Increased FGF-21 Improves Ectopic Lipid Deposition in the Liver and Skeletal Muscle
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Growth Characteristics
2.3. Tissue Sampling
2.4. Hematoxylin and Eosin (H&E) Staining
2.5. Cell Culture
2.6. Cell Transfection
2.7. Enzyme-Linked Immunosorbent Assay (Elisa)
2.8. Oil Red O Staining
2.9. Western Blot Analysis
2.10. Statistical Analysis
3. Results
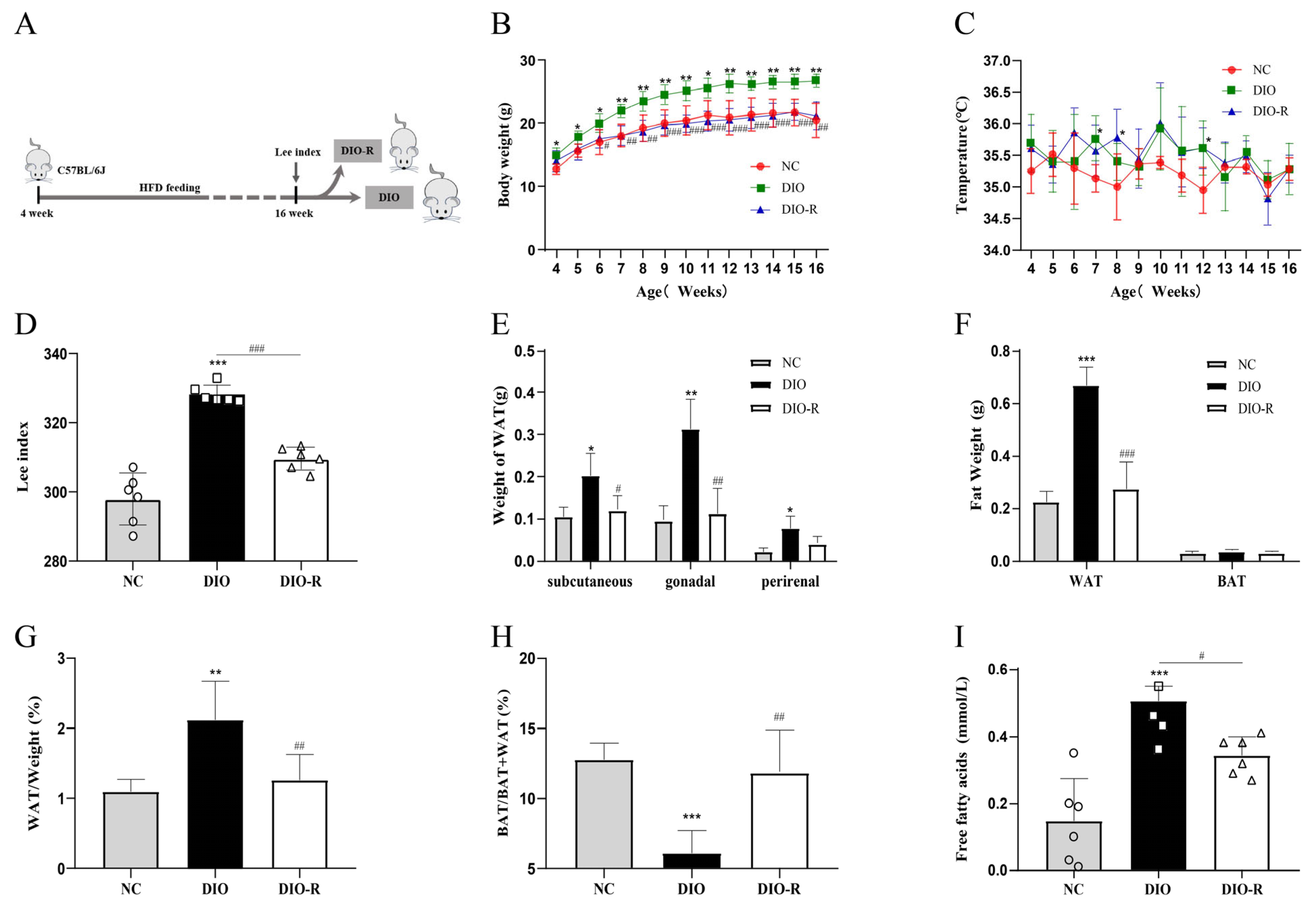
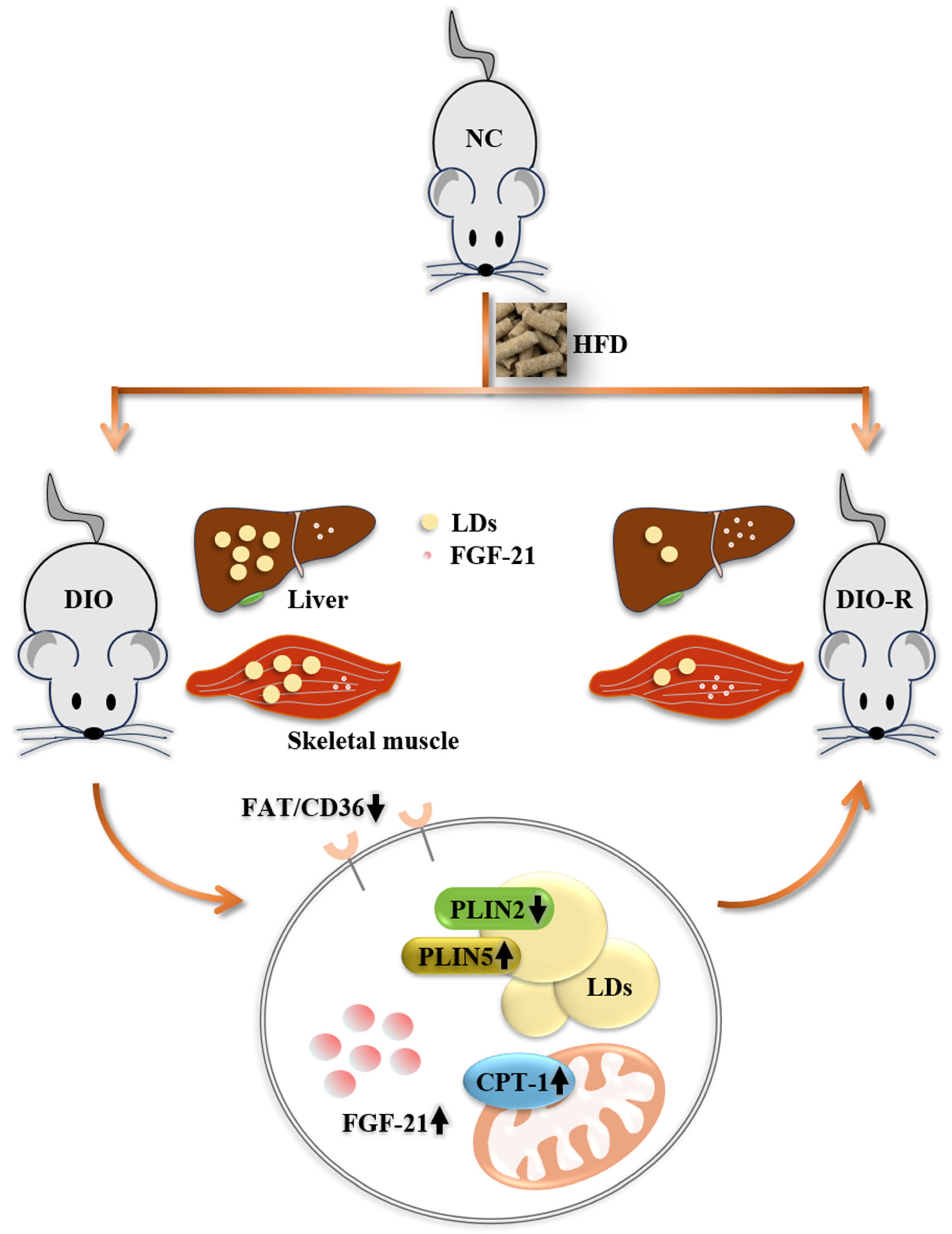
- Some of the mice fed a high-fat diet showed the characteristics to resist obesity.
- 2.
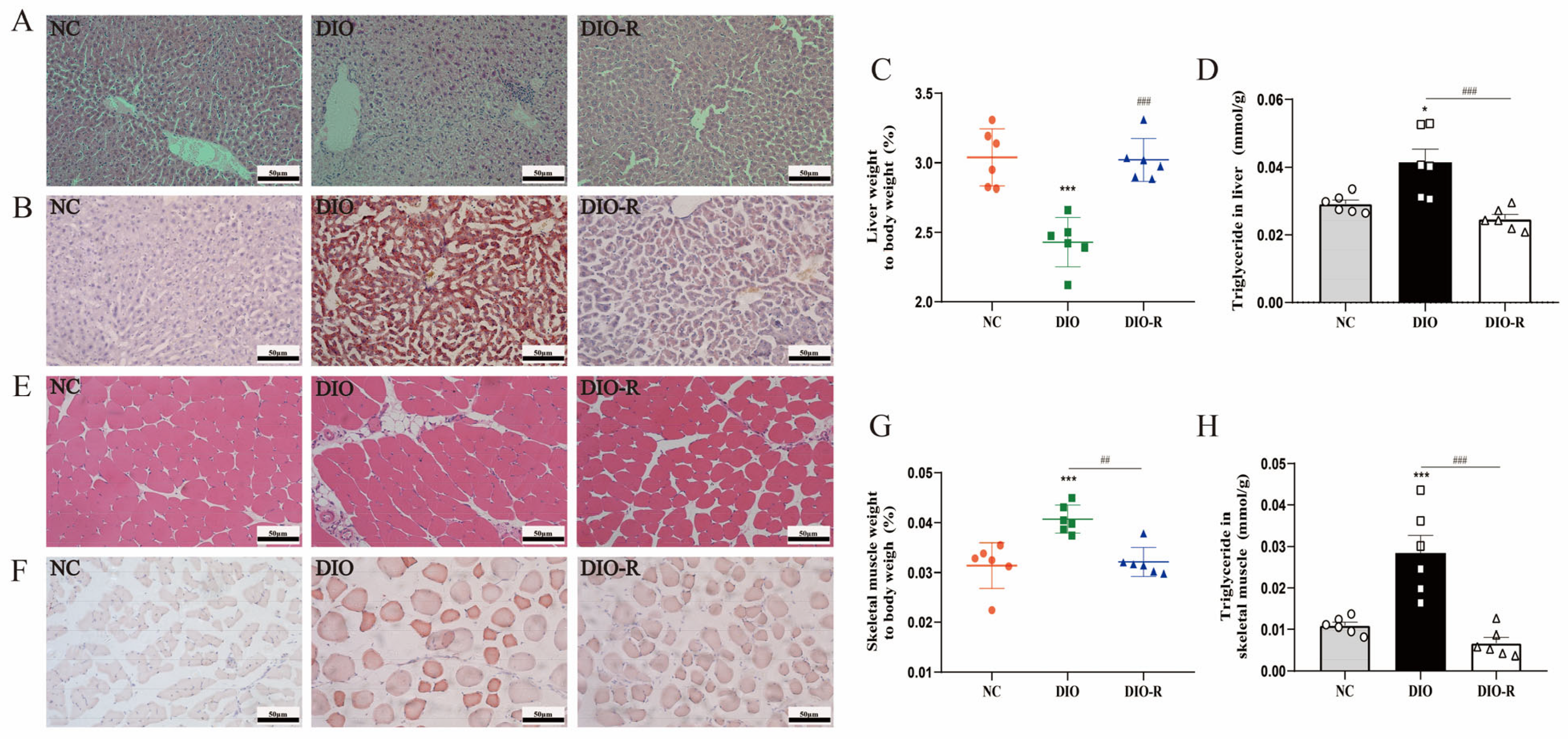
- Obesity-resistant mice exhibited reduced ectopic lipid deposition in the liver and skeletal muscles.
- 3.
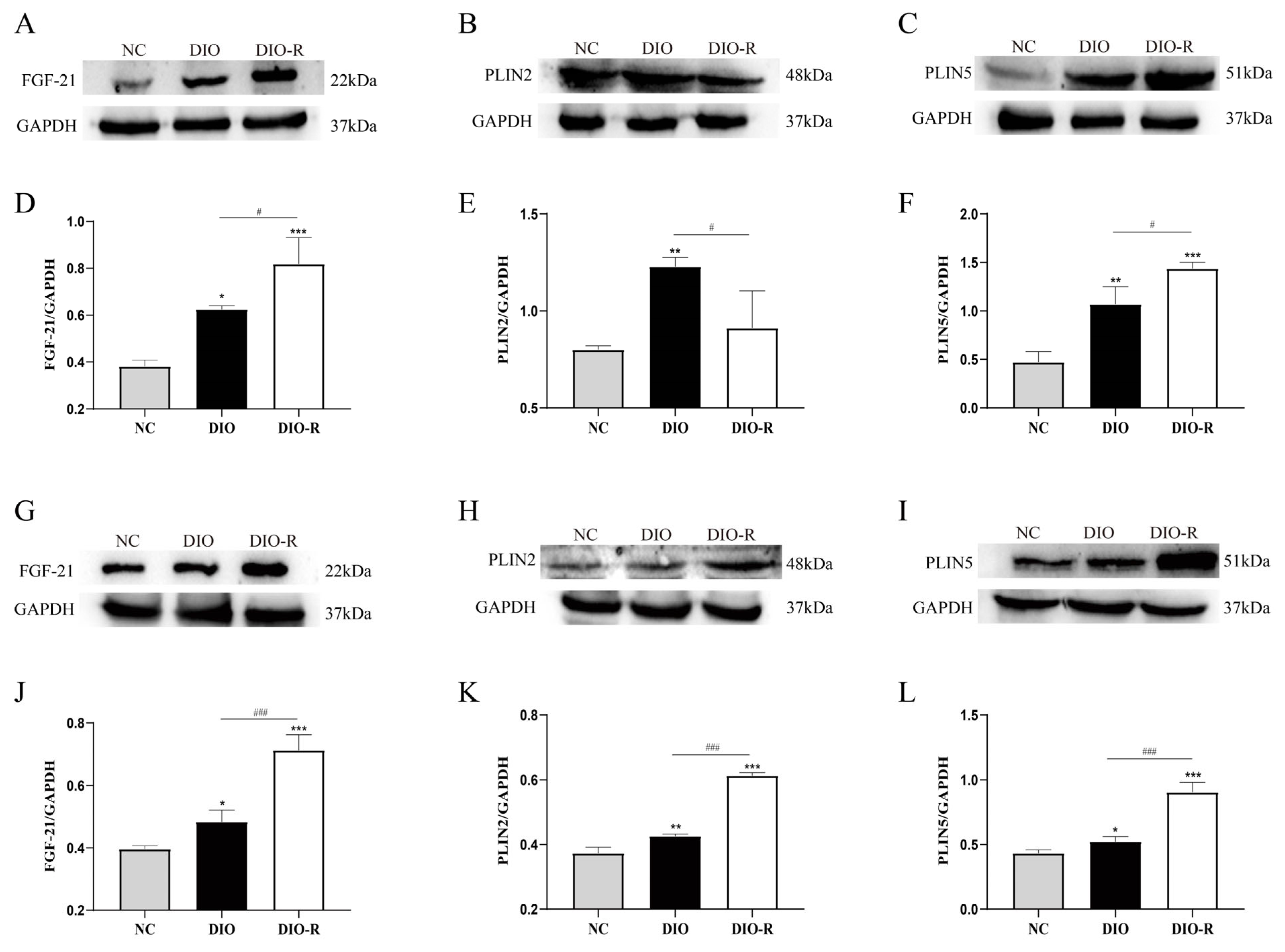
- FGF-21 increased in the liver and skeletal muscle of obesity-resistant mice.
- 4.
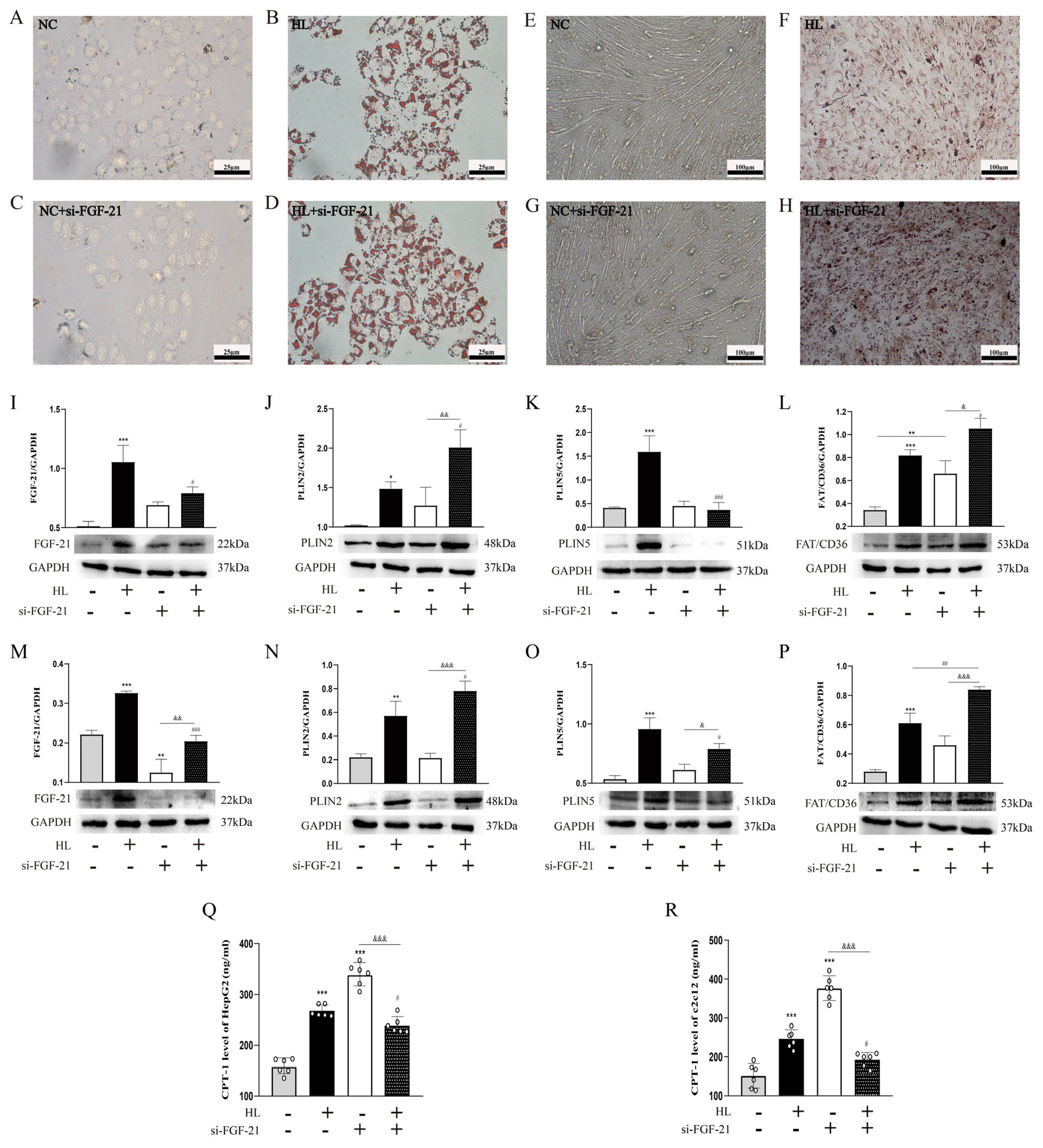
- FGF-21 regulated ectopic lipid deposition.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Su, K.; Yi, B.; Yao, B.Q.; Xia, T.; Yang, Y.F.; Zhang, Z.H.; Chen, C. Liraglutide attenuates renal tubular ectopic lipid deposition in rats with diabetic nephropathy by inhibiting lipid synthesis and promoting lipolysis. Pharmacol. Res. 2020, 156, 104778. [Google Scholar] [CrossRef] [PubMed]
- Caballero, B. Insulin resistance and amino acid metabolism in obesity. Ann. N. Y. Acad. Sci. 1987, 499, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Hana, C.A.; Klebermass, E.M.; Balber, T.; Mitterhauser, M.; Quint, R.; Hirtl, Y.; Klimpke, A.; Somloi, S.; Hutz, J.; Sperr, E.; et al. Inhibition of Lipid Accumulation in Skeletal Muscle and Liver Cells: A Protective Mechanism of Bilirubin against Diabetes Mellitus Type 2. Front. Pharmacol. 2021, 11, 636533. [Google Scholar] [CrossRef] [PubMed]
- Neeland, I.J.; Ross, R.; Després, J.P.; Matsuzawa, Y.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; et al. International Atherosclerosis Society, & International Chair on Cardiometabolic Risk Working Group on Visceral Obesity. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: A position statement. Lancet Diabetes Endocrinol. 2019, 7, 715–725. [Google Scholar] [PubMed]
- Liang, J.; Jia, Y.; Yu, H.; Yan, H.; Shen, Q.; Xu, Y.; Li, Y.; Yang, M. 5-Aza-2′-Deoxycytidine Regulates White Adipocyte Browning by Modulating miRNA-133a/Prdm16. Metabolites 2022, 12, 1131. [Google Scholar] [CrossRef] [PubMed]
- Soares, T.S.; Andreolla, A.P.; Miranda, C.A.; Klöppel, E.; Rodrigues, L.S.; Moraes-Souza, R.Q.; Damasceno, D.C.; Volpato, G.T.; Campos, K.E. Effect of the induction of transgenerational obesity on maternal-fetal parameters. Syst. Biol. Reprod. Med. 2018, 64, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Bass, J. Forever (FGF) 21. Nat. Med. 2013, 19, 1090–1092. [Google Scholar] [CrossRef] [PubMed]
- She, Q.Y.; Bao, J.F.; Wang, H.Z.; Liang, H.; Huang, W.; Wu, J.; Zhong, Y.; Ling, H.; Li, A.; Qin, S.L. Fibroblast growth factor 21: A “rheostat” for metabolic regulation? Metab. Clin. Exp. 2022, 130, 155166. [Google Scholar] [CrossRef] [PubMed]
- Kharitonenkov, A.; Shiyanova, T.L.; Koester, A.; Ford, A.M.; Micanovic, R.; Galbreath, E.J.; Sandusky, G.E.; Hammond, L.J.; Moyers, J.S.; Owens, R.A.; et al. FGF-21 as a novel metabolic regulator. J. Clin. Investig. 2005, 115, 1627–1635. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Kong, Y.; Peng, D. Fibroblast growth factor 21 in lipid metabolism and non-alcoholic fatty liver disease. Clin. Chim. Acta Int. J. Clin. Chem. 2019, 498, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Bag Soytas, R.; Suzan, V.; Arman, P.; Emiroglu Gedik, T.; Unal, D.; Cengiz, M.; Bolayirli, I.M.; Suna Erdincler, D.; Doventas, A.; Yavuzer, H. Association of FGF-19 and FGF-21 levels with primary sarcopenia. Geriatr. Gerontol. Int. 2021, 21, 959–962. [Google Scholar] [CrossRef] [PubMed]
- Szczepańska, E.; Gietka-Czernel, M. FGF21: A Novel Regulator of Glucose and Lipid Metabolism and Whole-Body Energy Balance. Horm. Metab. Res. 2022, 54, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Sherrier, M.; Li, H. Skeletal Muscle and Bone—Emerging Targets of Fibroblast Growth Factor-21. Front. Physiol. 2021, 12, 625287. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Han, Y.; Gao, P.; Yang, M.; Xiao, L.; Xiong, X.; Zhao, H.; Tang, C.; Chen, G.; Zhu, X.; et al. Disulfide-bond A oxidoreductase-like protein protects against ectopic fat deposition and lipid-related kidney damage in diabetic nephropathy. Kidney Int. 2019, 95, 880–895. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, S.; Cuervo, A.M. Degradation of lipid droplet-associated proteins by chaperone-mediated autophagy facilitates lipolysis. Nat. Cell Biol. 2015, 17, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Najt, C.P.; Devarajan, M.; Mashek, D.G. Perilipins at a glance. J. Cell Sci. 2022, 135, jcs259501. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, K.; Lee, Y.K.; Londos, C.; Raaka, B.M.; Dalen, K.T.; Kimmel, A.R. Perilipin family members preferentially sequester to either triacylglycerol-specific or cholesteryl-ester-specific intracellular lipid storage droplets. J. Cell Sci. 2012, 125 Pt 17, 4067–4076. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kang, K.; Shen, L.; Shen, L.; Zhou, Y. Integrative Analysis of the Predictive Value of Perilipin Family on Clinical Significance, Prognosis and Immunotherapy of Glioma. Biomedicines 2023, 11, 1009. [Google Scholar] [CrossRef] [PubMed]
- Carr, R.M.; Ahima, R.S. Pathophysiology of lipid droplet proteins in liver diseases. Exp. Cell Res. 2016, 340, 187–192. [Google Scholar] [CrossRef]
- Bosma, M.; Sparks, L.M.; Hooiveld, G.J.; Jorgensen, J.A.; Houten, S.M.; Schrauwen, P.; Kersten, S.; Hesselink, M.K. Overexpression of PLIN5 in skeletal muscle promotes oxidative gene expression and intramyocellular lipid content without compromising insulin sensitivity. Biochim. Biophys. Acta 2013, 1831, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.H.; Chen, E.; Li, L.; Saha, P.; Lee, H.J.; Huang, L.S.; Shelness, G.S.; Chan, L.; Chang, B.H. The constitutive lipid droplet protein PLIN2 regulates autophagy in liver. Autophagy 2017, 13, 1130–1144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, W.; Xu, R.; Wang, Z.; Zhang, X.; Wang, P.; Peng, K.; Li, M.; Li, J.; Tan, Y.; et al. Plin5 Bidirectionally Regulates Lipid Metabolism in Oxidative Tissues. Oxidative Med. Cell. Longev. 2022, 2022, 4594956. [Google Scholar] [CrossRef]
- Ramos-Jiménez, A.; Zavala-Lira, R.A.; Moreno-Brito, V.; González-Rodríguez, E. FAT/CD36 Participation in Human Skeletal Muscle Lipid Metabolism: A Systematic Review. J. Clin. Med. 2022, 12, 318. [Google Scholar] [CrossRef] [PubMed]
- Schlaepfer, I.R.; Joshi, M. CPT1A-mediated Fat Oxidation, Mechanisms, and Therapeutic Potential. Endocrinology 2020, 161, bqz046. [Google Scholar] [CrossRef] [PubMed]
- Schenk, S.; Horowitz, J.F. Coimmunoprecipitation of FAT/CD36 and CPT I in skeletal muscle increases proportionally with fat oxidation after endurance exercise training. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E254–E260. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Ruan, D.G.; Xu, X.Y. Effects of different types of exercise on lipid uptake, synthesis and oxidation in skeletal muscles of insulin-resistant rats. Sheng Li Xue Bao [Acta Physiol. Sin.] 2021, 73, 263–274. [Google Scholar] [PubMed]
- Hong, Q.; Xia, C.; Xiangying, H.; Quan, Y. Capsinoids suppress fat accumulation via lipid metabolism. Mol. Med. Rep. 2015, 11, 1669–1674. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, M.; Xu, J.; Yang, J. Uncarboxylated Osteocalcin Decreases SCD1 by Activating AMPK to Alleviate Hepatocyte Lipid Accumulation. Molecules 2023, 28, 3121. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Huang, S.; Yi, Q.; Liu, N.; Cui, T.; Duan, S.; Chen, J.; Li, J.; Li, J.; Wang, L.; et al. Hepatic Clstn3 Ameliorates Lipid Metabolism Disorders in High Fat Diet-Induced NAFLD through Activation of FXR. ACS Omega 2023, 8, 26158–26169. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.M. Subcutaneous and visceral adipose tissue: Structural and functional differences. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2010, 11, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Chen, X.; Zhang, P.; Liu, G. Perilipin2 is an Earlier Marker Than Perilipin1 for Identifying Adipocyte Regeneration in Fat Grafts. Aesthetic Surg. J. 2021, 41, NP646–NP652. [Google Scholar] [CrossRef] [PubMed]
- Frayn, K.N.; Arner, P.; Yki-Järvinen, H. Fatty acid metabolism in adipose tissue, muscle and liver in health and disease. Essays Biochem. 2006, 42, 89–103. [Google Scholar] [PubMed]
- Salles, J.; Tardif, N.; Landrier, J.F.; Mothe-Satney, I.; Guillet, C.; Boue-Vaysse, C.; Combaret, L.; Giraudet, C.; Patrac, V.; Bertrand-Michel, J.; et al. TNFα gene knockout differentially affects lipid deposition in liver and skeletal muscle of high-fat-diet mice. J. Nutr. Biochem. 2012, 23, 1685–1693. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Jia, Y.; Yan, H.; Shen, Q.; Bian, W.; Zhao, D.; Xu, Y.; Jin, Y.; Yang, M. Prdm16-Mediated Browning is Involved in Resistance to Diet-Induced and Monosodium Glutamate-Induced Obesity. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 4351–4360. [Google Scholar] [CrossRef] [PubMed]
- Bulik, C.M.; Allison, D.B. The genetic epidemiology of thinness. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2001, 2, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Diao, S.; Hu, M.; Zhang, L. Methylation of Hypothalamic Tsc1-mTOR Signaling in Regulation of Obesity and Obesity Resistance. BioMed Res. Int. 2020, 2020, 8723869. [Google Scholar] [CrossRef] [PubMed]
- Sá, F.G.; Lima-Leopoldo, A.P.; Jacobsen, B.B.; Ferron, A.J.; Estevam, W.M.; Campos, D.H.; Castardeli, E.; Cunha, M.R.; Cicogna, A.C.; Leopoldo, A.S. Obesity Resistance Promotes Mild Contractile Dysfunction Associated with Intracellular Ca2+ Handling. Arq. Bras. Cardiol. 2015, 105, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Guo, S.; Zou, Z. Resveratrol ameliorates metabolic disorders and insulin resistance in high-fat diet-fed mice. Life Sci. 2020, 242, 117212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Y. Fibroblast growth factor 21, the endocrine FGF pathway and novel treatments for metabolic syndrome. Drug Discov. Today 2014, 19, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Geng, L.; Ying, L.; Shu, L.; Ye, K.; Yang, R.; Liu, Y.; Wang, Y.; Cai, Y.; Jiang, X.; et al. FGF21-Sirtuin 3 Axis Confers the Protective Effects of Exercise against Diabetic Cardiomyopathy by Governing Mitochondrial Integrity. Circulation 2022, 146, 1537–1557. [Google Scholar] [CrossRef] [PubMed]
- Fougerat, A.; Schoiswohl, G.; Polizzi, A.; Régnier, M.; Wagner, C.; Smati, S.; Fougeray, T.; Lippi, Y.; Lasserre, F.; Raho, I.; et al. ATGL-dependent white adipose tissue lipolysis controls hepatocyte PPARα activity. Cell Rep. 2022, 39, 110910. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Khanal, P.; Norheim, F.; Hjorth, M.; Bjellaas, T.; Drevon, C.A.; Vaage, J.; Kimmel, A.R.; Dalen, K.T. Plin2 deletion increases cholesteryl ester lipid droplet content and disturbs cholesterol balance in adrenal cortex. J. Lipid Res. 2021, 62, 100048. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; You, W.; Chen, W.; Zhou, Y.; Nong, Q.; Valencak, T.G.; Wang, Y.; Shan, T. Single-cell RNA sequencing and lipidomics reveal cell and lipid dynamics of fat infiltration in skeletal muscle. J. Cachexia Sarcopenia Muscle 2021, 12, 109–129. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; You, W.; Valencak, T.G.; Shan, T. Bidirectional roles of skeletal muscle fibro-adipogenic progenitors in homeostasis and disease. Ageing Res. Rev. 2022, 80, 101682. [Google Scholar] [CrossRef] [PubMed]
- Wosczyna, M.N.; Perez Carbajal, E.E.; Wagner, M.W.; Paredes, S.; Konishi, C.T.; Liu, L.; Wang, T.T.; Walsh, R.A.; Gan, Q.; Morrissey, C.S.; et al. Targeting microRNA-mediated gene repression limits adipogenic conversion of skeletal muscle mesenchymal stromal cells. Cell Stem Cell 2021, 28, 1323–1334.e8. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, Y.; Yu, H.; Liang, J.; Zhang, Q.; Sun, J.; Yang, H.; Yan, H.; Zhang, S.; Li, Y.; Jin, Y.; et al. Increased FGF-21 Improves Ectopic Lipid Deposition in the Liver and Skeletal Muscle. Nutrients 2024, 16, 1254. https://doi.org/10.3390/nu16091254
Jia Y, Yu H, Liang J, Zhang Q, Sun J, Yang H, Yan H, Zhang S, Li Y, Jin Y, et al. Increased FGF-21 Improves Ectopic Lipid Deposition in the Liver and Skeletal Muscle. Nutrients. 2024; 16(9):1254. https://doi.org/10.3390/nu16091254
Chicago/Turabian StyleJia, Ying, Huixin Yu, Jia Liang, Qiang Zhang, Jiawei Sun, Hongqing Yang, Haijing Yan, Shuping Zhang, Yana Li, Yongjun Jin, and et al. 2024. "Increased FGF-21 Improves Ectopic Lipid Deposition in the Liver and Skeletal Muscle" Nutrients 16, no. 9: 1254. https://doi.org/10.3390/nu16091254
APA StyleJia, Y., Yu, H., Liang, J., Zhang, Q., Sun, J., Yang, H., Yan, H., Zhang, S., Li, Y., Jin, Y., & Yang, M. (2024). Increased FGF-21 Improves Ectopic Lipid Deposition in the Liver and Skeletal Muscle. Nutrients, 16(9), 1254. https://doi.org/10.3390/nu16091254




