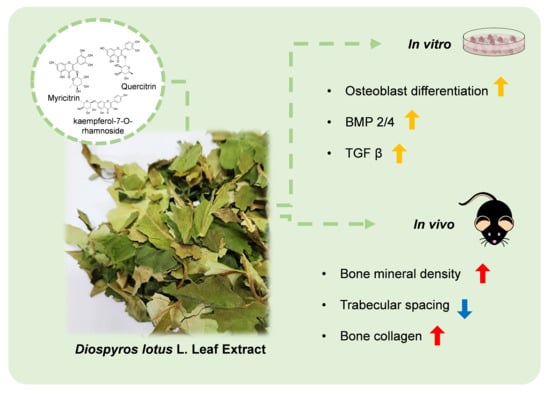
Osteogenic Effects of the Diospyros lotus L. Leaf Extract on MC3T3-E1 Pre-Osteoblasts and Ovariectomized Mice via BMP2/4 and TGF β Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. High-Performance Liquid Chromatography (HPLC) and Quadrupole Time-of-Flight (Q-TOF) Mass Spectrometry Analyses of Phytochemicals in DL
2.3. Cell Culture
2.4. ALP Activity
2.5. Luciferase Reporter Assays
2.6. Western Blotting
2.7. Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
2.8. Animal Surgery and Treatment
2.9. Micro-CT
2.10. Determination of Serum Osteocalcin (OCN) Levels
2.11. Statistical Analyses
3. Results and Discussions
3.1. DLE Enhances Osteoblast Differentiation by Inducing Osteogenic Signaling
3.2. DLE Contains Myricetrin, Quercetrin, and Kaempferol-7-O-Rhamnoside
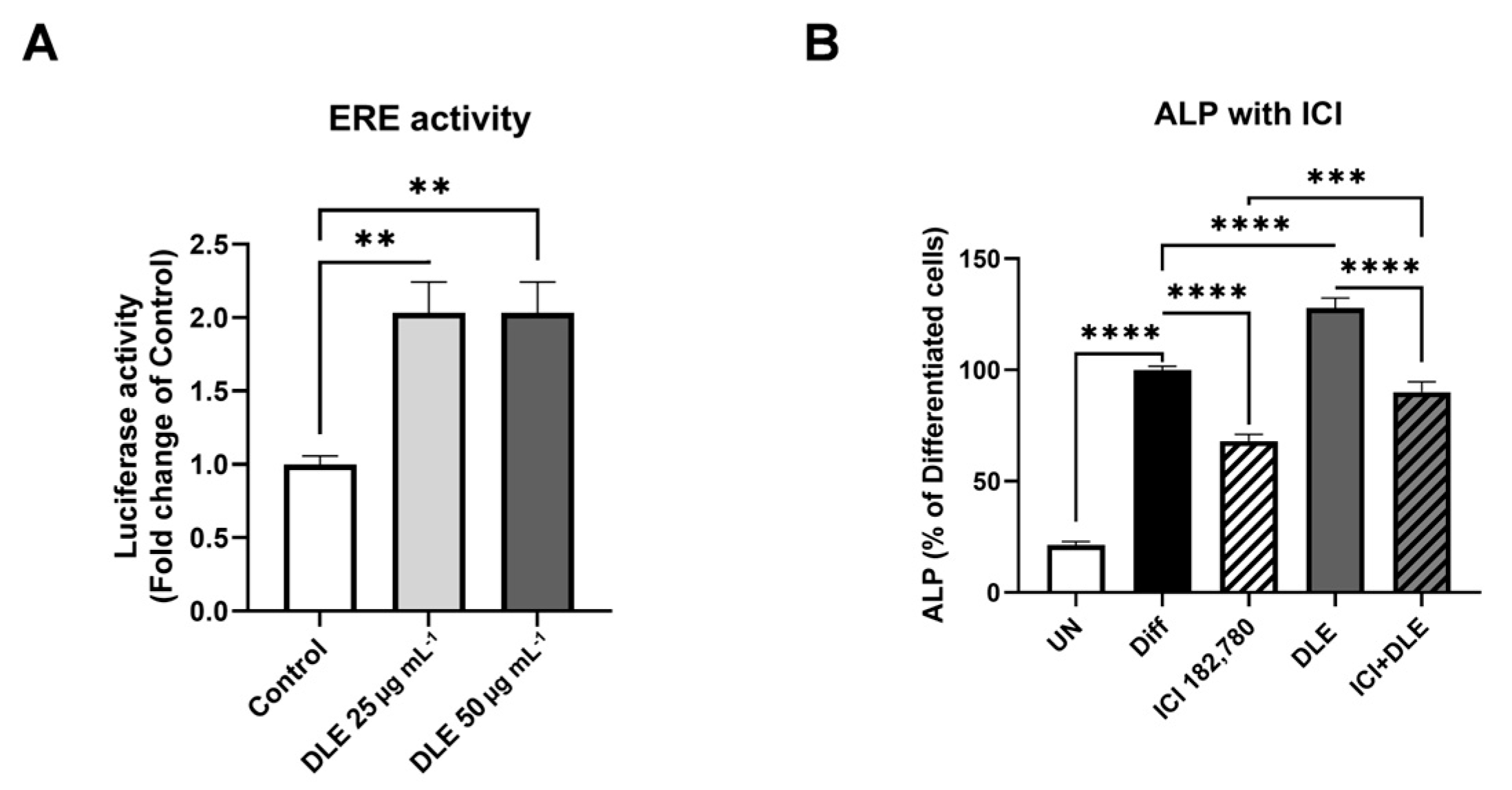
3.3. Estrogenic Activity of DLE Partially Mediates the Osteogenic Activity
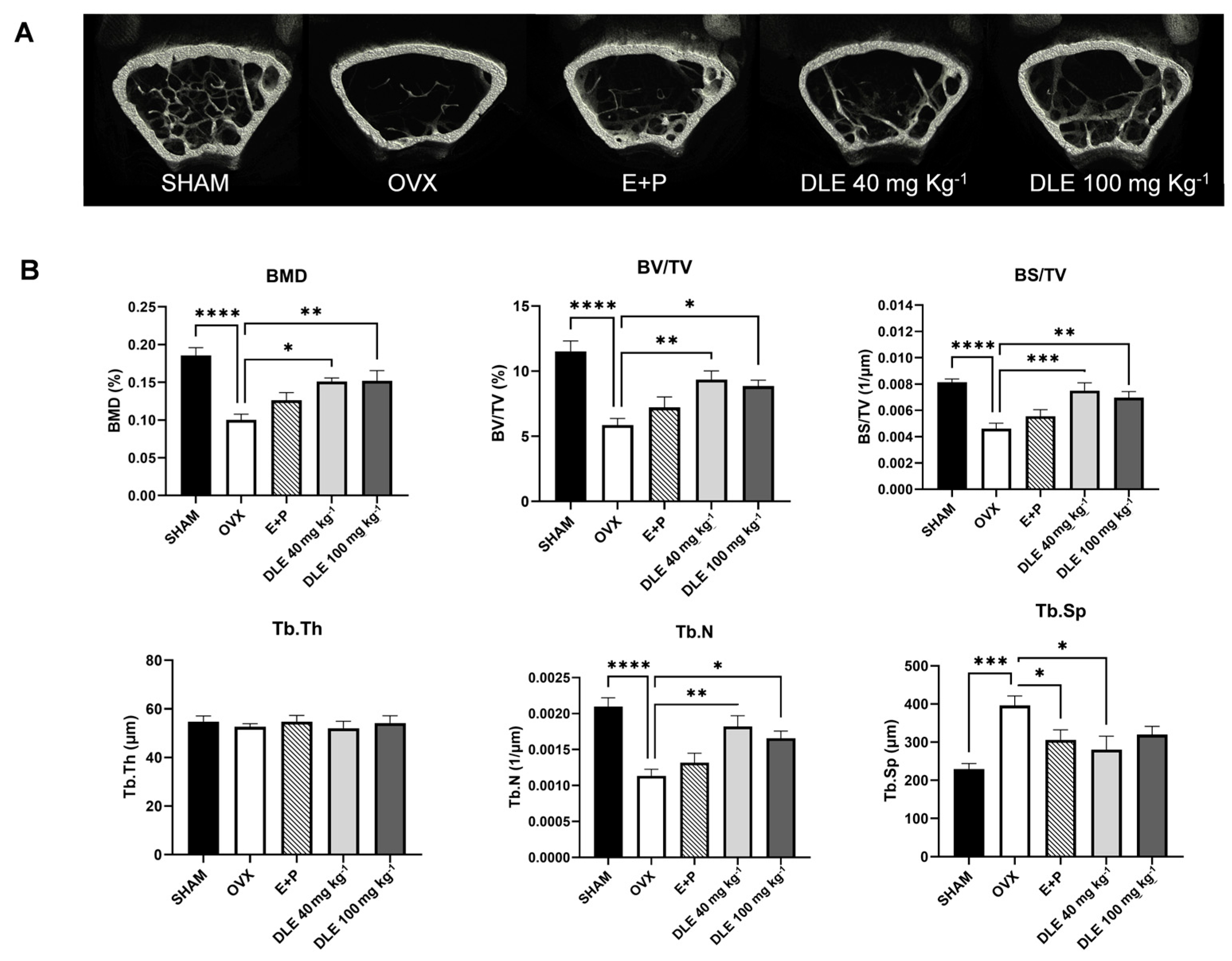
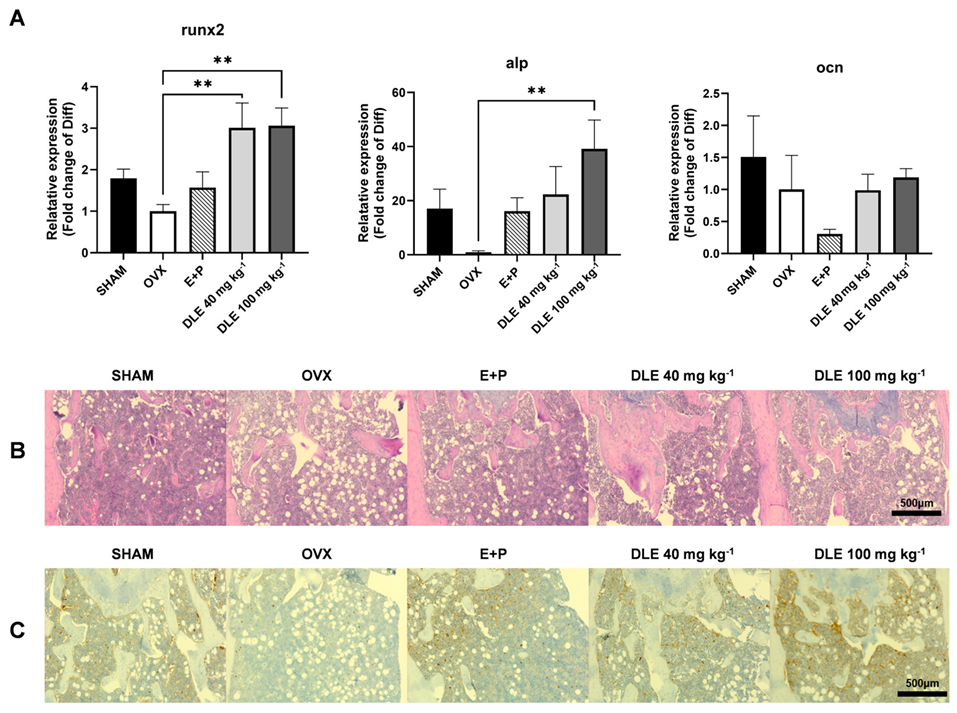
3.4. DLE Suppresses Osteoporosis in OVX Mice
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA 2001, 285, 785–795. [Google Scholar] [CrossRef]
- Sözen, T.; Özışık, L.; Başaran, N.Ç. An overview and management of osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.Y.; Yang, Y.; Jung, H. Molecular mechanisms and emerging therapeutics for osteoporosis. Int. J. Mol. Sci. 2020, 21, 7623. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.C.; Silva, A.M.; Santos, M.S.; Sardão, V.A. Phytoestrogens as alternative hormone replacement therapy in menopause: What is real, what is unknown. J. Steroid Biochem. Mol. Biol. 2014, 143, 61–71. [Google Scholar] [CrossRef]
- Hong, S.; Cha, K.H.; Park, J.H.; Jung, D.S.; Choi, J.H.; Yoo, G.; Nho, C.W. Cinnamic acid suppresses bone loss via induction of osteoblast differentiation with alteration of gut microbiota. J. Nutr. Biochem. 2022, 101, 108900. [Google Scholar] [CrossRef]
- Crockett, J.C.; Rogers, M.J.; Coxon, F.P.; Hocking, L.J.; Helfrich, M.H. Bone remodelling at a glance. J. Cell Sci. 2011, 124, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Hadjidakis, D.J.; Androulakis, I.I. Bone remodeling. Ann. N. Y Acad. Sci. 2006, 1092, 385–396. [Google Scholar] [CrossRef]
- Florencio-Silva, R.; Sasso, G.R.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of bone tissue: Structure, function, and factors that influence bone cells. BioMed Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef]
- Das, S.; Crockett, J.C. Osteoporosis—A current view of pharmacological prevention and treatment. Drug Des. Dev. Ther. 2013, 7, 435–448. [Google Scholar]
- Skjødt, M.K.; Frost, M.; Abrahamsen, B. Side effects of drugs for osteoporosis and metastatic bone disease. Br. J. Clin. Pharmacol. 2019, 85, 1063–1071. [Google Scholar] [CrossRef]
- Uddin, G.; Rauf, A.; Siddiqui, B.S.; Muhammad, N.; Khan, A.; Shah, S.U. Anti-nociceptive, anti-inflammatory and sedative activities of the extracts and chemical constituents of Diospyros lotus L. Phytomedicine 2014, 21, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Uddin, G.; Khan, H.; Raza, M.; Zafar, M.; Tokuda, H. Anti-tumour-promoting and thermal-induced protein denaturation inhibitory activities of β-sitosterol and lupeol isolated from Diospyros lotus L. Nat. Prod. Res. 2016, 30, 1205–1207. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Yan, H.H.; Qin, C.Q.; Li, H.R.; Li, X.; Ren, D.F. Protective effect of fermented Diospyros lotus L. extracts against the high glucose-induced apoptosis of min6 cells. J. Food Biochem. 2021, 45, e13685. [Google Scholar] [CrossRef]
- Cho, B.O.; Che, D.N.; Shin, J.Y.; Kang, H.J.; Kim, J.H.; Kim, H.Y.; Cho, W.G.; Jang, S.I. Ameliorative effects of Diospyros lotus leaf extract against UVB-induced skin damage in BALB/c mice. Biomed. Pharmacother. 2017, 95, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Che, D.N.; Kang, H.J.; Cho, B.O.; Shin, J.Y.; Jang, S.I. Combined effects of Diospyros lotus leaf and grape stalk extract in high-fat-diet-induced obesity in mice. Food Sci. Biotechnol. 2019, 28, 1207–1215. [Google Scholar] [CrossRef]
- Wong, S.K.; Chin, K.; Ima-Nirwana, S. The osteoprotective effects of kaempferol: The evidence from in vivo and in vitro studies. Drug Des. Dev. Ther. 2019, 13, 3497–3514. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Gao, X.; Chen, P.; Li, X. Myricetin ameliorates glucocorticoid-induced osteoporosis through the ERK signaling pathway. Life Sci. 2018, 207, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Cha, K.H.; Kwon, D.Y.; Son, Y.J.; Kim, S.M.; Choi, J.H.; Yoo, G.; Nho, C.W. Agastache rugosa ethanol extract suppresses bone loss via induction of osteoblast differentiation with alteration of gut microbiota. Phytomedicine 2021, 84, 153517. [Google Scholar] [CrossRef]
- Campbell, G.M.; Sophocleous, A. Quantitative analysis of bone and soft tissue by micro-computed tomography: Applications to ex vivo and in vivo studies. BoneKEy Rep. 2014, 3, 564. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.O.; Yin, H.H.; Park, S.H.; Byun, E.B.; Ha, H.Y.; Jang, S.I. Anti-inflammatory activity of myricetin from Diospyros lotus through suppression of NF-κB and STAT1 activation and Nrf2-mediated HO-1 induction in lipopolysaccharide-stimulated RAW264. 7 macrophages. Biosci. Biotechnol. Biochem. 2016, 80, 1520–1530. [Google Scholar] [CrossRef]
- Barlas, N.; Özer, S.; Karabulut, G. The estrogenic effects of apigenin, phloretin and myricetin based on uterotrophic assay in immature Wistar albino rats. Toxicol. Lett. 2014, 226, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Wang, W.; Xu, N.; Shi, C.; Xu, G.; Sun, J.; He, H.; Jiang, T. Myricetin loaded nano-micelles delivery system reduces bone loss induced by ovariectomy in rats through inhibition of osteoclast formation. J. Pharm. Sci. 2022, 111, 2341–2352. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.K.; Chin, K.; Ima-Nirwana, S. Quercetin as an agent for protecting the bone: A review of the current evidence. Int. J. Mol. Sci. 2020, 21, 6448. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Choi, Y.J.; Kang, M.K.; Park, S.H.; Antika, L.D.; Lee, E.J.; Kim, D.Y.; Kang, Y.H. Astragalin inhibits allergic inflammation and airway thickening in ovalbumin-challenged mice. J. Agric. Food Chem. 2017, 65, 836–845. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.H.; Lee, B.C.; Kim, D.; Cho, D.; Kim, T.S. Hydrophilic astragalin galactoside induces T helper type 1-mediated immune responses via dendritic cells. Int. J. Mol. Sci. 2018, 19, 3120. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Akhtar, N.; Jamil, H.M.; Banik, R.S.; Asaduzzaman, S.M. TGF-β/BMP signaling and other molecular events: Regulation of osteoblastogenesis and bone formation. Bone Res. 2015, 3, 15005. [Google Scholar] [CrossRef] [PubMed]
- Nohe, A.; Keating, E.; Knaus, P.; Petersen, N.O. Signal transduction of bone morphogenetic protein receptors. Cell. Signal. 2004, 16, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Chen, G.; Li, Y.P. TGF-beta and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016, 4, 16009. [Google Scholar] [CrossRef] [PubMed]
- Fischer, V.; Haffner-Luntzer, M. Interaction between bone and immune cells: Implications for postmenopausal osteoporosis. Semin. Cell Dev. Biol. 2022, 123, 14–21. [Google Scholar] [CrossRef]
- Tyagi, A.M.; Srivastava, K.; Mansoori, M.N.; Trivedi, R.; Chattopadhyay, N.; Singh, D. Estrogen deficiency induces the differentiation of IL-17 secreting Th17 cells: A new candidate in the pathogenesis of osteoporosis. PLoS ONE 2012, 7, e44552. [Google Scholar] [CrossRef]
- Wu, D.; Cline-Smith, A.; Shashkova, E.; Perla, A.; Katyal, A.; Aurora, R. T-cell mediated inflammation in postmenopausal osteoporosis. Front. Immunol. 2021, 12, 687551. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Animal models for osteoporosis. Eur. J. Pharmacol. 2015, 759, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Jia, X.; Mo, L.; Liu, C.; Zheng, L.; Yuan, Q.; Zhou, X. Intestinal microbiota: A potential target for the treatment of postmenopausal osteoporosis. Bone Res. 2017, 5, 17046. [Google Scholar] [CrossRef] [PubMed]



| Gene | Forward (5′-3′) | Tm | Reverse (5′-3′) | Tm |
|---|---|---|---|---|
| runx2 | TCCACAAGGACAGAGTCAGATTAC | 58.4 | TGGCTCAGATAGGAGGGGTA | 57.4 |
| alp | GATCATTCCCACGTTTTCAC | 53.4 | TGCGGGCTTGTGGGACCTGC | 63.6 |
| Ocn | AGACTCCGGCGCTACCTT | 59.4 | CTCGTCACAAGCAGGGTTAAG | 58 |
| Gapdh | AAG AGG GAT GCT GCC CTT AC | 57.4 | CCATTTTGTCTACGGGACGA | 55.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, S.; Lazerka, N.; Jeon, B.J.; Kim, J.D.; Erdenebileg, S.; Nho, C.W.; Yoo, G. Osteogenic Effects of the Diospyros lotus L. Leaf Extract on MC3T3-E1 Pre-Osteoblasts and Ovariectomized Mice via BMP2/4 and TGF β Pathways. Nutrients 2024, 16, 1247. https://doi.org/10.3390/nu16081247
Hong S, Lazerka N, Jeon BJ, Kim JD, Erdenebileg S, Nho CW, Yoo G. Osteogenic Effects of the Diospyros lotus L. Leaf Extract on MC3T3-E1 Pre-Osteoblasts and Ovariectomized Mice via BMP2/4 and TGF β Pathways. Nutrients. 2024; 16(8):1247. https://doi.org/10.3390/nu16081247
Chicago/Turabian StyleHong, Soyeon, Nadzeya Lazerka, Byeong Jun Jeon, Jeong Do Kim, Saruul Erdenebileg, Chu Won Nho, and Gyhye Yoo. 2024. "Osteogenic Effects of the Diospyros lotus L. Leaf Extract on MC3T3-E1 Pre-Osteoblasts and Ovariectomized Mice via BMP2/4 and TGF β Pathways" Nutrients 16, no. 8: 1247. https://doi.org/10.3390/nu16081247
APA StyleHong, S., Lazerka, N., Jeon, B. J., Kim, J. D., Erdenebileg, S., Nho, C. W., & Yoo, G. (2024). Osteogenic Effects of the Diospyros lotus L. Leaf Extract on MC3T3-E1 Pre-Osteoblasts and Ovariectomized Mice via BMP2/4 and TGF β Pathways. Nutrients, 16(8), 1247. https://doi.org/10.3390/nu16081247