Lactation | Maternal protein restriction | SD rat/M | 8 | Hepatic insulin signaling and gene expression were prevented | [116] |
| Citrulline | ||||||
| 2.5 g/L in drinking water | Pregnancy and lactation | Maternal caloric restriction | SD rat/M | 12 | Kidney disease was prevented | [117] |
| 2.5 g/L in drinking water | Pregnancy and lactation | Antenatal dexamethasone exposure | SD rat/M | 16 | Hypertension was prevented | [118] |
| 2.5 g/L in drinking water | Pregnancy and lactation | STZ-induced diabetes | SD rat/M | 12 | Hypertension and kidney disease were prevented | [119] |
| 2.5 g/L in drinking water | Pregnancy and lactation | Maternal L-NAME exposure | SD rat/M | 12 | Hypertension was prevented | [120] |
| 2.5 g/L in drinking water | Pregnancy and lactation | Maternal CKD | SD rat/M | 12 | Hypertension was prevented | [121] |
| 2.5 g/L in drinking water | From gestational day 7 to postnatal week 6 | Genetic hypertension model | SHR/M and F | 50 | Hypertension was prevented | [62] |
| Taurine | ||||||
| 1.5% in drinking water | Pregnancy and lactation | Maternal high-fat/high-fructose diet | Wistar rat/M and F | 21 | Obesity was prevented in M | [122] |
| 2.5% in drinking water | Pregnancy and lactation | Genetic hypertension model | NOD mice/M | 50 | Onset time of diabetes was postponed | [123] |
| 3% in drinking water | Pregnancy and lactation | Maternal high-sugar diet | SD rat/F | 8 | Hypertension was prevented | [124] |
| 3% in drinking water | Pregnancy and lactation | Maternal CKD | SD rat/M | 12 | Hypertension and renal hypertrophy were prevented | [125] |
| 3% in drinking water | Pregnancy and lactation | STZ-induced diabetes | Wistar rat/M and F | 16 | Hypertension was prevented | [126] |
| 3% in drinking water | Pregnancy and lactation | Maternal dyslipidemia | Wistar rat/M and F | 16 | Obesity, dyslipidemia, and hypertension were ameliorated | [127] |
| 3% in drinking water | Pregnancy and lactation | Genetic hypertension model | SHR/M | 22 | Hypertension was prevented and diabetic retinopathy was attenuated | [128] |
| 5% in drinking water | Pregnancy and lactation | Genetic hypertension model | SHRSP/M | 12 | Hypertension was prevented | [129] |
| Cysteine | ||||||
| L- or D-cysteine, 8 mmol/kg/day | Pregnancy | Maternal CKD | SD rat/M | 12 | Hypertension was prevented | [130] |
| NAC, 1% in drinking water | Pregnancy and lactation | Prenatal dexamethasone and postnatal high-fat diet | SD rat/M | 12 | Hypertension was prevented | [131] |
| NAC, 1% in drinking water | Pregnancy and lactation | Suramin-induced preeclampsia | SD rat/M | 12 | Hypertension was prevented | [132] |
| NAC, 1% in drinking water | Pregnancy and lactation | Maternal L-NAME exposure | SD rat/M | 12 | Hypertension was prevented | [133] |
| NAC, 500 mg/kg/day in drinking water | From gestational day 4 to postnatal day 10 | Maternal nicotine exposure | SD rat/M | 32 | Hypertension was prevented | [134] |
| Glycine | ||||||
| 3% in chow | Pregnancy and lactation | Maternal protein restriction | Wistar/M | 4 | Hypertension was prevented | [135] |
| BCAAs | ||||||
| BCAA-supplemented diets | Pregnancy | Maternal caloric restriction | SD rat/M | 16 | Hypertension was prevented | [136] |
| 1.5% in chow | Pregnancy and lactation | Maternal and post-weaning high-fat diet | C57BL/6 mice/M | 16 | Obesity and glucose intolerance were alleviated | [137] |
| Tryptophan | ||||||
| 200 mg/kg/day | Pregnancy | Maternal CKD | SD rat/M | 12 | Hypertension was prevented | [138] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tain, Y.-L.; Hsu, C.-N. Amino Acids during Pregnancy and Offspring Cardiovascular–Kidney–Metabolic Health. Nutrients 2024, 16, 1263. https://doi.org/10.3390/nu16091263
Tain Y-L, Hsu C-N. Amino Acids during Pregnancy and Offspring Cardiovascular–Kidney–Metabolic Health. Nutrients. 2024; 16(9):1263. https://doi.org/10.3390/nu16091263
Chicago/Turabian StyleTain, You-Lin, and Chien-Ning Hsu. 2024. "Amino Acids during Pregnancy and Offspring Cardiovascular–Kidney–Metabolic Health" Nutrients 16, no. 9: 1263. https://doi.org/10.3390/nu16091263
APA StyleTain, Y.-L., & Hsu, C.-N. (2024). Amino Acids during Pregnancy and Offspring Cardiovascular–Kidney–Metabolic Health. Nutrients, 16(9), 1263. https://doi.org/10.3390/nu16091263








