L-Carnitine in the Treatment of Psychiatric and Neurological Manifestations: A Systematic Review
Abstract
1. Background
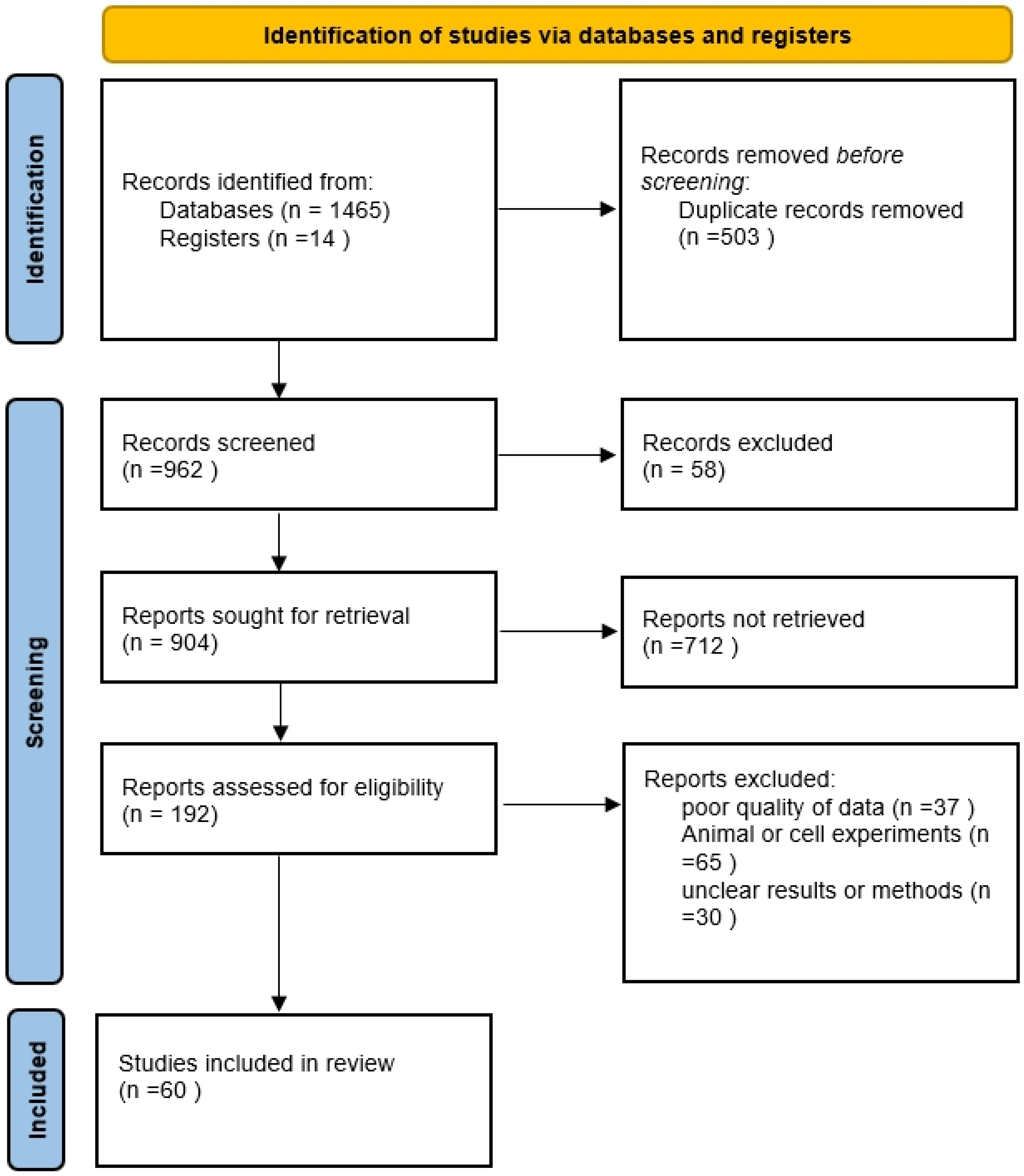
2. Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Level-of-Evidence Ratings
3. Results
3.1. Evidence of Effectiveness of LC in the Treatment of Psychiatric and Neurological Disorders
3.1.1. Neurodegenerative Diseases
Alzheimer’s Disease
Down Syndrome
3.1.2. Amyotrophic Lateral Sclerosis
3.1.3. Ataxia
3.1.4. Attention Deficit Hyperactivity Disorder
3.1.5. Carpal Tunnel Syndrome
3.1.6. Cognitive Dysfunction
3.1.7. Depressive Disorder
3.1.8. Fatigue Syndrome, Chronic (CFS)
3.1.9. Hepatic Encephalopathy (HE)
3.1.10. Migraine Disorder
3.1.11. Multiple Sclerosis
3.1.12. Neurofibromatosis
3.1.13. Peripheral Nervous System Diseases
3.1.14. Rett Syndrome
3.1.15. Sciatica
3.1.16. Stroke
3.2. AEs Reported in Controlled Clinical Trials
3.3. Potential Mechanisms of Action
3.3.1. Oxidative Stress
3.3.2. Inflammatory Mediators
3.3.3. Mitochondrial Dysfunction
3.3.4. Dopamine Neurotransmission
3.3.5. Cholinergic Neurotransmission
3.3.6. Glutamate Neurotransmission
3.3.7. Fatty Acid Transport
4. Discussion
4.1. Dosage and Formulations
4.2. Potential Adverse Effects
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Alhasaniah, A.H. l-carnitine: Nutrition, pathology, and health benefits. Saudi J. Biol. Sci. 2023, 30, 103555. [Google Scholar] [CrossRef]
- Almannai, M.; Alfadhel, M.; El-Hattab, A.W. Carnitine Inborn Errors of Metabolism. Molecules 2019, 24, 3251. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.L.; McDonald, D.A.; Borum, P.R. Acylcarnitines: Role in brain. Prog. Lipid Res. 2010, 49, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Marcovina, S.M.; Sirtori, C.; Peracino, A.; Gheorghiade, M.; Borum, P.; Remuzzi, G.; Ardehali, H. Translating the basic knowledge of mitochondrial functions to metabolic therapy: Role of L-carnitine. Transl. Res. J. Lab. Clin. Med. 2013, 161, 73–84. [Google Scholar] [CrossRef]
- Ferreira, G.C.; McKenna, M.C. L-Carnitine and Acetyl-L-carnitine Roles and Neuroprotection in Developing Brain. Neurochem. Res. 2017, 42, 1661–1675. [Google Scholar] [CrossRef]
- Group OCEBM Levels of Evidence Working. The Oxford 2011 Levels of Evidence; Oxford Centre for Evidence-Based Medicine: Oxford, UK, 2011. [Google Scholar]
- Durieux, N.; Vandenput, S.; Pasleau, F. OCEBM levels of evidence system. Rev. Med. Liege 2013, 68, 644–649. [Google Scholar]
- Chen, Z.-Y.; Zhang, Y. Animal models of Alzheimer’s disease: Applications, evaluation, and perspectives. Zool. Res. 2022, 43, 1026. [Google Scholar] [CrossRef]
- Antonarakis, S.E.; Skotko, B.G.; Rafii, M.S.; Strydom, A.; Pape, S.E.; Bianchi, D.W.; Sherman, S.L.; Reeves, R.H. Down syndrome. Nat. Rev. Dis. Primers 2020, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Down, J.L. Observations on an ethnic classification of idiots. 1866. Ment. Retard. 1995, 33, 54–56. [Google Scholar]
- Livingston, G.A.; Sax, K.B.; McClenahan, Z.; Blumenthal, E.; Foley, K.; Willison, J.; Mann, A.H.; James, I.M. Acetyl-L-Carnitine in dementia. Int. J. Geriatr. Psychiatry 1991, 6, 853–860. [Google Scholar] [CrossRef]
- Bowman, B.A.B. Acetyl-Carnitine and Alzheimer’s Disease. Nutr. Rev. 1992, 50, 142–144. [Google Scholar] [CrossRef] [PubMed]
- Pettegrew, J.W.; Klunk, W.E.; Panchalingam, K.; Kanfer, J.N.; McClure, R.J. Clinical and neurochemical effects of acetyl-L-carnitine in Alzheimer’s disease. Neurobiol. Aging 1995, 16, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Thal, L.J.; Carta, A.; Clarke, W.R.; Ferris, S.H.; Friedland, R.P.; Petersen, R.C.; Pettegrew, J.W.; Pfeiffer, E.; Raskind, M.A.; Sano, M.; et al. A 1-year multicenter placebo-controlled study of acetyl-L-carnitine in patients with Alzheimer’s disease. Neurology 1996, 47, 705–711. [Google Scholar] [CrossRef]
- Brooks, J.O., 3rd; Yesavage, J.A.; Carta, A.; Bravi, D. Acetyl L-carnitine slows decline in younger patients with Alzheimer’s disease: A reanalysis of a double-blind, placebo-controlled study using the trilinear approach. Int. Psychogeriatr. 1998, 10, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Thal, L.J.; Calvani, M.; Amato, A.; Carta, A. A 1-year controlled trial of acetyl-l-carnitine in early-onset AD. Neurology 2000, 55, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Gavrilova, S.I.; Kalyn Ia, B.; Kolykhalov, I.V.; Roshchina, I.F.; Selezneva, N.D. Acetyl-L-carnitine (carnicetine) in the treatment of early stages of Alzheimer’s disease and vascular dementia. Zhurnal Nevrol. Psikhiatrii Im. S.S. Korsakova 2011, 111, 16–22. [Google Scholar]
- Yang, Y.; Choi, H.; Lee, C.N.; Kim, Y.B.; Kwak, Y.T. A Multicenter, Randomized, Double-blind, Placebo-controlled Clinical Trial for Efficacy of Acetyl-L-carnitine in Patients with Dementia Associated with Cerebrovascular Disease. Dement. Neurocogn. Disord. 2018, 17, 1–10. [Google Scholar] [CrossRef]
- Pueschel, S.M. The effect of acetyl-L-carnitine administration on persons with Down syndrome. Res. Dev. Disabil. 2006, 27, 599–604. [Google Scholar] [CrossRef]
- van Es, M.A.; Hardiman, O.; Chio, A.; Al-Chalabi, A.; Pasterkamp, R.J.; Veldink, J.H.; van den Berg, L.H. Amyotrophic lateral sclerosis. Lancet 2017, 390, 2084–2098. [Google Scholar] [CrossRef]
- Beghi, E.; Pupillo, E.; Bonito, V.; Buzzi, P.; Caponnetto, C.; Chiò, A.; Corbo, M.; Giannini, F.; Inghilleri, M.; Bella, V.L.; et al. Randomized double-blind placebo-controlled trial of acetyl-L-carnitine for ALS. Amyotroph. Lateral Scler. Front. Degener. 2013, 14, 397–405. [Google Scholar] [CrossRef]
- Sassi, S.; Bianchi, E.; Diamanti, L.; Tornabene, D.; Sette, E.; Medici, D.; Matà, S.; Leccese, D.; Sperti, M.; Martinelli, I.; et al. Retrospective observational study on the use of acetyl-L-carnitine in ALS. J. Neurol. 2023, 270, 5344–5357. [Google Scholar] [CrossRef] [PubMed]
- Sorbi, S.; Forleo, P.; Fani, C.; Piacentini, S. Double-blind, crossover, placebo-controlled clinical trial with L-acetylcarnitine in patients with degenerative cerebellar ataxia. Clin. Neuropharmacol. 2000, 23, 114–118. [Google Scholar] [CrossRef]
- Ashizawa, T.; Xia, G. Ataxia. Continuum (Minneapolis, Minn.) 2016, 22, 1208–1226. [Google Scholar] [CrossRef] [PubMed]
- Thapar, A.; Cooper, M. Attention deficit hyperactivity disorder. Lancet 2016, 387, 1240–1250. [Google Scholar] [CrossRef]
- Arnold, L.E.; Amato, A.; Bozzolo, H.; Hollway, J.; Cook, A.; Ramadan, Y.; Crowl, L.; Zhang, D.; Thompson, S.; Testa, G.; et al. Acetyl-L-carnitine (ALC) in attention-deficit/hyperactivity disorder: A multi-site, placebo-controlled pilot trial. J. Child Adolesc. Psychopharmacol. 2007, 17, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Padua, L.; Coraci, D.; Erra, C.; Pazzaglia, C.; Paolasso, I.; Loreti, C.; Caliandro, P.; Hobson-Webb, L.D. Carpal tunnel syndrome: Clinical features, diagnosis, and management. Lancet Neurol. 2016, 15, 1273–1284. [Google Scholar] [CrossRef]
- Cruccu, G.; Di Stefano, G.; Fattapposta, F.; Jann, S.; Padua, L.; Schenone, A.; Truini, A. L-Acetyl-carnitine in Patients with Carpal Tunnel Syndrome: Effects on Nerve Protection, Hand Function and Pain. CNS Drugs 2017, 31, 1103–1111. [Google Scholar] [CrossRef]
- Atique-Ur-Rehman, H.; Neill, J.C. Cognitive dysfunction in major depression: From assessment to novel therapies. Pharmacol. Ther. 2019, 202, 53–71. [Google Scholar] [CrossRef]
- Benton, D.; Donohoe, R.T. The influence on cognition of the interactions between lecithin, carnitine and carbohydrate. Psychopharmacology 2004, 175, 84–91. [Google Scholar] [CrossRef]
- Malaguarnera, M.; Gargante, M.P.; Cristaldi, E.; Colonna, V.; Messano, M.; Koverech, A.; Neri, S.; Vacante, M.; Cammalleri, L.; Motta, M. Acetyl L-carnitine (ALC) treatment in elderly patients with fatigue. Arch. Gerontol. Geriatr. 2008, 46, 181–190. [Google Scholar] [CrossRef]
- Malaguarnera, G.; Catania, V.E.; Bertino, G.; Chisari, L.M.; Castorina, M.; Bonfiglio, C.; Cauli, O.; Malaguarnera, M. Acetyl-L-carnitine Slows the Progression from Prefrailty to Frailty in Older Subjects: A Randomized Interventional Clinical Trial. Curr. Pharm. Des. 2022, 28, 3158–3166. [Google Scholar] [CrossRef] [PubMed]
- Dimidjian, S.; Barrera, M., Jr.; Martell, C.; Muñoz, R.F.; Lewinsohn, P.M. The origins and current status of behavioral activation treatments for depression. Annu. Rev. Clin. Psychol. 2011, 7, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Palazidou, E. The neurobiology of depression. Br. Med. Bull. 2012, 101, 127–145. [Google Scholar] [CrossRef]
- Salvioli, G.; Neri, M. L-acetylcarnitine treatment of mental decline in the elderly. Drugs Exp. Clin. Res. 1994, 20, 169–176. [Google Scholar] [PubMed]
- Bersani, G.; Meco, G.; Denaro, A.; Liberati, D.; Colletti, C.; Nicolai, R.; Bersani, F.S.; Koverech, A. L-Acetylcarnitine in dysthymic disorder in elderly patients: A double-blind, multicenter, controlled randomized study vs. fluoxetine. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 2013, 23, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Holmes, G.P.; Kaplan, J.E.; Gantz, N.M.; Komaroff, A.L.; Schonberger, L.B.; Straus, S.E.; Jones, J.F.; Dubois, R.E.; Cunningham-Rundles, C.; Pahwa, S.; et al. Chronic fatigue syndrome: A working case definition. Ann. Intern. Med. 1988, 108, 387–389. [Google Scholar] [CrossRef]
- Lloyd, A.R.; Wakefield, D.; Boughton, C.; Dwyer, J. What is myalgic encephalomyelitis? Lancet 1988, 331, 1286–1287. [Google Scholar] [CrossRef]
- Lloyd, A.R.; Hickie, I.; Boughton, C.R.; Spencer, O.; Wakefield, D. Prevalence of chronic fatigue syndrome in an Australian population. Med. J. Aust. 1990, 153, 522–528. [Google Scholar] [CrossRef]
- Sharpe, M.C.; Archard, L.C.; Banatvala, J.E.; Borysiewicz, L.K.; Clare, A.W.; David, A.; Edwards, R.H.; Hawton, K.E.; Lambert, H.P.; Lane, R.J.; et al. A report--chronic fatigue syndrome: Guidelines for research. J. R. Soc. Med. 1991, 84, 118–121. [Google Scholar] [CrossRef]
- Fukuda, K.; Straus, S.E.; Hickie, I.; Sharpe, M.C.; Dobbins, J.G.; Komaroff, A. The chronic fatigue syndrome: A comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann. Intern. Med. 1994, 121, 953–959. [Google Scholar] [CrossRef]
- Vermeulen, R.C.; Scholte, H.R. Exploratory open label, randomized study of acetyl- and propionylcarnitine in chronic fatigue syndrome. Psychosom. Med. 2004, 66, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Vilstrup, H.; Amodio, P.; Bajaj, J.; Cordoba, J.; Ferenci, P.; Mullen, K.D.; Weissenborn, K.; Wong, P. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology 2014, 60, 715–735. [Google Scholar] [CrossRef] [PubMed]
- Balzano, T.; Forteza, J.; Borreda, I.; Molina, P.; Giner, J.; Leone, P.; Urios, A.; Montoliu, C.; Felipo, V. Histological Features of Cerebellar Neuropathology in Patients With Alcoholic and Nonalcoholic Steatohepatitis. J. Neuropathol. Exp. Neurol. 2018, 77, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Forton, D.M.; Allsop, J.M.; Main, J.; Foster, G.R.; Thomas, H.C.; Taylor-Robinson, S.D. Evidence for a cerebral effect of the hepatitis C virus. Lancet 2001, 358, 38–39. [Google Scholar] [CrossRef]
- Mosher, V.A.L.; Swain, M.G.; Pang, J.X.Q.; Kaplan, G.G.; Sharkey, K.A.; MacQueen, G.M.; Goodyear, B.G. Magnetic resonance imaging evidence of hippocampal structural changes in patients with primary biliary cholangitis. Clin. Transl. Gastroenterol. 2018, 9, 169. [Google Scholar] [CrossRef] [PubMed]
- Grover, V.P.; Southern, L.; Dyson, J.K.; Kim, J.U.; Crossey, M.M.; Wylezinska-Arridge, M.; Patel, N.; Fitzpatrick, J.A.; Bak-Bol, A.; Waldman, A.D.; et al. Early primary biliary cholangitis is characterised by brain abnormalities on cerebral magnetic resonance imaging. Aliment. Pharmacol. Ther. 2016, 44, 936–945. [Google Scholar] [CrossRef]
- Cecere, A.; Ciaramella, F.; Tancredi, L.; Romano, C.; Gattoni, A. Efficacy of L-carnitine in reducing hyperammonaemia and improving neuropsychological test performance in patients with hepatic cirrhosis: Results of a randomised trial. Clin. Drug Investig. 2002, 22, 7–14. [Google Scholar] [CrossRef]
- Malaguarnera, M.; Pistone, G.; Astuto, M.; Dell’Arte, S.; Finocchiaro, G.; Lo Giudice, E.; Pennisi, G. L-Carnitine in the treatment of mild or moderate hepatic encephalopathy. Dig. Dis. 2003, 21, 271–275. [Google Scholar] [CrossRef]
- Malaguarnera, M.; Pistone, G.; Elvira, R.; Leotta, C.; Scarpello, L.; Liborio, R. Effects of L-carnitine in patients with hepatic encephalopathy. World J. Gastroenterol. 2005, 11, 7197–7202. [Google Scholar] [CrossRef]
- Malaguarnera, M.; Pistone, G.; Astuto, M.; Vecchio, I.; Raffaele, R.; Lo Giudice, E.; Rampello, L. Effects of L-acetylcarnitine on cirrhotic patients with hepatic coma: Randomized double-blind, placebo-controlled trial. Dig. Dis. Sci. 2006, 51, 2242–2247. [Google Scholar] [CrossRef]
- Siciliano, M.; Annicchiarico, B.E.; Lucchese, F.; Bombardieri, G. Effects of a single, short intravenous dose of acetyl-L-carnitine on pattern-reversal visual-evoked potentials in cirrhotic patients with hepatic encephalopathy. Clin. Exp. Pharmacol. Physiol. 2006, 33, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, M.; Gargante, M.P.; Cristaldi, E.; Vacante, M.; Risino, C.; Cammalleri, L.; Pennisi, G.; Rampello, L. Acetyl-L-carnitine treatment in minimal hepatic encephalopathy. Dig. Dis. Sci. 2008, 53, 3018–3025. [Google Scholar] [CrossRef]
- Malaguarnera, M.; Bella, R.; Vacante, M.; Giordano, M.; Malaguarnera, G.; Gargante, M.P.; Motta, M.; Mistretta, A.; Rampello, L.; Pennisi, G. Acetyl-L-carnitine reduces depression and improves quality of life in patients with minimal hepatic encephalopathy. Scand. J. Gastroenterol. 2011, 46, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, M.; Vacante, M.; Giordano, M.; Pennisi, G.; Bella, R.; Rampello, L.; Malaguarnera, M.; Li Volti, G.; Galvano, F. Oral acetyl-L-carnitine therapy reduces fatigue in overt hepatic encephalopathy: A randomized, double-blind, placebo-controlled study. Am. J. Clin. Nutr. 2011, 93, 799–808. [Google Scholar] [CrossRef]
- Malaguarnera, M.; Vacante, M.; Motta, M.; Giordano, M.; Malaguarnera, G.; Bella, R.; Nunnari, G.; Rampello, L.; Pennisi, G. Acetyl-L-carnitine improves cognitive functions in severe hepatic encephalopathy: A randomized and controlled clinical trial. Metab. Brain Dis. 2011, 26, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Hirano, H.; Yano, Y.; Momose, K.; Yoshida, M.; Azuma, T. Serum level of taurine would be associated with the amelioration of minimal hepatic encephalopathy in cirrhotic patients. Hepatol. Res. Off. J. Jpn. Soc. Hepatol. 2016, 46, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Yoon, E.L.; Ahn, S.B.; Jun, D.W.; Cho, Y.K.; Song, D.S.; Jeong, J.Y.; Kim, H.Y.; Jung, Y.K.; Song, M.J.; Kim, S.E.; et al. Effect of L-carnitine on quality of life in covert hepatic encephalopathy: A randomized, double-blind, placebo-controlled study. Korean J. Intern. Med. 2022, 37, 757–767. [Google Scholar] [CrossRef]
- Levin, M. The International Classification of Headache Disorders, 3rd Edition (ICHD III)—Changes and Challenges. Headache J. Head Face Pain 2013, 53, 1383–1395. [Google Scholar] [CrossRef]
- Giffin, N.J.; Ruggiero, L.; Lipton, R.B.; Silberstein, S.D.; Tvedskov, J.F.; Olesen, J.; Altman, J.; Goadsby, P.J.; Macrae, A. Premonitory symptoms in migraine: An electronic diary study. Neurology 2003, 60, 935–940. [Google Scholar] [CrossRef]
- Karsan, N.; Prabhakar, P.; Goadsby, P.J. Characterising the premonitory stage of migraine in children: A clinic-based study of 100 patients in a specialist headache service. J. Headache Pain 2016, 17, 94. [Google Scholar] [CrossRef]
- Dodick, D.W. Migraine. Lancet 2018, 391, 1315–1330. [Google Scholar] [CrossRef]
- Tarighat Esfanjani, A.; Mahdavi, R.; Ebrahimi Mameghani, M.; Talebi, M.; Nikniaz, Z.; Safaiyan, A. The effects of magnesium, L-carnitine, and concurrent magnesium-L-carnitine supplementation in migraine prophylaxis. Biol. Trace Elem. Res. 2012, 150, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Hagen, K.; Brenner, E.; Linde, M.; Gravdahl, G.B.; Tronvik, E.A.; Engstrøm, M.; Sonnewald, U.; Helde, G.; Stovner, L.J.; Sand, T. Acetyl-l-carnitine versus placebo for migraine prophylaxis: A randomized, triple-blind, crossover study. Cephalalgia Int. J. Headache 2015, 35, 987–995. [Google Scholar] [CrossRef]
- McFarland, H.F.; Martin, R. Multiple sclerosis: A complicated picture of autoimmunity. Nat. Immunol. 2007, 8, 913–919. [Google Scholar] [CrossRef]
- Trapp, B.D.; Peterson, J.; Ransohoff, R.M.; Rudick, R.; Mörk, S.; Bö, L. Axonal transection in the lesions of multiple sclerosis. N. Engl. J. Med. 1998, 338, 278–285. [Google Scholar] [CrossRef]
- Lublin, F.D.; Reingold, S.C. Defining the clinical course of multiple sclerosis: Results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology 1996, 46, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Tomassini, V.; Pozzilli, C.; Onesti, E.; Pasqualetti, P.; Marinelli, F.; Pisani, A.; Fieschi, C. Comparison of the effects of acetyl L-carnitine and amantadine for the treatment of fatigue in multiple sclerosis: Results of a pilot, randomised, double-blind, crossover trial. J. Neurol. Sci. 2004, 218, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Ferner, R.E.; O’Doherty, M.J. Neurofibroma and schwannoma. Curr. Opin. Neurol. 2002, 15, 679–684. [Google Scholar] [CrossRef]
- Vasiljevski, E.R.; Burns, J.; Bray, P.; Donlevy, G.; Mudge, A.J.; Jones, K.J.; Summers, M.A.; Biggin, A.; Munns, C.F.; McKay, M.J.; et al. L-carnitine supplementation for muscle weakness and fatigue in children with neurofibromatosis type 1: A Phase 2a clinical trial. Am. J. Med. Genet. Part A 2021, 185, 2976–2985. [Google Scholar] [CrossRef]
- Hanewinckel, R.; Ikram, M.A.; Van Doorn, P.A. Chapter 15—Peripheral neuropathies. In Handbook of Clinical Neurology; Aminoff, M.J., Boller, F., Swaab, D.F., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 138, pp. 263–282. [Google Scholar]
- De Grandis, D.; Minardi, C. Acetyl-L-carnitine (levacecarnine) in the treatment of diabetic neuropathy. A long-term, randomised, double-blind, placebo-controlled study. Drugs R&D 2002, 3, 223–231. [Google Scholar] [CrossRef]
- Sima, A.A.; Calvani, M.; Mehra, M.; Amato, A. Acetyl-L-carnitine improves pain, nerve regeneration, and vibratory perception in patients with chronic diabetic neuropathy: An analysis of two randomized placebo-controlled trials. Diabetes Care 2005, 28, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Ulvi, H.; Aygül, R.; Demir, R. Effect of L-carnitine on diabetic neuropathy and ventricular dispersion in patients with diabetes mellitus. Turk. J. Med. Sci. 2010, 40, 169–175. [Google Scholar] [CrossRef]
- Sun, Y.; Shu, Y.; Liu, B.; Liu, P.; Wu, C.; Zheng, R.; Zhang, X.; Zhuang, Z.; Deng, Y.; Zheng, L.; et al. A prospective study to evaluate the efficacy and safety of oral acetyl-L-carnitine for the treatment of chemotherapy-induced peripheral neuropathy. Exp. Ther. Med. 2016, 12, 4017–4024. [Google Scholar] [CrossRef]
- Li, S.; Chen, X.; Li, Q.; Du, J.; Liu, Z.; Peng, Y.; Xu, M.; Li, Q.; Lei, M.; Wang, C.; et al. Effects of acetyl-L-carnitine and methylcobalamin for diabetic peripheral neuropathy: A multicenter, randomized, double-blind, controlled trial. J. Diabetes Investig. 2016, 7, 777–785. [Google Scholar] [CrossRef]
- Chahrour, M.; Zoghbi, H.Y. The story of Rett syndrome: From clinic to neurobiology. Neuron 2007, 56, 422–437. [Google Scholar] [CrossRef] [PubMed]
- Guy, J.; Cheval, H.; Selfridge, J.; Bird, A. The role of MeCP2 in the brain. Annu. Rev. Cell Dev. Biol. 2011, 27, 631–652. [Google Scholar] [CrossRef]
- Percy, A.K. Rett syndrome: Exploring the autism link. Arch. Neurol. 2011, 68, 985–989. [Google Scholar] [CrossRef]
- Banerjee, A.; Castro, J.; Sur, M. Rett syndrome: Genes, synapses, circuits, and therapeutics. Front. Psychiatry 2012, 3, 34. [Google Scholar] [CrossRef] [PubMed]
- Katz, D.M.; Bird, A.; Coenraads, M.; Gray, S.J.; Menon, D.U.; Philpot, B.D.; Tarquinio, D.C. Rett Syndrome: Crossing the Threshold to Clinical Translation. Trends Neurosci. 2016, 39, 100–113. [Google Scholar] [CrossRef]
- Leonard, H.; Cobb, S.; Downs, J. Clinical and biological progress over 50 years in Rett syndrome. Nat. Reviews Neurol. 2017, 13, 37–51. [Google Scholar] [CrossRef]
- Banerjee, A.; Miller, M.T.; Li, K.; Sur, M.; Kaufmann, W.E. Towards a better diagnosis and treatment of Rett syndrome: A model synaptic disorder. Brain J. Neurol. 2019, 142, 239–248. [Google Scholar] [CrossRef]
- Ellaway, C.; Williams, K.; Leonard, H.; Higgins, G.; Wilcken, B.; Christodoulou, J. Rett syndrome: Randomized controlled trial of L-carnitine. J. Child Neurol. 1999, 14, 162–167. [Google Scholar] [CrossRef]
- Ellaway, C.J.; Peat, J.; Williams, K.; Leonard, H.; Christodoulou, J. Medium-term open label trial of L-carnitine in Rett syndrome. Brain Dev. 2001, 23 (Suppl. S1), S85–S89. [Google Scholar] [CrossRef]
- Guideri, F.; Acampa, M.; Hayek, Y.; Zappella, M. Effects of acetyl-L-carnitine on cardiac dysautonomia in Rett syndrome: Prevention of sudden death? Pediatr. Cardiol. 2005, 26, 574–577. [Google Scholar] [CrossRef]
- Pearce, J.M. A brief history of sciatica. Spinal Cord 2007, 45, 592–596. [Google Scholar] [CrossRef]
- Frymoyer, J.W. Back pain and sciatica. N. Engl. J. Med. 1988, 318, 291–300. [Google Scholar] [CrossRef] [PubMed]
- MIXTER, W.J.; BARR, J.S. Rupture of the Intervertebral Disc with Involvement of the Spinal Canal. N. Engl. J. Med. 1934, 211, 210–215. [Google Scholar] [CrossRef]
- Porchet, F.; Wietlisbach, V.; Burnand, B.; Daeppen, K.; Villemure, J.-G.; Vader, J.-P. Relationship between severity of lumbar disc disease and disability scores in sciatica patients. Neurosurgery 2002, 50, 1253–1259; discussion 1259–1260. [Google Scholar] [CrossRef]
- Ropper, A.H.; Zafonte, R.D. Sciatica. N. Engl. J. Med. 2015, 372, 1240–1248. [Google Scholar] [CrossRef]
- Memeo, A.; Loiero, M. Thioctic acid and acetyl-L-carnitine in the treatment of sciatic pain caused by a herniated disc: A randomized, double-blind, comparative study. Clin. Drug Investig. 2008, 28, 495–500. [Google Scholar] [CrossRef]
- Aho, K.; Harmsen, P.; Hatano, S.; Marquardsen, J.; Smirnov, V.E.; Strasser, T. Cerebrovascular disease in the community: Results of a WHO collaborative study. Bull. World Health Organ. 1980, 58, 113. [Google Scholar] [PubMed]
- Rodgers, H. Chapter 36—Stroke. In Handbook of Clinical Neurology; Barnes, M.P., Good, D.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 110, pp. 427–433. [Google Scholar]
- Fedotova, A.V.; Mironova, O.P.; Fedin, A.I. Use of L-Carnitine in Patients with Chronic Cerebral Ischemia. Neurosci. Behav. Physiol. 2014, 44, 939–944. [Google Scholar] [CrossRef]
- Chichanovskaya, L.V.; Bakhareva, O.N.; Sorokina, K.B. A study of the efficacy and safety of L-carnitine in patients with ischemic stroke in the early rehabilitation period. Zhurnal Nevrol. Psikhiatrii Im. S.S. Korsakova 2017, 117, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Kazemian, K.; Ala, S.; Mojtahedzadeh, M.; Abedini, M.; Alipour, A.; Abediankenari, S.; Rafati, M.; Abaskhanidavanloo, A.; Mohajerani, F. Evaluation of Neuroprtective Effects of L-Carnitine and Fat Emulsion in the CVA Patients: A Prospective, Randomized, Double Blind, Clinical Trial. Iran. J. Pharm. Res. IJPR 2020, 19, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Mazdeh, M.; Abolfathi, P.; Sabetghadam, M.; Mohammadi, Y.; Mehrpooya, M. Clinical Evidence of Acetyl-L-Carnitine Efficacy in the Treatment of Acute Ischemic Stroke: A Pilot Clinical Trial. Oxidative Med. Cell. Longev. 2022, 2022, 2493053. [Google Scholar] [CrossRef]
- Gandhi, S.; Abramov, A.Y. Mechanism of Oxidative Stress in Neurodegeneration. Oxidative Med. Cell. Longev. 2012, 2012, 428010. [Google Scholar] [CrossRef]
- Maes, M.; Galecki, P.; Chang, Y.S.; Berk, M. A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro)degenerative processes in that illness. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 676–692. [Google Scholar] [CrossRef] [PubMed]
- Behr, G.A.; Moreira, J.C.; Frey, B.N. Preclinical and clinical evidence of antioxidant effects of antidepressant agents: Implications for the pathophysiology of major depressive disorder. Oxidative Med. Cell. Longev. 2012, 2012, 609421. [Google Scholar] [CrossRef] [PubMed]
- Markesbery, W.R. The role of oxidative stress in Alzheimer disease. Arch. Neurol. 1999, 56, 1449–1452. [Google Scholar] [CrossRef]
- Smaga, I.; Pomierny, B.; Krzyżanowska, W.; Pomierny-Chamioło, L.; Miszkiel, J.; Niedzielska, E.; Ogórka, A.; Filip, M. N-acetylcysteine possesses antidepressant-like activity through reduction of oxidative stress: Behavioral and biochemical analyses in rats. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2012, 39, 280–287. [Google Scholar] [CrossRef]
- Chen, Z.; Zhong, C. Oxidative stress in Alzheimer’s disease. Neurosci. Bull. 2014, 30, 271–281. [Google Scholar] [CrossRef]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef]
- Bhatt, S.; Nagappa, A.N.; Patil, C.R. Role of oxidative stress in depression. Drug Discov. Today 2020, 25, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Moylan, S.; Berk, M.; Dean, O.M.; Samuni, Y.; Williams, L.J.; O’Neil, A.; Hayley, A.C.; Pasco, J.A.; Anderson, G.; Jacka, F.N.; et al. Oxidative & nitrosative stress in depression: Why so much stress? Neurosci. Biobehav. Rev. 2014, 45, 46–62. [Google Scholar] [CrossRef]
- Ye, Y.X.; Chen, H.G.; Sun, B.; Chen, Y.J.; Duan, P.; Meng, T.Q.; Xiong, C.L.; Wang, Y.X.; Pan, A. Associations between depression, oxidative stress, and semen quality among 1,000 healthy men screened as potential sperm donors. Fertil. Steril. 2022, 117, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Louwerse, E.S.; Weverling, G.J.; Bossuyt, P.M.; Meyjes, F.E.; de Jong, J.M. Randomized, double-blind, controlled trial of acetylcysteine in amyotrophic lateral sclerosis. Arch. Neurol. 1995, 52, 559–564. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.; Zhou, J.; Li, Y.; Wu, K.; Chen, Z.; Luo, Z.; Zhang, X.; Liang, Y.; Esteban, M.A.; Zhou, Y.; et al. TDP-43 aggregation induced by oxidative stress causes global mitochondrial imbalance in ALS. Nat. Struct. Mol. Biol. 2021, 28, 132–142. [Google Scholar] [CrossRef]
- Barber, S.C.; Shaw, P.J. Oxidative stress in ALS: Key role in motor neuron injury and therapeutic target. Free. Radic. Biol. Med. 2010, 48, 629–641. [Google Scholar] [CrossRef]
- Tam, O.H.; Rozhkov, N.V.; Shaw, R.; Kim, D.; Hubbard, I.; Fennessey, S.; Propp, N.; Fagegaltier, D.; Harris, B.T.; Ostrow, L.W.; et al. Postmortem Cortex Samples Identify Distinct Molecular Subtypes of ALS: Retrotransposon Activation, Oxidative Stress, and Activated Glia. Cell Rep. 2019, 29, 1164–1177.e1165. [Google Scholar] [CrossRef]
- Areti, A.; Yerra, V.G.; Naidu, V.; Kumar, A. Oxidative stress and nerve damage: Role in chemotherapy induced peripheral neuropathy. Redox Biol. 2014, 2, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Li, K.; Chen, Y.; Xie, J.; Wu, C.; Cui, C.; Deng, B. Oxidative Stress in Diabetic Peripheral Neuropathy: Pathway and Mechanism-Based Treatment. Mol. Neurobiol. 2023, 60, 4574–4594. [Google Scholar] [CrossRef] [PubMed]
- Eftekharpour, E.; Fernyhough, P. Oxidative Stress and Mitochondrial Dysfunction Associated with Peripheral Neuropathy in Type 1 Diabetes. Antioxid. Redox Signal. 2022, 37, 578–596. [Google Scholar] [CrossRef] [PubMed]
- Gawryluk, J.W.; Wang, J.F.; Andreazza, A.C.; Shao, L.; Young, L.T. Decreased levels of glutathione, the major brain antioxidant, in post-mortem prefrontal cortex from patients with psychiatric disorders. Int. J. Neuropsychopharmacol. 2011, 14, 123–130. [Google Scholar] [CrossRef]
- Virmani, A.; Binienda, Z. Role of carnitine esters in brain neuropathology. Mol. Asp. Med. 2004, 25, 533–549. [Google Scholar] [CrossRef] [PubMed]
- Bigini, P.; Larini, S.; Pasquali, C.; Muzio, V.; Mennini, T. Acetyl-L-carnitine shows neuroprotective and neurotrophic activity in primary culture of rat embryo motoneurons. Neurosci. Lett. 2002, 329, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Bertamini, M.; Marzani, B.; Guarneri, R.; Guarneri, P.; Bigini, P.; Mennini, T.; Curti, D. Mitochondrial oxidative metabolism in motor neuron degeneration (mnd) mouse central nervous system. Eur. J. Neurosci. 2002, 16, 2291–2296. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, C.; Zhang, X.; Wang, S.; Meng, Q.; Wu, S.; Yang, H.; Xia, Y.; Chen, R. An acetyl-L-carnitine switch on mitochondrial dysfunction and rescue in the metabolomics study on aluminum oxide nanoparticles. Part. Fibre Toxicol. 2016, 13, 4. [Google Scholar] [CrossRef] [PubMed]
- Kocsis, K.; Frank, R.; Szabó, J.; Knapp, L.; Kis, Z.; Farkas, T.; Vécsei, L.; Toldi, J. Acetyl-l-carnitine restores synaptic transmission and enhances the inducibility of stable LTP after oxygen-glucose deprivation. Neuroscience 2016, 332, 203–211. [Google Scholar] [CrossRef]
- Kazak, F.; Yarim, G.F. Neuroprotective effects of acetyl-l-carnitine on lipopolysaccharide-induced neuroinflammation in mice: Involvement of brain-derived neurotrophic factor. Neurosci. Lett. 2017, 658, 32–36. [Google Scholar] [CrossRef]
- Altun, Z.; Olgun, Y.; Ercetin, P.; Aktas, S.; Kirkim, G.; Serbetcioglu, B.; Olgun, N.; Guneri, E.A. Protective effect of acetyl-l-carnitine against cisplatin ototoxicity: Role of apoptosis-related genes and pro-inflammatory cytokines. Cell Prolif. 2014, 47, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zeng, X.; Li, L.; Ou, Z.L. Carnitine promotes recovery from oxidative stress and extends lifespan in C. elegans. Aging 2020, 13, 813–830. [Google Scholar] [CrossRef] [PubMed]
- Moeinian, M.; Ghasemi-Niri, S.F.; Mozaffari, S.; Abdollahi, M. Synergistic effect of probiotics, butyrate and l-Carnitine in treatment of IBD. J. Med. Hypotheses Ideas 2013, 7, 50–53. [Google Scholar] [CrossRef]
- Lee, B.J.; Lin, J.S.; Lin, Y.C.; Lin, P.T. Effects of L-carnitine supplementation on lipid profiles in patients with coronary artery disease. Lipids Health Dis. 2016, 15, 107. [Google Scholar] [CrossRef] [PubMed]
- Nachvak, S.M.; Shabanpur, M.; Mostafai, R.; Heidari Moghaddam, R.; Moludi, J. L-Carnitine supplementation reduces biomarkers of inflammatory and oxidative stress in patients with coronary artery disease: A randomised controlled trial. Arch. Physiol. Biochem. 2023, 129, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Modanloo, M.; Shokrzadeh, M. Analyzing Mitochondrial Dysfunction, Oxidative Stress, and Apoptosis: Potential Role of L-carnitine. Iran. J. Kidney Dis. 2019, 13, 74–86. [Google Scholar] [PubMed]
- Montesano, A.; Senesi, P.; Luzi, L.; Benedini, S.; Terruzzi, I. Potential therapeutic role of L-carnitine in skeletal muscle oxidative stress and atrophy conditions. Oxidative Med. Cell. Longev. 2015, 2015, 646171. [Google Scholar] [CrossRef]
- Kita, K.; Kato, S.; Amanyaman, M.; Okumura, J.; Yokota, H. Dietary L-carnitine increases plasma insulin-like growth factor-I concentration in chicks fed a diet with adequate dietary protein level. Br. Poult. Sci. 2002, 43, 117–121. [Google Scholar] [CrossRef]
- Keller, J.; Ringseis, R.; Eder, K. Supplemental carnitine affects the microRNA expression profile in skeletal muscle of obese Zucker rats. BMC Genom. 2014, 15, 512. [Google Scholar] [CrossRef]
- Keller, J.; Couturier, A.; Haferkamp, M.; Most, E.; Eder, K. Supplementation of carnitine leads to an activation of the IGF-1/PI3K/Akt signalling pathway and down regulates the E3 ligase MuRF1 in skeletal muscle of rats. Nutr. Metab. 2013, 10, 28. [Google Scholar] [CrossRef]
- Gülçin, I. Antioxidant and antiradical activities of L-carnitine. Life Sci. 2006, 78, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Anisman, H.; Hayley, S.; Turrin, N.; Merali, Z. Cytokines as a stressor: Implications for depressive illness. Int. J. Neuropsychopharmacol. 2002, 5, 357–373. [Google Scholar] [CrossRef]
- Tiemeier, H.; Hofman, A.; van Tuijl, H.R.; Kiliaan, A.J.; Meijer, J.; Breteler, M.M. Inflammatory proteins and depression in the elderly. Epidemiology 2003, 14, 103–107. [Google Scholar] [CrossRef]
- Penninx, B.W.; Kritchevsky, S.B.; Yaffe, K.; Newman, A.B.; Simonsick, E.M.; Rubin, S.; Ferrucci, L.; Harris, T.; Pahor, M. Inflammatory markers and depressed mood in older persons: Results from the Health, Aging and Body Composition study. Biol. Psychiatry 2003, 54, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Empana, J.P.; Sykes, D.H.; Luc, G.; Juhan-Vague, I.; Arveiler, D.; Ferrieres, J.; Amouyel, P.; Bingham, A.; Montaye, M.; Ruidavets, J.B.; et al. Contributions of depressive mood and circulating inflammatory markers to coronary heart disease in healthy European men: The Prospective Epidemiological Study of Myocardial Infarction (PRIME). Circulation 2005, 111, 2299–2305. [Google Scholar] [CrossRef]
- Berk, M.; Williams, L.J.; Jacka, F.N.; O’Neil, A.; Pasco, J.A.; Moylan, S.; Allen, N.B.; Stuart, A.L.; Hayley, A.C.; Byrne, M.L.; et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013, 11, 200. [Google Scholar] [CrossRef]
- Nerurkar, L.; Siebert, S.; McInnes, I.B.; Cavanagh, J. Rheumatoid arthritis and depression: An inflammatory perspective. Lancet Psychiatry 2019, 6, 164–173. [Google Scholar] [CrossRef]
- Hop, P.J.; Zwamborn, R.A.J.; Hannon, E.; Shireby, G.L.; Nabais, M.F.; Walker, E.M.; van Rheenen, W.; van Vugt, J.; Dekker, A.M.; Westeneng, H.J.; et al. Genome-wide study of DNA methylation shows alterations in metabolic, inflammatory, and cholesterol pathways in ALS. Sci. Transl. Med. 2022, 14, eabj0264. [Google Scholar] [CrossRef]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef]
- Twarowski, B.; Herbet, M. Inflammatory Processes in Alzheimer’s Disease-Pathomechanism, Diagnosis and Treatment: A Review. Int. J. Mol. Sci. 2023, 24, 6518. [Google Scholar] [CrossRef]
- Qian, X.H.; Song, X.X.; Liu, X.L.; Chen, S.D.; Tang, H.D. Inflammatory pathways in Alzheimer’s disease mediated by gut microbiota. Ageing Res. Rev. 2021, 68, 101317. [Google Scholar] [CrossRef]
- Jain, L.; Sharma, B.C.; Sharma, P.; Srivastava, S.; Agrawal, A.; Sarin, S.K. Serum endotoxin and inflammatory mediators in patients with cirrhosis and hepatic encephalopathy. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2012, 44, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Butterworth, R.F. Hepatic encephalopathy: A central neuroinflammatory disorder? Hepatology 2011, 53, 1372–1376. [Google Scholar] [CrossRef] [PubMed]
- Rajkowska, G.; Miguel-Hidalgo, J.J. Gliogenesis and glial pathology in depression. CNS Neurol. Disord. Drug Targets 2007, 6, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorso, S.; Marino, V.; Puzella, A.; Pasquini, M.; Biondi, M.; Artini, M.; Almerighi, C.; Verkerk, R.; Meltzer, H.; Maes, M. Increased depressive ratings in patients with hepatitis C receiving interferon-alpha-based immunotherapy are related to interferon-alpha-induced changes in the serotonergic system. J. Clin. Psychopharmacol. 2002, 22, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Capuron, L.; Ravaud, A.; Miller, A.H.; Dantzer, R. Baseline mood and psychosocial characteristics of patients developing depressive symptoms during interleukin-2 and/or interferon-alpha cancer therapy. Brain Behav. Immun. 2004, 18, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Bodaghi-Namileh, V.; Sepand, M.R.; Omidi, A.; Aghsami, M.; Seyednejad, S.A.; Kasirzadeh, S.; Sabzevari, O. Acetyl-l-carnitine attenuates arsenic-induced liver injury by abrogation of mitochondrial dysfunction, inflammation, and apoptosis in rats. Environ. Toxicol. Pharmacol. 2018, 58, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; Deng, R.; Li, J.; Chi, W.; Su, Z.; Lin, J.; Pflugfelder, S.C.; Li, D.Q. Protective Effects of L-Carnitine Against Oxidative Injury by Hyperosmolarity in Human Corneal Epithelial Cells. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5503–5511. [Google Scholar] [CrossRef]
- Salama, S.A.; Arab, H.H.; Omar, H.A.; Gad, H.S.; Abd-Allah, G.M.; Maghrabi, I.A.; Al Robaian, M.M. L-carnitine mitigates UVA-induced skin tissue injury in rats through downregulation of oxidative stress, p38/c-Fos signaling, and the proinflammatory cytokines. Chem. -Biol. Interact. 2018, 285, 40–47. [Google Scholar] [CrossRef]
- Siomek, A. NF-κB signaling pathway and free radical impact. Acta Biochim. Pol. 2012, 59, 323–331. [Google Scholar] [CrossRef]
- Fathizadeh, H.; Milajerdi, A.; Reiner, Ž.; Amirani, E.; Asemi, Z.; Mansournia, M.A.; Hallajzadeh, J. The effects of L-carnitine supplementation on indicators of inflammation and oxidative stress: A systematic review and meta-analysis of randomized controlled trials. J. Diabetes Metab. Disord. 2020, 19, 1879–1894. [Google Scholar] [CrossRef] [PubMed]
- Duranay, M.; Akay, H.; Yilmaz, F.M.; Senes, M.; Tekeli, N.; Yücel, D. Effects of L-carnitine infusions on inflammatory and nutritional markers in haemodialysis patients. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2006, 21, 3211–3214. [Google Scholar] [CrossRef] [PubMed]
- Yeun, J.Y.; Levine, R.A.; Mantadilok, V.; Kaysen, G.A. C-Reactive protein predicts all-cause and cardiovascular mortality in hemodialysis patients. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2000, 35, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Malek Mahdavi, A.; Mahdavi, R.; Kolahi, S. Effects of l-Carnitine Supplementation on Serum Inflammatory Factors and Matrix Metalloproteinase Enzymes in Females with Knee Osteoarthritis: A Randomized, Double-Blind, Placebo-Controlled Pilot Study. J. Am. Coll. Nutr. 2016, 35, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Koc, A.; Ozkan, T.; Karabay, A.Z.; Sunguroglu, A.; Aktan, F. Effect of L-carnitine on the synthesis of nitric oxide in RAW 264·7 murine macrophage cell line. Cell Biochem. Funct. 2011, 29, 679–685. [Google Scholar] [CrossRef]
- Sahebkar, A. Effect of L-carnitine Supplementation on Circulating C-reactive Protein Levels: A Systematic Review and Meta-Analysis. J. Med. Biochem. 2015, 34, 151–159. [Google Scholar] [CrossRef]
- Neale, E.P.; Tapsell, L.C.; Guan, V.; Batterham, M.J. The effect of nut consumption on markers of inflammation and endothelial function: A systematic review and meta-analysis of randomised controlled trials. BMJ Open 2017, 7, e016863. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhao, F.; Ma, X.; Perry, G.; Zhu, X. Mitochondria dysfunction in the pathogenesis of Alzheimer’s disease: Recent advances. Mol. Neurodegener. 2020, 15, 30. [Google Scholar] [CrossRef]
- Shoshan-Barmatz, V.; Nahon-Crystal, E.; Shteinfer-Kuzmine, A.; Gupta, R. VDAC1, mitochondrial dysfunction, and Alzheimer’s disease. Pharmacol. Res. 2018, 131, 87–101. [Google Scholar] [CrossRef]
- Li, Y.; Xia, X.; Wang, Y.; Zheng, J.C. Mitochondrial dysfunction in microglia: A novel perspective for pathogenesis of Alzheimer’s disease. J. Neuroinflamm. 2022, 19, 248. [Google Scholar] [CrossRef]
- Ke, J.; Tian, Q.; Xu, Q.; Fu, Z.; Fu, Q. Mitochondrial dysfunction: A potential target for Alzheimer’s disease intervention and treatment. Drug Discov. Today 2021, 26, 1991–2002. [Google Scholar] [CrossRef] [PubMed]
- Lifshitz, J.; Sullivan, P.G.; Hovda, D.A.; Wieloch, T.; McIntosh, T.K. Mitochondrial damage and dysfunction in traumatic brain injury. Mitochondrion 2004, 4, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Mira, R.G.; Lira, M.; Cerpa, W. Traumatic Brain Injury: Mechanisms of Glial Response. Front. Physiol. 2021, 12, 740939. [Google Scholar] [CrossRef] [PubMed]
- Beal, M.F. Mitochondria and neurodegeneration. Novartis Found. Symp. 2007, 287, 183–192; discussion 192–186. [Google Scholar] [CrossRef]
- Subramaniam, S.R.; Chesselet, M.F. Mitochondrial dysfunction and oxidative stress in Parkinson’s disease. Prog. Neurobiol. 2013, 106–107, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J.; Tan, E.K. Parkinson’s disease: Etiopathogenesis and treatment. J. Neurol. Neurosurg. Psychiatry 2020, 91, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Giannoccaro, M.P.; La Morgia, C.; Rizzo, G.; Carelli, V. Mitochondrial DNA and primary mitochondrial dysfunction in Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2017, 32, 346–363. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.E.; Akther, M.; Azam, S.; Kim, I.S.; Lin, Y.; Lee, Y.H.; Choi, D.K. Targeting α-synuclein aggregation and its role in mitochondrial dysfunction in Parkinson’s disease. Br. J. Pharmacol. 2022, 179, 23–45. [Google Scholar] [CrossRef] [PubMed]
- Witte, M.E.; Mahad, D.J.; Lassmann, H.; van Horssen, J. Mitochondrial dysfunction contributes to neurodegeneration in multiple sclerosis. Trends Mol. Med. 2014, 20, 179–187. [Google Scholar] [CrossRef]
- Wang, P.F.; Jiang, F.; Zeng, Q.M.; Yin, W.F.; Hu, Y.Z.; Li, Q.; Hu, Z.L. Mitochondrial and metabolic dysfunction of peripheral immune cells in multiple sclerosis. J. Neuroinflamm. 2024, 21, 28. [Google Scholar] [CrossRef]
- Yong, H.Y.F.; Yong, V.W. Mechanism-based criteria to improve therapeutic outcomes in progressive multiple sclerosis. Nat. Rev. Neurol. 2022, 18, 40–55. [Google Scholar] [CrossRef]
- Cozzolino, M.; Carrì, M.T. Mitochondrial dysfunction in ALS. Prog. Neurobiol. 2012, 97, 54–66. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, X.; Huo, Z.; Chen, Y.; Liu, J.; Zhao, Z.; Meng, F.; Su, Q.; Bao, W.; Zhang, L.; et al. The Impact of Mitochondrial Dysfunction in Amyotrophic Lateral Sclerosis. Cells 2022, 11, 2049. [Google Scholar] [CrossRef] [PubMed]
- Chia, R.; Chiò, A.; Traynor, B.J. Novel genes associated with amyotrophic lateral sclerosis: Diagnostic and clinical implications. Lancet Neurol. 2018, 17, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Cao, H.; Zuo, C.; Gu, Z.; Huang, Y.; Miao, J.; Fu, Y.; Guo, Y.; Jiang, Y.; Wang, F. Mitochondrial dysfunction: A fatal blow in depression. Biomed. Pharmacother. 2023, 167, 115652. [Google Scholar] [CrossRef] [PubMed]
- Bansal, Y.; Kuhad, A. Mitochondrial Dysfunction in Depression. Curr. Neuropharmacol. 2016, 14, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Fries, G.R.; Saldana, V.A.; Finnstein, J.; Rein, T. Molecular pathways of major depressive disorder converge on the synapse. Mol. Psychiatry 2023, 28, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Orth, M.; Schapira, A.H. Mitochondria and degenerative disorders. Am. J. Med. Genet. 2001, 106, 27–36. [Google Scholar] [CrossRef]
- Shao, L.; Martin, M.V.; Watson, S.J.; Schatzberg, A.; Akil, H.; Myers, R.M.; Jones, E.G.; Bunney, W.E.; Vawter, M.P. Mitochondrial involvement in psychiatric disorders. Ann. Med. 2008, 40, 281–295. [Google Scholar] [CrossRef]
- Fattal, O.; Budur, K.; Vaughan, A.J.; Franco, K. Review of the literature on major mental disorders in adult patients with mitochondrial diseases. Psychosomatics 2006, 47, 1–7. [Google Scholar] [CrossRef]
- Fattal, O.; Link, J.; Quinn, K.; Cohen, B.H.; Franco, K. Psychiatric comorbidity in 36 adults with mitochondrial cytopathies. CNS Spectr. 2007, 12, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Volz, H.P.; Rzanny, R.; May, S.; Hegewald, H.; Preussler, B.; Hajek, M.; Kaiser, W.A.; Sauer, H. 31P magnetic resonance spectroscopy in the dorsolateral prefrontal cortex of schizophrenics with a volume selective technique--preliminary findings. Biol. Psychiatry 1997, 41, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Kegeles, L.S.; Humaran, T.J.; Mann, J.J. In vivo neurochemistry of the brain in schizophrenia as revealed by magnetic resonance spectroscopy. Biol. Psychiatry 1998, 44, 382–398. [Google Scholar] [CrossRef] [PubMed]
- Prince, J.A.; Harro, J.; Blennow, K.; Gottfries, C.G.; Oreland, L. Putamen mitochondrial energy metabolism is highly correlated to emotional and intellectual impairment in schizophrenics. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2000, 22, 284–292. [Google Scholar] [CrossRef][Green Version]
- Buchsbaum, M.S.; Hazlett, E.A. Positron emission tomography studies of abnormal glucose metabolism in schizophrenia. Schizophr. Bull. 1998, 24, 343–364. [Google Scholar] [CrossRef]
- Strakowski, S.M.; DelBello, M.P.; Adler, C.; Cecil, D.M.; Sax, K.W. Neuroimaging in bipolar disorder. Bipolar Disord. 2000, 2, 148–164. [Google Scholar] [CrossRef] [PubMed]
- Karry, R.; Klein, E.; Ben Shachar, D. Mitochondrial complex I subunits expression is altered in schizophrenia: A postmortem study. Biol. Psychiatry 2004, 55, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Jun, D.W.; Cho, W.K.; Jun, J.H.; Kwon, H.J.; Jang, K.S.; Kim, H.J.; Jeon, H.J.; Lee, K.N.; Lee, H.L.; Lee, O.Y.; et al. Prevention of free fatty acid-induced hepatic lipotoxicity by carnitine via reversal of mitochondrial dysfunction. Liver Int. Off. J. Int. Assoc. Study Liver 2011, 31, 1315–1324. [Google Scholar] [CrossRef]
- Calvani, M.; Reda, E.; Arrigoni-Martelli, E. Regulation by carnitine of myocardial fatty acid and carbohydrate metabolism under normal and pathological conditions. Basic Res. Cardiol. 2000, 95, 75–83. [Google Scholar] [CrossRef]
- Shug, A.L.; Schmidt, M.J.; Golden, G.T.; Fariello, R.G. The distribution and role of carnitine in the mammalian brain. Life Sci. 1982, 31, 2869–2874. [Google Scholar] [CrossRef]
- Nzwalo, H.; Carrapatoso, L.; Ferreira, F.; Basilio, C. Valproic acid-induced hyperammonaemic coma and unrecognised portosystemic shunt. Epileptic Disord. 2013, 15, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Berk, M.; Dodd, S.; Kauer-Sant’anna, M.; Malhi, G.S.; Bourin, M.; Kapczinski, F.; Norman, T. Dopamine dysregulation syndrome: Implications for a dopamine hypothesis of bipolar disorder. Acta Psychiatr. Scand. 2007, 116, 41–49. [Google Scholar] [CrossRef]
- Heinz, A.; Schlagenhauf, F. Dopaminergic dysfunction in schizophrenia: Salience attribution revisited. Schizophr. Bull. 2010, 36, 472–485. [Google Scholar] [CrossRef]
- Hyman, S.E.; Malenka, R.C.; Nestler, E.J. Neural mechanisms of addiction: The role of reward-related learning and memory. Annu. Rev. Neurosci. 2006, 29, 565–598. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xiao, H.; Sun, H.; Zou, L.; Zhu, L.Q. Role of dopamine receptors in ADHD: A systematic meta-analysis. Mol. Neurobiol. 2012, 45, 605–620. [Google Scholar] [CrossRef] [PubMed]
- Deepmala; Slattery, J.; Kumar, N.; Delhey, L.; Berk, M.; Dean, O.; Spielholz, C.; Frye, R. Clinical trials of N-acetylcysteine in psychiatry and neurology: A systematic review. Neurosci. Biobehav. Rev. 2015, 55, 294–321. [Google Scholar] [CrossRef]
- Duman, R.S.; Sanacora, G.; Krystal, J.H. Altered Connectivity in Depression: GABA and Glutamate Neurotransmitter Deficits and Reversal by Novel Treatments. Neuron 2019, 102, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Sesack, S.R.; Carr, D.B.; Omelchenko, N.; Pinto, A. Anatomical substrates for glutamate-dopamine interactions: Evidence for specificity of connections and extrasynaptic actions. Ann. N. Y. Acad. Sci. 2003, 1003, 36–52. [Google Scholar] [CrossRef]
- Hastings, T.G. The role of dopamine oxidation in mitochondrial dysfunction: Implications for Parkinson’s disease. J. Bioenerg. Biomembr. 2009, 41, 469–472. [Google Scholar] [CrossRef]
- Rabinovic, A.D.; Lewis, D.A.; Hastings, T.G. Role of oxidative changes in the degeneration of dopamine terminals after injection of neurotoxic levels of dopamine. Neuroscience 2000, 101, 67–76. [Google Scholar] [CrossRef]
- Ares-Santos, S.; Granado, N.; Moratalla, R. The role of dopamine receptors in the neurotoxicity of methamphetamine. J. Intern. Med. 2013, 273, 437–453. [Google Scholar] [CrossRef] [PubMed]
- Bozzi, Y.; Borrelli, E. Dopamine in neurotoxicity and neuroprotection: What do D2 receptors have to do with it? Trends Neurosci. 2006, 29, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Sanjari Moghaddam, H.; Zare-Shahabadi, A.; Rahmani, F.; Rezaei, N. Neurotransmission systems in Parkinson’s disease. Rev. Neurosci. 2017, 28, 509–536. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Mishra, A.; Srivastava, N.; Shukla, R.; Shukla, S. Acetyl-L-Carnitine via Upegulating Dopamine D1 Receptor and Attenuating Microglial Activation Prevents Neuronal Loss and Improves Memory Functions in Parkinsonian Rats. Mol. Neurobiol. 2018, 55, 583–602. [Google Scholar] [CrossRef] [PubMed]
- Ando, S.; Tadenuma, T.; Tanaka, Y.; Fukui, F.; Kobayashi, S.; Ohashi, Y.; Kawabata, T. Enhancement of learning capacity and cholinergic synaptic function by carnitine in aging rats. J. Neurosci. Res. 2001, 66, 266–271. [Google Scholar] [CrossRef]
- Birks, J.S.; Harvey, R.J. Donepezil for dementia due to Alzheimer’s disease. Cochrane Database Syst. Rev. 2018, 6, Cd001190. [Google Scholar] [CrossRef]
- Giacobini, E.; Cuello, A.C.; Fisher, A. Reimagining cholinergic therapy for Alzheimer’s disease. Brain J. Neurol. 2022, 145, 2250–2275. [Google Scholar] [CrossRef] [PubMed]
- Stanciu, G.D.; Luca, A.; Rusu, R.N.; Bild, V.; Beschea Chiriac, S.I.; Solcan, C.; Bild, W.; Ababei, D.C. Alzheimer’s Disease Pharmacotherapy in Relation to Cholinergic System Involvement. Biomolecules 2019, 10, 40. [Google Scholar] [CrossRef]
- Tiepolt, S.; Meyer, P.M.; Patt, M.; Deuther-Conrad, W.; Hesse, S.; Barthel, H.; Sabri, O. PET Imaging of Cholinergic Neurotransmission in Neurodegenerative Disorders. J. Nucl. Med. 2022, 63, 33s–44s. [Google Scholar] [CrossRef]
- Kanel, P.; Bedard, M.A.; Aghourian, M.; Rosa-Neto, P.; Soucy, J.P.; Albin, R.L.; Bohnen, N.I. Molecular Imaging of the Cholinergic System in Alzheimer and Lewy Body Dementias: Expanding Views. Curr. Neurol. Neurosci. Rep. 2021, 21, 52. [Google Scholar] [CrossRef]
- Perez-Lloret, S.; Barrantes, F.J. Deficits in cholinergic neurotransmission and their clinical correlates in Parkinson’s disease. NPJ Park. Dis. 2016, 2, 16001. [Google Scholar] [CrossRef] [PubMed]
- Mancini, M.; Chung, K.; Zajack, A.; Martini, D.N.; Ramsey, K.; Lapidus, J.; Horak, F.B.; Nutt, J.G. Effects of augmenting cholinergic neurotransmission on balance in Parkinson’s disease. Park. Relat. Disord. 2019, 69, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Schliebs, R.; Arendt, T. The significance of the cholinergic system in the brain during aging and in Alzheimer’s disease. J. Neural Transm. 2006, 113, 1625–1644. [Google Scholar] [CrossRef]
- Ghelardini, C.; Galeotti, N.; Bartolini, A. Amitriptyline and clomipramine activate Gi-protein signaling pathway in the induction of analgesia. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2002, 365, 1–7. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Imperato, A.; Ramacci, M.T.; Angelucci, L. Acetyl-L-carnitine enhances acetylcholine release in the striatum and hippocampus of awake freely moving rats. Neurosci. Lett. 1989, 107, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Danbolt, N.C. Glutamate as a neurotransmitter in the healthy brain. J. Neural Transm. 2014, 121, 799–817. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.W.; Johnston, M.V. Physiological and pathophysiological roles of excitatory amino acids during central nervous system development. Brain Res. Brain Res. Rev. 1990, 15, 41–70. [Google Scholar] [CrossRef] [PubMed]
- Baj, A.; Moro, E.; Bistoletti, M.; Orlandi, V.; Crema, F.; Giaroni, C. Glutamatergic Signaling Along The Microbiota-Gut-Brain Axis. Int. J. Mol. Sci. 2019, 20, 1482. [Google Scholar] [CrossRef]
- Robinson, M.B.; Coyle, J.T. Glutamate and related acidic excitatory neurotransmitters: From basic science to clinical application. FASEB J. 1987, 1, 446–455. [Google Scholar] [CrossRef]
- Verma, M.; Lizama, B.N.; Chu, C.T. Excitotoxicity, calcium and mitochondria: A triad in synaptic neurodegeneration. Transl. Neurodegener. 2022, 11, 3. [Google Scholar] [CrossRef]
- Niswender, C.M.; Conn, P.J. Metabotropic glutamate receptors: Physiology, pharmacology, and disease. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 295–322. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, S.; Fu, P.; Zhang, Z.; Lin, K.; Ko, J.K.; Yung, K.K. Roles of Glutamate Receptors in Parkinson’s Disease. Int. J. Mol. Sci. 2019, 20, 4391. [Google Scholar] [CrossRef] [PubMed]
- Campanelli, F.; Natale, G.; Marino, G.; Ghiglieri, V.; Calabresi, P. Striatal glutamatergic hyperactivity in Parkinson’s disease. Neurobiol. Dis. 2022, 168, 105697. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Bose, S.K.; Pavese, N.; Ramlackhansingh, A.; Turkheimer, F.; Hotton, G.; Hammers, A.; Brooks, D.J. Glutamate NMDA receptor dysregulation in Parkinson’s disease with dyskinesias. Brain 2011, 134, 979–986. [Google Scholar] [CrossRef]
- Celli, R.; Santolini, I.; Van Luijtelaar, G.; Ngomba, R.T.; Bruno, V.; Nicoletti, F. Targeting metabotropic glutamate receptors in the treatment of epilepsy: Rationale and current status. Expert Opin. Ther. Targets 2019, 23, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Sankar, R.; Shin, D.H.; Liu, H.; Mazarati, A.; Pereira de Vasconcelos, A.; Wasterlain, C.G. Patterns of status epilepticus-induced neuronal injury during development and long-term consequences. J. Neurosci. 1998, 18, 8382–8393. [Google Scholar] [CrossRef] [PubMed]
- Dorsett, C.R.; McGuire, J.L.; DePasquale, E.A.; Gardner, A.E.; Floyd, C.L.; McCullumsmith, R.E. Glutamate Neurotransmission in Rodent Models of Traumatic Brain Injury. J. Neurotrauma 2017, 34, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Witkin, J.M.; Shafique, H.; Cerne, R.; Smith, J.L.; Marini, A.M.; Lipsky, R.H.; Delery, E. Mechanistic and therapeutic relationships of traumatic brain injury and γ-amino-butyric acid (GABA). Pharmacol. Ther. 2024, 256, 108609. [Google Scholar] [CrossRef] [PubMed]
- Boccuni, I.; Bas-Orth, C.; Bruehl, C.; Draguhn, A.; Fairless, R. Glutamate transporter contribution to retinal ganglion cell vulnerability in a rat model of multiple sclerosis. Neurobiol. Dis. 2023, 187, 106306. [Google Scholar] [CrossRef]
- Errico, F.; Gilio, L.; Mancini, A.; Nuzzo, T.; Bassi, M.S.; Bellingacci, L.; Buttari, F.; Dolcetti, E.; Bruno, A.; Galifi, G.; et al. Cerebrospinal fluid, brain, and spinal cord levels of L-aspartate signal excitatory neurotransmission abnormalities in multiple sclerosis patients and experimental autoimmune encephalomyelitis mouse model. J. Neurochem. 2023, 166, 534–546. [Google Scholar] [CrossRef]
- Iovino, L.; Tremblay, M.E.; Civiero, L. Glutamate-induced excitotoxicity in Parkinson’s disease: The role of glial cells. J. Pharmacol. Sci. 2020, 144, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.V.; Schousboe, A.; Verkhratsky, A. Astrocyte energy and neurotransmitter metabolism in Alzheimer’s disease: Integration of the glutamate/GABA-glutamine cycle. Prog. Neurobiol. 2022, 217, 102331. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elrahman, K.S.; Ferguson, S.S.G. Noncanonical Metabotropic Glutamate Receptor 5 Signaling in Alzheimer’s Disease. Annu. Rev. Pharmacol. Toxicol. 2022, 62, 235–254. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.V.; Markussen, K.H.; Jakobsen, E.; Schousboe, A.; Waagepetersen, H.S.; Rosenberg, P.A.; Aldana, B.I. Glutamate metabolism and recycling at the excitatory synapse in health and neurodegeneration. Neuropharmacology 2021, 196, 108719. [Google Scholar] [CrossRef] [PubMed]
- Jamwal, S.; Kumar, P. Insight Into the Emerging Role of Striatal Neurotransmitters in the Pathophysiology of Parkinson’s Disease and Huntington’s Disease: A Review. Curr. Neuropharmacol. 2019, 17, 165–175. [Google Scholar] [CrossRef]
- Lewerenz, J.; Maher, P. Chronic Glutamate Toxicity in Neurodegenerative Diseases-What is the Evidence? Front. Neurosci. 2015, 9, 469. [Google Scholar] [CrossRef] [PubMed]
- Vukolova, M.N.; Yen, L.Y.; Khmyz, M.I.; Sobolevsky, A.I.; Yelshanskaya, M.V. Parkinson’s disease, epilepsy, and amyotrophic lateral sclerosis-emerging role of AMPA and kainate subtypes of ionotropic glutamate receptors. Front. Cell Dev. Biol. 2023, 11, 1252953. [Google Scholar] [CrossRef] [PubMed]
- Llansola, M.; Erceg, S.; Hernández-Viadel, M.; Felipo, V. Prevention of ammonia and glutamate neurotoxicity by carnitine: Molecular mechanisms. Metab. Brain Dis. 2002, 17, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Felipo, V.; Miñana, M.D.; Cabedo, H.; Grisolía, S. L-carnitine increases the affinity of glutamate for quisqualate receptors and prevents glutamate neurotoxicity. Neurochem. Res. 1994, 19, 373–377. [Google Scholar] [CrossRef]
- Breitkreutz, R.; Babylon, A.; Hack, V.; Schuster, K.; Tokus, M.; Böhles, H.; Hagmüller, E.; Edler, L.; Holm, E.; Dröge, W. Effect of carnitine on muscular glutamate uptake and intramuscular glutathione in malignant diseases. Br. J. Cancer 2000, 82, 399–403. [Google Scholar] [CrossRef]
- Kao, Y.C.; Ho, P.C.; Tu, Y.K.; Jou, I.M.; Tsai, K.J. Lipids and Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 1505. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, M.S. Current Insights into Fatty Acid Transport in the Brain. J. Membr. Biol. 2020, 253, 375–379. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, R.; Yang, T.; Xu, N.; Chen, J.; Gao, Y.; Stetler, R.A. Fatty acid transporting proteins: Roles in brain development, aging, and stroke. Prostaglandins Leukot. Essent. Fat. Acids 2018, 136, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, M.S.; Jackson, J.; Sheu, S.H.; Chang, C.L.; Weigel, A.V.; Liu, H.; Pasolli, H.A.; Xu, C.S.; Pang, S.; Matthies, D.; et al. Neuron-Astrocyte Metabolic Coupling Protects against Activity-Induced Fatty Acid Toxicity. Cell 2019, 177, 1522–1535.e1514. [Google Scholar] [CrossRef] [PubMed]
- Pennetta, G.; Welte, M.A. Emerging Links between Lipid Droplets and Motor Neuron Diseases. Dev. Cell 2018, 45, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, T.; Ji, Y.J.; Johnson, K.; Liu, H.; Johnson, K.; Bailey, S.; Suk, Y.; Lu, Y.N.; Liu, M.; et al. A C9orf72-CARM1 axis regulates lipid metabolism under glucose starvation-induced nutrient stress. Genes Dev. 2018, 32, 1380–1397. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.L.; Weigel, A.V.; Ioannou, M.S.; Pasolli, H.A.; Xu, C.S.; Peale, D.R.; Shtengel, G.; Freeman, M.; Hess, H.F.; Blackstone, C.; et al. Spastin tethers lipid droplets to peroxisomes and directs fatty acid trafficking through ESCRT-III. J. Cell Biol. 2019, 218, 2583–2599. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Vicente, M.; Talloczy, Z.; Wong, E.; Tang, G.; Koga, H.; Kaushik, S.; de Vries, R.; Arias, E.; Harris, S.; Sulzer, D.; et al. Cargo recognition failure is responsible for inefficient autophagy in Huntington’s disease. Nat. Neurosci. 2010, 13, 567–576. [Google Scholar] [CrossRef]
- Outeiro, T.F.; Lindquist, S. Yeast cells provide insight into alpha-synuclein biology and pathobiology. Science 2003, 302, 1772–1775. [Google Scholar] [CrossRef]
- Morris, A.J.; Carey, E.M. Postnastal changes in the concentration of carnitine and acylcarnitines in the rat brain. Brain Res. 1983, 284, 381–384. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Karimzadeh, I.; Sagheb, M.M.; Khalili, H. The Renal Safety of L-Carnitine, L-Arginine, and Glutamine in Athletes and Bodybuilders. J. Ren. Nutr. 2019, 29, 221–234. [Google Scholar] [CrossRef] [PubMed]

| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Written in English Meta-analysis, human clinical trials that included randomized controlled trials, nonrandomized trials Studies on humans Studies on psychiatric and neurological disorders reporting a direct clinical effect of LC as an outcome | Case–control experiments, case reports Studies other than on humans Did not present new or unique data (review articles, letter to the editor, duplicate article) Did not measure a clinical outcome related to effects of LC Articles published before 1980 |
| Level | Description |
|---|---|
| 1a | SR or meta-analysis of RCTs with homogeneity or Cochrane review with favorable findings |
| 1b | Prospective high-quality RCT (medium-sized with N between 50 and 100 or large-sized with N over 100, and/or higher validity trials based on adequate follow-up, intent-to-treat analysis, baseline similarity, equal treatment and dropout rates) |
| 2a | SR of cohort (prospective, nonrandomized) studies with homogeneity |
| 2b | Individual cohort (prospective, nonrandomized) study or low-quality RCT (small-sized with N less than 50 and/or lower validity trials based on adequate follow-up, intent-to-treat analysis, baseline similarity, equal treatment and dropout rates) |
| 3a | SR of case–control (retrospective) studies with homogeneity |
| 3b | Individual case–control (retrospective) studies |
| 4 | Open-label trials, case series or reports |
| 5 | Expert opinion without critical appraisal or based on physiology or bench research |
| Grade | Description |
|---|---|
| A | At least one level 1a study or two level 1b studies |
| B | At least one level 1b, 2a, or 3a study, or two level 2b or 3b studies |
| C | At least one level 2b or 3b study, or two level 4 studies |
| D | Level 5 evidence, or troublingly inconsistent or inconclusive studies of any level, or studies reporting no improvements |
| N | No studies identified |
| Psychiatric and Neurological Condition | Uncontrolled Studies Positive% (Positive/Total) | Controlled Studies Positive% (Positive/Total) | Grade of Recommendation | Recommendation for Treatment |
|---|---|---|---|---|
| Amyotrophic Lateral Sclerosis | 0% (0/1) | 50% (0.5/1) | C | No |
| Ataxia | 100% (1/1) | C | None | |
| Attention Deficit Disorder with Hyperactivity | 50% (0.5/1) | C | Mixed | |
| Carpal Tunnel Syndrome | 100% (1/1) | B | Mixed | |
| Cognitive Dysfunction | 67% (2/3) | B | Mixed | |
| Depressive Disorder | 100% (1/1) | 100% (1/1) | C | Mixed |
| Fatigue Syndrome, Chronic | 50% (0.5/1) | C | Mixed | |
| Hepatic Encephalopathy | 100% (2/2) | 100% (10/10) | A | Mixed |
| Migraine Disorder | 50% (1/2) | B | Mixed | |
| Multiple Sclerosis | 100% (1/1) | C | None | |
| Neurodegenerative Diseases—Alzheimer’s Disease | 100% (1/1) | 71% (5/7) | B | Mixed |
| Neurodegenerative Diseases—Down Syndrome | 0% (0/1) | C | None | |
| Neurofibromatosis | 100% (1/1) | B | Mixed | |
| Peripheral Nervous System Diseases | 50% (1/2) | 87.5% (3.5/4) | B | Mixed |
| Rett Syndrome | 100% (3/3) | B | Mixed | |
| Sciatica | 50% (0.5/1) | C | No | |
| Stroke | 80% (4/5) | B | Mixed |
| Study | Participants #Group (M, F); Age (SD) | Treatment | Study Design | Outcome Measure | Effect of LC | AEs | Level/Point |
|---|---|---|---|---|---|---|---|
| AD Livingston et al. (1991) | 71 (13M,58F) Over 65, specific ages NR LC: 35 PB: 36 | LC or PB for 12 wk and 24 wk. Packets of tablets were collected each week and a pill count was completed. | DBPC parallel | NART CGI | Improvement in some psychological tests | Exanthema | 2b/0.5 |
| Barbara et al. (1992) | LC: 63 (19M,44F) PB: 67 (20M,47F) Mean age: 75 | 2 g/d LC or PB for a year | DBPC parallel | DSM-III | Significant difference in all outcomes | Psychomotor agitation | 1b/0.5 |
| Pettegrew J.W. et al. (1994) | LC1: 7 (3M,4F); 70.7 (3.3), LC2: 5 (1M,4F); 64.2 (2.6) PB: 21 (11M,F10); 70.5 (1.3) | 3 g/d LC or PB for a year | Case series | MMS ADAS 31P MRS | Significant changes in MMS and ADAS No significant change in 31P MRS Examination | NR | 2b/0.5 |
| L. J. Thal, MD et al. (1996) | LC: 212 (95M,F117); 71 (8) PB: 207 (88M,F119); 72 (7) | 3 g/d LC or PB for a year | DBPC parallel | ADAS-NonCog, MMSE ADL, IADL CGI-S, CGI-C | No significant differences in all outcomes | NR | 1b/0 |
| John. O. Brooks III et al. (1998) | LC: 165 (69M,F96); 71.34 (6.67) PB: 169 (79M,F90); 70.82 (7.88) | 3 g/d LC or PB for a year | DBPC crossover | ADAS | Significant changes in ADAS | Body odor, flatulence, increased appetite, exanthema | 1b/0.5 |
| L.J. Thal, MD et al. (2000) | LC: 111 (58M,F53); 59 (45–65) PB: 116 (61M,F55); 58 (47–65) | 3 g/d LC or PB for a year | DBPC parallel | ADAS-Cog, CDR, ADAS-NonCog, MMSE, ADL, CIBIC | Significant changes in MMSE | Hyperleukocyte | 1b/0.5 |
| S.I. Gavrilova et al. (2011) | LC: 30 (14M,16F); 70.9 (7.0) PB: 30 (7M,23F); 70.9 (7.5) | 2250 mg/d–3000 mg/d LC or PB for a year | DBPC parallel | MMSE, CGI, MDRS, IADL | Significant difference in all outcomes, especially in CGI | Not significant | 2b/1 |
| Young Soon Yang et al. (2018) | LC: 30 (25M,5F); 73.0 (3.8) PB: 26 (22M,4F); 73.2 (4.0) | 1500 mg/d LC for 28 weeks | Open label | MoCA-K, K-MMSE, Korean-Color Word Stroop | Significant changes in MoCA-K | NR | 2b/0.5 |
| DS Siegfried M Pueschel et al. (2004) | LC: 20 (20M,0F); 20.2 (19.3–22.8) PB: 20 (20M,0F); 21.5 (19.9–23.2) | 10 mg/kg/d LC in the first month, 20 mg/kg/d LC in the second month, and 30 mg/kg/d later for a total of 6 months, PB ditto | DBPC parallel | SBIS (4th Edition), HNVMT, WIS for Children, KAB WIS, VABS, CBC | No significant differences in all outcomes | Not significant | 2b/0 |
| Study | Participants #Group (M, F); Age (SD) | Treatment | Study Design | Outcome Measure | Effect of LC | AEs | Level/Point |
|---|---|---|---|---|---|---|---|
| Ettore beghi et al. (2013) | LC: 42 (24M,18F); 61 (38–74) PB: 40 (26M,14F); 63 (39–73) | 1000 mg/d LC or PB for 48 weeks. | DBPC parallel | MRC, ALSFRS-R, FVC, MPQSF | Significant differences in all outcomes | Stomachache, diarrhea, stomach discomfort | 1b/0.5 |
| Serena Sassi et al. (2023) | LC: 45 (31M,14F); 65.2 (60.1–71.1) PB: 45 (31M,14F); 66.1 (60.5–70.8) | 3 g/d LC or PB for 24 months. | CCS | ALSFRS-R, FVC | No significant differences in all outcomes | NR | 3a/0 |
| Study | Participants #Group (M, F); Age (SD) | Treatment | Study Design | Outcome Measure | Effect of LC | Aes | Level/Point |
|---|---|---|---|---|---|---|---|
| Sandro Sorbi et al. (2000) | LC: 12; 61 (38–74) PB: 12; 63 (39–73) | 2000 mg/d LC for 6 months and then a 1 month washout period followed by placebo | DSC | ARS, GST | Peripheral signs and muscle tone were significantly improved, and other indicators were not significantly different | NR | 3b/1 |
| Study | Participants #Group (M, F); Age (SD) | Treatment | Study Design | Outcome Measure | Effect of LC | Aes | Level/Point |
|---|---|---|---|---|---|---|---|
| L. Eugene Arnold et al. (2007) | LC: 53 (41M,12F); 8.4 (2.3) PB: 59 (42M,17F); 8.3 (2.2) | 13.5–30 kg = 0.5 g/day; 30–50 kg = 1.0 g LC OR PB for 16 weeks, and greater than 50 kg = 1.5 g LC OR PB for 16 weeks. | MPDRT | DISC-IV | No significant differences in DSM-IV | Negligible | 2b/0.5 |
| Study | Participants #Group (M, F); Age (SD) | Treatment | Study Design | Outcome Measure | Effect of LC | Aes | Level/Point |
|---|---|---|---|---|---|---|---|
| Giorgio Cruccu et al. (2017) | LC: 82 (25M,57F); 47.1 (9.0) | 1000 mg/d LC intramuscularly for the first 10 days; 1000 mg/d LC orally for the next 110 days. | MSS | BCTQ, DN4, NPSI | Significant differences in all outcomes | NR | 2a/1 |
| Study | Participants #Group (M, F); Age (SD) | Treatment | Study Design | Outcome Measure | Effect of LC | Aes | Level/Point |
|---|---|---|---|---|---|---|---|
| David Benton et al. (2003) | PB: 100 (0M,400F); 21.8 LC: 100 (0M,400F); 21.8 Lecithin: 100 (0M,400F); 21.8 LC + Lecithin: 100 (0M,400F); 21.8 | PB group: the same amount of placebo LC group: 500 mg of LC plus placebo Lecithin group: 1.6 g Lecithin plus placebo LC + Lecithin group: 500 mg + 1.6 g Lecithin | DBPC | Cognitive tests, POMS | LC enhanced the cognitive function of patients, but the effect on the decision-making ability of patients needs to be viewed with caution and remains to be discussed. | Tiredness, hunger, headache, stomachache | 1b/0.5 |
| Michele Malaguarnera et al. (2008) | LC: 48 (23M,25F); 76.2 (7.6) PB: 48 (24M,24F); 78.4 (6.4) | 4 g/d LC or PB for 180 days | SRDCC | WPS, FSS, PF, MMSE | LC reduced physical and mental fatigue and improved cognitive status and physical function. | NR | 2b/1 |
| Giulia Malaguarnera et al. (2022) | LC: 46 (NR); NR PB: 46 (NR); NR | 3 g/d LC or PB for 3 months | DBPC | CRP, SFC, MMSE, 6-WT | Significant differences in all outcomes | NR | 2a/1 |
| Study | Participants #Group (M, F); Age (SD) | Treatment | Study Design | Outcome Measure | Effect of LC | AEs | Level/Point |
|---|---|---|---|---|---|---|---|
| G Salvioli et al. (1994) | 481 (NR) NR | Stages T1 and T2: LAC 1500 mg/d for 90 days. Stage T3 received the same amount of placebo for 30 days. | SCS | MMSE, GDS, HDRS | MMSE, GDS, and HRS were improved in the treatment group, and the changes were statistically significant. | NR | 3a/1 |
| Giuseppe Bersani et al. (2012) | LC: 41 (9M,32F); 72.23 (9.33) PB: 39 (12M,27F); 71.22 (7.83) | 1 g/d LC or fluoxetine for 7 weeks | MDRCS | MMS, HAMD, HAM-A, CGI, BDI, TPT | LC group showed statistically significant improvements in HAM-D, HAM-A, BDI, and TPT scales. | NR | 2b/1 |
| Study | Participants #Group (M, F); Age (SD) | Treatment | Study Design | Outcome Measure | Effect of LC | AEs | Level/Point |
|---|---|---|---|---|---|---|---|
| Ruud C. W et al. (2004) | ALC: 30 (7M,23F); 37 (11) PLC: 30 (7M,23F); 38 (11) ALC + PLC: (7M,23F); 42 (12) | Group 1 was given 4 g of ALC daily for 24 weeks, Group 2 was given the same amount of PLC for 24 weeks, and Group 3 was given the same amount of ALC plus PLC for 24 weeks. | ROCT | CGI, MFI, Stroop Test MPQ-DLV, TMS | ALC had a major effect on mental fatigue, PLC had a major effect on general fatigue. The effect was better and statistically significant. | Overstimulation Insomnia | 2a/1 |
| Study | Participants #Group (M, F); Age (SD) | Treatment | Study Design | Outcome Measure | Effect of LC | AEs | Level/Point |
|---|---|---|---|---|---|---|---|
| Angelo Cecere et al. (2002) | LC: 16 (7M,9F); 64.3 (8.1) PB: 11 (5M,6F); 67.4 (6.9) | 6 g/d LC or PB for 4 weeks | DBPC | AM, BDT, DST, HNTB | The experimental group had a significant reduction in serum ammonia levels and overall improvement in psychological test results. | Negligible | 2b/1 |
| Mariano Malaguarnera et al. (2003) | LC: 40 (20M,20F); 51.7 (11.8) PB: 38 (16M,22F); 52.4 (10.4) | 4 g/d LC or PB for 60 days | DBPC | NCT-A, DFW, HWHC | Significantly reduced the blood ammonia concentration; 60-day intervention was more significant than the 30-day intervention. Significantly better in NCT-A. | NR | 2a/1 |
| Mariano Malaguarnera et al. (2005) | LC: 75 (50M,25F); 51.7 (9.6) PB: 75 (45M,30F); 53.2 (9.2) | 4 g/d LC or PB for 90 days | DBPC | EEG, TMT, WAIS, BDT, SDMT | Significantly reduced fasting serum NH4; significant difference in symbolic digital modal test versus block design. | NR | 1b/1 |
| Massimo Siciliano et al. (2006) | LC: 18 (10M,8F); 63.78 (9.64) PB: 6 (3M,3F); 66 (6.20) | 0.5 g of LC was injected together in 50 mL isotonic saline, and the indexes were measured after 15, 30, 60, and 90 min. | SPCE | P100 latency | LC neuronal function after a single intravenous injection. | NR | 2b/0.5 |
| Mariano Malaguarnera et al. (2006) | LC: 13 (9M,4F); 51.4 (9.1) PB: 11 (7M,4F); 50.2 (8.9) | 4 g/d LC + Glycosylated or Glycosylated solution for 30 days | DPBC | EEG, DFW | Significant differences in neurological function scores and blood ammonia levels. | NR | 2b/1 |
| Mariano Malaguarnera et al. (2008) | LC: 60 (33M,27F); 48 (10) PB: 55 (35M,20F); 45 (11) | 4 g/d LC or PB for 90 days | DPBC | TMT, WAIS, MMS, AVL, EEG, CP, DFW | Significant differences in neurological function scores and blood ammonia levels. | NR | 1b/1 |
| Michele Malaguarnera et al. (2011) | LC: 61 (32M,29F); (40–66) PB: 60 (33M,27F); (41–67) | 4 g/d LC or PB for 90 days | DPBC | EEG, Fatigue Severity Scale (FSS), WPT, 7-d PAR, 6MWT, SPPB, CP, DFW | Significant differences in neurological function scores and blood ammonia levels, especially blood ammonia levels. | NR | 2a/1 |
| Michele Malaguarnera et al.(2011) | LC: 30 (14M,16F); (37–64) PB: 30 (15M,15F); (35–65) | 4 g/d LC or PB for 90 days | DPBC | SSM, EMQ, HVOT, EEG, TMT, MMSE, COWAT, JLO, CP, DFW | Significant differences in blood ammonia levels, EEG, SSM, etc. | NR | 2b/0.5 |
| Mariano Malaguarnera et al. (2011) | LC: 33 (20M,13F); (37–65) PB: 34 (19M,15F); (34–67) | 4 g/d LC or PB for 90 days | DPBC | PHES, TMT, SF-36, BDI, STAI, EEG, CP, DFW. | Significant differences in blood ammonia levels, MMSE, BDI, SF-36, etc. | NR | 3a/1 |
| Masaya Saito et al. (2015) | LC: 11 (4M,7F); 73 (53–85) PB: 13 (6M,7F); 71 (53–85) | 1.8 g/d LC or PB for 3 months | PCS | NCT, RTT, BBI | Significant differences in blood ammonia levels and some indicators of neurological function. | Negligible | 2b/0.5 |
| Study | Participants #Group (M, F); Age (SD) | Treatment | Study Design | Outcome Measure | Effect of LC | AEs | Level/Point |
|---|---|---|---|---|---|---|---|
| Ali Tarighat Esfanjani et al. (2012) | LC: 35 (8M,27F); 34.09 (1.70) PB: 35 (5M,30F); 36.54 (1.54) | 500 mg/d LC or PB for 12 weeks. | DBPC | TKT | Significant differences in all outcomes | NR | 2a/0.5 |
| Knut Hagen et al. (2015) | LC: 71 (8M,63F); 39 (13) PB: 70 (7M,63F); 39 (13) | 500 mg/d LC or PB for 12 weeks. | TCCT | Number of days with moderate or severe headache per four-week period. Headache days, duration of headache, proportion of responders | No significant differences in all outcomes | Abdominal pain nausea, vomiting, headache | 1b/0 |
| Study | Participants #Group (M, F); Age (SD) | Treatment | Study Design | Outcome Measure | Effect of LC | AEs | Level/Point |
|---|---|---|---|---|---|---|---|
| Valentina Tomassini et al. (2004) | LC: 18 (6M,12F); 44.5 (10.9) PB: 18 (6M,12F); 43.1 (11.7) | 2 g/d LC or ATD for 3 months Washout period for 3 months 2 g/d LC or ATD for 3 months | SPRDCT | FSS, FIS, BDI, SEC | Significant differences in FSS, FIS | Insomnia Nervousness Nausea Dizziness | 3b/0.5 |
| Study | Participants #Group (M, F); Age (SD) | Treatment | Study Design | Outcome Measure | Effect of LC | AEs | Level/Point |
|---|---|---|---|---|---|---|---|
| Emily R. Vasiljevski et al. (2021) | LC: 6 (4M,2F); 10.7 (1.2) | 1000 mg/d LC or PB for 12 weeks. | Open-label, single-center, Phase 2a Clinical trial | Safety, compliance, BSA, FMF, GMF | Significant difference in muscle strength and energy levels. Phase 3 clinical trials will confirm the effectiveness of the treatment. | NR | 4/0.5 |
| Study | Participants #Group (M, F); Age (SD) | Treatment | Study Design | Outcome Measure | Effect of LC | AEs | Level/Point |
|---|---|---|---|---|---|---|---|
| Domenico De Grandis et al. (2002) | LC: 167 (85M,62F); 56 (25–75) PB: 166 (81M,66F); 59 (28–72) | 1000 mg/d LC, or PB for 10 days 2000 mg/d LC, or PB for 355 days | DBPC | ECG, SNCV, MNCV, VAS | Significant differences in improved neurophysiological parameters and reduced pain aspects | Headache, vomiting, facial paresthesia, nausea, cold sore infections, retching, biliary colic, upper abdominal pain, gastrointestinal diseases | 1b/1 |
| Anders A.F. Sima et al. (2004) | LC1: 208 (NR); NR (NR) LC2: 256 (NR); NR (NR) PB: 218 (NR); NR (NR) | 500 mg/d LC or PB for 52 weeks 1000 mg/d LC or PB for 52 weeks | MDRT | NCV, OBR, VP, CSC, VAS | Significant differences in alleviating pain, improving nerve fiber regeneration and vibration perception, among other aspects. | Pain, paresthesia, hyperesthesia, cardiovascular and gastrointestinal symptoms. | 1b/1 |
| Hizir Ulvi et al. (2010) | LC: 30 (12M,18F); Male age: 49.92 (10.66) Female age: 53.26 (8.08) | 2 g/d LC o for 10 months | SST | EE | Significant differences in improved peripheral neuropathy and ventricular dispersion. | NR | 3b/1 |
| YUANJUE SUN et al.(2015) | LC: 118 (NR); 44.5 (NR) PB: 118 (NR); NR (NR) | 3000 mg/d LC or Lactose for 8 weeks | DBPC parallel | CFC, KPS, EE | Significant differences in improved peripheral sensory neuropathy, reduced fatigue, and improved physical condition. | Vomiting, flatulence, diarrhea, decreased white blood cell count, liver dysfunction, insomnia | 2b/0.5 |
| Sheyu Li et al. (2016) | LC: 117 (57M,60F); 57.82 (8.72) MC: 115 (65M,50F); 57.75 (7.92) | 500 mg/d LC or MC for 24 weeks | DBPC parallel | NSS, NDS, NSS + NDS NCV NRR | Significant differences in reduced neuropathy, symptom score, and neuropathy disability score | Bloating, Belching, Nausea | 1a/0.5 |
| Study | Participants #Group (M, F); Age (SD) | Treatment | Study Design | Outcome Measure | Effect of LC | AEs | Level/Point |
|---|---|---|---|---|---|---|---|
| Carolyn Ellaway et al. (1999) | LC: 31 (NR); Under 20 PB: 31 (7M,63F); Under 20 | 100 mg/kg/d LC or PB for 8 weeks Washout period for 8 weeks 1000 mg/kg/d LC or PB for 8 weeks | RCCT | RMBA, HAS PWI | Significant difference in improved hand apraxia scale indicators and well-being. | Bowel movements, body smell of fish or urine | 3a/0.5 |
| Carolyn J. Ellaway et al. (2001) | LC: 21 (NR); 7–41 (14.4) PB: 62 (NR); 43.1 (11.7) | 100 mg/kg/d LC or PB for 6 months | ORCT | RMBA, HAS, 7-NSD, SF-36HS, TRE | Significant improvement in sleep efficiency, energy level, communication skills, and language expression. | Bowel movements, body smell of fish or urine | 3a/0.5 |
| F. Guideri et al. (2005) | LC: 10 (0M,10F); 6.3 (4.3) PB: 12 (0M,12F); 6.3 (4.0) | 50 mg/kg/d LC or PB for 6 months 20 mg/kg/d CMZP for seizure prevention | DBPC parallel | HRV, QTc, QTcD | Significant improvement in heart rate variability. Reduced risk of sudden death. | NR | 3b/0.5 |
| Study | Participants #Group (M, F); Age (SD) | Treatment | Study Design | Outcome Measure | Effect of LC | AEs | Level/Point |
|---|---|---|---|---|---|---|---|
| Antonio Memeo et al. (2008) | LC: 33 (14M,19F); 60 (15) TTA: 31 (14M,17F); 62 (16) | 1180 mg/d LC or 600 mg TTA for 60 days. | DBPC | NIS-LL, NSC-LL, TSS, EMG | Significant improvements in neuropathy and electromyography | NR | 1b/0.5 |
| Study | Participants #Group (M, F); Age (SD) | Treatment | Study Design | Outcome Measure | Effect of LC | AEs | Level/Point |
|---|---|---|---|---|---|---|---|
| A. V. Fedotova et al. (2013) | LC1: 20 (7M,13F); 61.2 (8.2) LC2: 20 (7M,13F); 61.2 (8.2) PB: 20 (8M,12F); 61.2 (8.2) | 1000 mg/d or 2000 mg/d LC or PB for 60 days. | DBPC | MMS, ST, MFI-20 | Significant differences in MMSE total score; differences in the “concentration of attention” and “memory” subscales. | NR | 2b/0.5 |
| L.V. Chichanovskaya et al. (2017) | LC: 30 (NR); 66.7 (1.7) PB: 30 (NR); 65.1 (1.9) | 1000 mg/d LC or PB for 3 weeks | DBPC | NIHSS, BI, MFI-20 HADS, VAS | Significant differences in BI and NIHSS. | NR | 2a/0.5 |
| Kaveh Kazemian et al. (2020) | LC: 25 (11M,14F); 62.48 (8.76) LC + Lipofundin: 25 (18M,7F); 60.56 (8.55) Lipofundin: 25 (11M,14F); 63.24 (9.35) PB: 25 (8M,17F); 63.44 (6.54) | 1000 mg/d LC or LFD for 1, 2, or 3 days | PDBPC | Biomarker S100B | Significant differences in reducing serum levels of the biomarker S100B and protecting nerves. | NR | 2b/1 |
| Mehrdokht Mazdeh et al. (2022) | LC: 34 (19M,15F); 65.24 (12.89) PB: 35 (13M,22F); 70.37 (13.58) | 1000 mg/d LC or PB for 90 days | SDBPC | NIHSS, MRS, BM | Significant differences in increased NIHSS score and MRS score. | Nausea, upset stomach, diarrhea | 3a/0.5 |
| AEs | ALS | Ataxia | ADHD | CTS | CD | DD | CFS | HE | MD | MS | NDs | NF | PNSD | RS | Sciatica | Stroke | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AD | DS | ||||||||||||||||
| Gastrointestinal AEs | |||||||||||||||||
| Abdominal pain | x | x | x | ||||||||||||||
| Mild discomfort | x | x | |||||||||||||||
| Flatulence | x | x | |||||||||||||||
| Nausea | x | x | x | ||||||||||||||
| Vomiting | x | x | |||||||||||||||
| Diarrhea | x | x | x | ||||||||||||||
| Hiccup | x | ||||||||||||||||
| Peristalsis | x | ||||||||||||||||
| Neurological AEs | |||||||||||||||||
| Headaches | x | x | x | ||||||||||||||
| Insomnia | x | x | x | ||||||||||||||
| Anxiety | x | ||||||||||||||||
| Excited | x | x | |||||||||||||||
| Other system AEs | |||||||||||||||||
| Exanthema | x | ||||||||||||||||
| Body odor | x | x | |||||||||||||||
| Leukopenia | x | ||||||||||||||||
| Hepatic dysfunction | x | ||||||||||||||||
| Severe AEs needing discontinuation | |||||||||||||||||
| Hypereosinophilia | x | ||||||||||||||||
| Overstimulation | x | ||||||||||||||||
| Neurogenic tonus | x | ||||||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Pan, D.; Liu, Q.; Chen, X.; Wang, S. L-Carnitine in the Treatment of Psychiatric and Neurological Manifestations: A Systematic Review. Nutrients 2024, 16, 1232. https://doi.org/10.3390/nu16081232
Wang W, Pan D, Liu Q, Chen X, Wang S. L-Carnitine in the Treatment of Psychiatric and Neurological Manifestations: A Systematic Review. Nutrients. 2024; 16(8):1232. https://doi.org/10.3390/nu16081232
Chicago/Turabian StyleWang, Wenbo, Da Pan, Qi Liu, Xiangjun Chen, and Shaokang Wang. 2024. "L-Carnitine in the Treatment of Psychiatric and Neurological Manifestations: A Systematic Review" Nutrients 16, no. 8: 1232. https://doi.org/10.3390/nu16081232
APA StyleWang, W., Pan, D., Liu, Q., Chen, X., & Wang, S. (2024). L-Carnitine in the Treatment of Psychiatric and Neurological Manifestations: A Systematic Review. Nutrients, 16(8), 1232. https://doi.org/10.3390/nu16081232









