The Association between Circulating Cytokines and Body Composition in Frail Patients with Cardiovascular Disease
Abstract
1. Introduction
2. Materials and Methods
- -
- Slowness—reduced gait speed at a distance of 5 m at the usual pace. A patient must repeat three times, and the results are averaged. The result is classified as positive if it is >6 s.
- -
- Weakness is assessed with a maximal handgrip strength test. It is carried out in the dominant arm. We use an electronic hand dynamometer EH101 (VETEK AB, Sweden). The patient must repeat three times, and the maximal value is recorded. The test is positive for frailty if it is <30 kg for men and <20 kg for women.
- -
- Low physical activity is assessed by the Minnesota Leisure Time Activity questionnaire. The result is positive when calorie expenditure per week is lower than 270 kcal/week in women and less than 383 kcal/week in men. We have prepared a Microsoft Excel-based template for rapid questioning and easy calculation of all activities and respective calorie expenditures. We are assessing the physical activity from the recent twelve years.
- -
- Exhaustion is self-reported by a patient. The patient has to answer the following questions from the CESD-R scale: “How often in the past week did you feel like everything you did was an effort? How often in the past week did you feel like you could not get going?” The possible answers are often (3 or more days) or not often, when the feeling is present in 0–2 days. The positive answer is when the patient says “often”.
- -
- The last criterion is unintentional weight loss exceeding 10 pounds (appr. 4.5 kg) in the past year.
2.1. Cytokines Analysis
2.2. Evaluation of the Cytokines’ Concentrations
2.3. Statistical Analysis
3. Results
3.1. Characteristics of Frail Population
3.2. The Analysis of Cytokines
3.3. Predictors of Reduced Fat-Free Mass
4. Discussion
4.1. Study Limitations
4.2. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mensah, G.A.; Roth, G.A.; Fuster, V. The Global Burden of Cardiovascular Diseases and Risk Factors: 2020 and Beyond. J. Am. Coll. Cardiol. 2019, 74, 2529–2532. [Google Scholar] [CrossRef] [PubMed]
- World Heart Report. 2023. Available online: https://world-heart-federation.org/wp-content/uploads/World-Heart-Report-2023.pdf (accessed on 24 June 2023).
- Ziaeian, B.; Fonarow, G.C. Epidemiology and aetiology of heart failure. Nat. Rev. Cardiol. 2016, 13, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Savarese, G.; Becher, P.M.; Lund, L.H.; Seferovic, P.; Rosano, G.M.C.; Coats, A.J.S. Global burden of heart failure: A comprehensive and updated review of epidemiology. Cardiovasc. Res. 2023, 118, 3272–3287. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E.; Vellas, B.; van Kan, G.A.; Anker, S.D.; Bauer, J.M.; Bernabei, R.; Cesari, M.; Chumlea, W.C.; Doehner, W.; Evans, J.; et al. Frailty consensus: A call to action. J. Am. Med. Dir. Assoc. 2013, 14, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Richter, D.; Guasti, L.; Walker, D.; Lambrinou, E.; Lionis, C.; Abreu, A.; Savelieva, I.; Fumagalli, S.; Bo, M.; Rocca, B.; et al. Frailty in cardiology: Definition, assessment and clinical implications for general cardiology. A consensus document of the Council for Cardiology Practice (CCP), Association for Acute Cardio Vascular Care (ACVC), Association of Cardiovascular Nursing and Allied Professions (ACNAP), European Association of Preventive Cardiology (EAPC), European Heart Rhythm Association (EHRA), Council on Valvular Heart Diseases (VHD), Council on Hypertension (CHT), Council of Cardio-Oncology (CCO), Working Group (WG) Aorta and Peripheral Vascular Diseases, WG e-Cardiology, WG Thrombosis, of the European Society of Cardiology, European Primary Care Cardiology Society (EPCCS). Eur. J. Prev. Cardiol. 2022, 29, 216–227. [Google Scholar] [CrossRef] [PubMed]
- O’Caoimh, R.; Galluzzo, L.; Rodriguez-Laso, A.; Van der Heyden, J.; Ranhoff, A.H.; Lamprini-Koula, M.; Ciutan, M.; Lopez-Samaniego, L.; Carcaillon-Bentata, L.; Kennelly, S.; et al. Prevalence of frailty at population level in European ADVANTAGE Joint Action Member States: A systematic review and meta-analysis. Ann. Dell’Istituto Super. Sanita 2018, 54, 226–238. [Google Scholar] [CrossRef]
- Marinus, N.; Vigorito, C.; Giallauria, F.; Haenen, L.; Jansegers, T.; Dendale, P.; Feys, P.; Meesen, R.; Timmermans, A.; Spildooren, J.; et al. Frailty is highly prevalent in specific cardiovascular diseases and females, but significantly worsens prognosis in all affected patients: A systematic review. Ageing Res. Rev. 2021, 66, 101233. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Lee, Y.S.; Zimmers, T.A.; Soleimani, A.; Matzuk, M.M.; Tsuchida, K.; Cohn, R.D.; Barton, E.R. Regulation of muscle mass by follistatin and activins. Mol. Endocrinol. 2010, 24, 1998–2008. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Salvioli, S.; Basile, M.S.; Bencivenga, L.; Carrino, S.; Conte, M.; Damanti, S.; De Lorenzo, R.; Fiorenzato, E.; Gialluisi, A.; Ingannato, A.; et al. Biomarkers of aging in frailty and age-associated disorders: State of the art and future perspective. Ageing Res. Rev. 2023, 91, 102044. [Google Scholar] [CrossRef] [PubMed]
- Boirie, Y. Physiopathological mechanism of sarcopenia. J. Nutr. Health Aging 2009, 13, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Walston, J.; Hadley, E.C.; Ferrucci, L.; Guralnik, J.M.; Newman, A.B.; Studenski, S.A.; Ershler, W.B.; Harris, T.; Fried, L.P. Research agenda for frailty in older adults: Toward a better understanding of physiology and etiology: Summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J. Am. Geriatr. Soc. 2006, 54, 991–1001. [Google Scholar] [CrossRef]
- García-Giménez, J.L.; Mena-Molla, S.; Tarazona-Santabalbina, F.J.; Viña, J.; Gomez-Cabrera, M.C.; Pallardó, F.V. Implementing Precision Medicine in Human Frailty through Epigenetic Biomarkers. Int. J. Environ. Res. Public Health 2021, 18, 1883. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Woloszyn-Horak, E.; Salamon, R.; Chojnacka, K.; Brzosko, A.; Bieda, L.; Standera, J.; Ploszaj, K.; Stepien, E.; Nowalany-Kozielska, E.; Tomasik, A. Frailty syndrome in daily practice of interventional cardiology ward-rationale and design of the FRAPICA trial: A STROBE-compliant prospective observational study. Medicine 2020, 99, e18935. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.S.; Pollock, M.L. Practical Assessment of Body Composition. Physician Sportsmed. 1985, 13, 76–90. [Google Scholar] [CrossRef] [PubMed]
- De Jager, W.; Rijkers, G.T. Solid-phase and bead-based cytokine immunoassay: A comparison. Methods 2006, 38, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Faresjö, M. A Useful Guide for Analysis of Immune Mediators in Cancer by Fluorochrome (Luminex) Technique. Methods Mol. Biol. 2020, 2108, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Grudzińska, E.; Grzegorczyn, S.; Czuba, Z.P. Chemokines and Growth Factors Produced by Lymphocytes in the Incompetent Great Saphenous Vein. Mediat. Inflamm. 2019, 2019, 7057303. [Google Scholar] [CrossRef] [PubMed]
- Idzik, M.; Poloczek, J.; Skrzep-Poloczek, B.; Dróżdż, E.; Chełmecka, E.; Czuba, Z.; Jochem, J.; Stygar, D. The Effects of 21-Day General Rehabilitation after Hip or Knee Surgical Implantation on Plasma Levels of Selected Interleukins, VEGF, TNF-α, PDGF-BB, and Eotaxin-1. Biomolecules 2022, 12, 605. [Google Scholar] [CrossRef]
- Bronikowska, J.; Kłósek, M.; Janeczko, T.; Kostrzewa-Susłow, E.; Czuba, Z.P. The modulating effect of methoxy-derivatives of 2′-hydroxychalcones on the release of IL-8, MIF, VCAM-1 and ICAM-1 by colon cancer cells. Biomed. Pharmacother. 2022, 145, 112428. [Google Scholar] [CrossRef]
- Snedecor, G.; Cochrane, W. Statistical Methods, 7th ed.; Iowa: Ames, IA, USA, 1980. [Google Scholar]
- Knapp, R.G.; Miller, M.C., III. Clinical Epidemiology and Biostatistics; Williams & Wilkins: Baltimore, MD, USA, 1992. [Google Scholar]
- Barzilay, J.I.; Blaum, C.; Moore, T.; Xue, Q.L.; Hirsch, C.H.; Walston, J.D.; Fried, L.P. Insulin resistance and inflammation as precursors of frailty: The Cardiovascular Health Study. Arch. Intern. Med. 2007, 167, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Hsu, B.; Hirani, V.; Cumming, R.G.; Naganathan, V.; Blyth, F.M.; Wright, F.C.; Waite, L.M.; Seibel, M.J.; Handelsman, D.J.; Le Couteur, D.G. Cross-Sectional and Longitudinal Relationships Between Inflammatory Biomarkers and Frailty in Community-dwelling Older Men: The Concord Health and Ageing in Men Project. J. Gerontol. Ser. A 2017, 74, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Travison, T.G.; Nguyen, A.H.; Naganathan, V.; Stanaway, F.F.; Blyth, F.M.; Cumming, R.G.; Le Couteur, D.G.; Sambrook, P.N.; Handelsman, D.J. Changes in reproductive hormone concentrations predict the prevalence and progression of the frailty syndrome in older men: The concord health and ageing in men project. J. Clin. Endocrinol. Metab. 2011, 96, 2464–2474. [Google Scholar] [CrossRef] [PubMed]
- Walston, J.; McBurnie, M.A.; Newman, A.; Tracy, R.P.; Kop, W.J.; Hirsch, C.H.; Gottdiener, J.; Fried, L.P.; Cardiovascular Health, S. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: Results from the Cardiovascular Health Study. Arch. Intern. Med. 2002, 162, 2333–2341. [Google Scholar] [CrossRef] [PubMed]
- Beben, T.; Ix, J.H.; Shlipak, M.G.; Sarnak, M.J.; Fried, L.F.; Hoofnagle, A.N.; Chonchol, M.; Kestenbaum, B.R.; de Boer, I.H.; Rifkin, D.E. Fibroblast Growth Factor-23 and Frailty in Elderly Community-Dwelling Individuals: The Cardiovascular Health Study. J. Am. Geriatr. Soc. 2016, 64, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Holecki, M.; Chudek, J.; Owczarek, A.; Olszanecka-Glinianowicz, M.; Bożentowicz-Wikarek, M.; Duława, J.; Mossakowska, M.; Zdrojewski, T.; Skalska, A.; Więcek, A. Inflammation but not obesity or insulin resistance is associated with increased plasma fibroblast growth factor 23 concentration in the elderly. Clin. Endocrinol. 2015, 82, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Echeverria, I.; Besga, A.; Sanz, B.; Amasene, M.; Hervás, G.; Barroso, J.; Rodriguez-Larrad, A.; Irazusta, J. Identification of frailty and sarcopenia in hospitalised older people. Eur. J. Clin. Investig. 2021, 51, e13420. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, R.E.; O’Mahony, M.S.; Calver, B.L.; Woodhouse, K.W. Nutrition, inflammation, and leptin levels in aging and frailty. J. Am. Geriatr. Soc. 2008, 56, 279–284. [Google Scholar] [CrossRef]
- Kohara, K.; Ochi, M.; Tabara, Y.; Nagai, T.; Igase, M.; Miki, T. Leptin in sarcopenic visceral obesity: Possible link between adipocytes and myocytes. PLoS ONE 2011, 6, e24633. [Google Scholar] [CrossRef]
- Lana, A.; Valdés-Bécares, A.; Buño, A.; Rodríguez-Artalejo, F.; Lopez-Garcia, E. Serum Leptin Concentration is Associated with Incident Frailty in Older Adults. Aging Dis. 2017, 8, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Boreskie, K.F.; Oldfield, C.J.; Thompson, L.A.; Moffatt, T.L.; Hiebert, B.M.; Arora, R.C.; Duhamel, T.A. Bone Metabolism Analytes as Biomarkers of Pre-Frailty and Cardiovascular Disease Risk in Females. Adv. Geriatr. Med. Res. 2020, 2, e200025. [Google Scholar] [CrossRef]
- Pacho, C.; Domingo, M.; Núñez, R.; Lupón, J.; Núñez, J.; Barallat, J.; Moliner, P.; de Antonio, M.; Santesmases, J.; Cediel, G.; et al. Predictive biomarkers for death and rehospitalization in comorbid frail elderly heart failure patients. BMC Geriatr. 2018, 18, 109. [Google Scholar] [CrossRef] [PubMed]
- Alawo, D.O.A.; Tahir, T.A.; Fischer, M.; Bates, D.G.; Amirova, S.R.; Brindle, N.P.J. Regulation of Angiopoietin Signalling by Soluble Tie2 Ectodomain and Engineered Ligand Trap. Sci. Rep. 2017, 7, 3658. [Google Scholar] [CrossRef] [PubMed]
- Woodfin, A.; Voisin, M.-B.; Nourshargh, S. PECAM-1: A Multi-Functional Molecule in Inflammation and Vascular Biology. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2514–2523. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.R.; Ward, A.C.; Russell, A.P. Granulocyte Colony-Stimulating Factor and Its Potential Application for Skeletal Muscle Repair and Regeneration. Mediat. Inflamm. 2017, 2017, 7517350. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Ge, J.; Huang, C.; Liu, H.; Jiang, H. Application of mesenchymal stem cell therapy for aging frailty: From mechanisms to therapeutics. Theranostics 2021, 11, 5675–5685. [Google Scholar] [CrossRef]
- Sun, X.L.; Hao, Q.K.; Tang, R.J.; Xiao, C.; Ge, M.L.; Dong, B.R. Frailty and Rejuvenation with Stem Cells: Therapeutic Opportunities and Clinical Challenges. Rejuvenation Res. 2019, 22, 484–497. [Google Scholar] [CrossRef]
- Esposito, K.; Ciotola, M.; Sasso, F.C.; Cozzolino, D.; Saccomanno, F.; Assaloni, R.; Ceriello, A.; Giugliano, D. Effect of a single high-fat meal on endothelial function in patients with the metabolic syndrome: Role of tumor necrosis factor-alpha. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Marcucci, M.; Franchi, C.; Nobili, A.; Mannucci, P.M.; Ardoino, I. REPOSI Investigators. Defining Aging Phenotypes and Related Outcomes: Clues to Recognize Frailty in Hospitalized Older Patients. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 395–402. [Google Scholar] [CrossRef] [PubMed]
| All Patients (n = 78) | Robust (n = 20) | Pre-frail (n = 43) | Frail (n = 15) | Significance (Kruskal–Wallis ANOVA) | |
|---|---|---|---|---|---|
| Age, years, median (IQR) | 69.0 (9.0) | 69.0 (5.5) | 68.0 (11.0) | 72.0 (9.0) | p = 0.458 |
| Gender, M/F, n | 49/39 | 15/5 | 29/14 | 5/10 | p < 0.05 |
| Height, m, median (IQR) | 1.700 (0.14) | 1.725 (0.14) | 1.700 (0.14) | 1.640 (0.13) | p = 0.125 |
| Weight, kg, median (IQR) | 81.0 (19.0) | 84.50 (22.50) | 78.000 (17.00) | 82.00 (32.00) | p = 0.324 |
| BMI, kg/m2, median (IQR) | 28.4 (5.4) | 29.1 (5.7) | 27.8 (4.6) | 30.7 (10.0) | p = 0.253 |
| Fat-free mass, kg, median (IQR) | 53.00 (14.00) | 58.65 (16.05) | 53.00 (12.4) | 42.1 (9.7) | p < 0.001 |
| Coronary artery disease, n (%) | 73 (94) | 19 (95) | 41 (95) | 13 (87) | p = 0.48 |
| Hypertension, n (%) | 66 (85) | 17 (85) | 37 (86) | 12 (80) | p = 0.856 |
| Diabetes, n (%) | 33 (42) | 7 (35) | 14 (33) | 12 (80) | p < 0.05 |
| Chronic obstructive pulmonary disease, n (%) | 12 (15) | 3 (15) | 7 (16) | 2 (13) | p = 0.466 |
| Chronic renal failure, n (%) | 4 (5) | 0 (0) | 2 (5) | 2 (13) | p = 0.303 |
| Smoking, n (%) | 21 (27) | 6 (30) | 13 (30) | 2 (13) | p = 0.712 |
| All Patients (n = 78) | Robust (n = 20) | Pre-Frail (n = 43) | Frail (n = 15) | Significance (Kruskal–Wallis ANOVA) | |
|---|---|---|---|---|---|
| sST2, ng/mL | 25.688 (18.42) | 30.566 (15.59) | 23.879 (16.01) | 33.450 (26.34) | p = 0.065 |
| sEGFR | 8011.630 (7258.03) | 8668.745 (8376.51) | 7760.650 (7410.95) | 7907.200 (4554.41) | p = 0.959 |
| FGF basic | 89.150 (52.17) | 78.835 (53.42) | 88.320 (56.07) | 104.130 (77.44) | p = 0.0546 |
| Follistatin | 273.730 (306.67) | 177.865 (197.90) | 280.580 (323.29) | 464.420 (372.58) | p = 0.0714 |
| G-CSF | 81.320 (33.92) | 76.450 (36.22) | 81.110 (33.12) | 94.780 (59.54) | p = 0.46 |
| sHER-2new | 3163.665 (2406.52) | 3095.760 (2310.75) | 3058.740 (2629.98) | 3464.110 (2505.51) | p = 0.655 |
| HGF | 693.280 (485.28) | 665.595 (538.96) | 696.520 (574.41) | 736.210 (777.58) | p = 0.627 |
| sIL-6RA | 7667.835 (3166.27) | 6755.965 (2182.74) | 8038.640 (3672.75) | 7965.730 (2834.65) | p = 0.352 |
| Leptin | 4626.890 (10,153.93) | 4405.740 (6743.39) | 4418.710 (10,897.44) | 6708.200 (10,519.41) | p = 0.486 |
| Osteopontin | 4231.720 (7736.82) | 4209.705 (11,171.82) | 2884.290 (7736.82) | 6533.970 (41,661.13) | p = 0.204 |
| PDGF-AB | 898.420 (1709.68) | 742.400 (1683.09) | 794.120 (1594.41) | 1408.680 (1872.71) | p = 0.925 |
| PECAM-1 | 4453.135 (1815.60) | 4151.790 (2047.52) | 4556.280 (1821.25) | 4797.900 (2026.70) | p = 0.161 |
| Prolactin | 3941.705 (4131.73) | 2931.650 (4686.14) | 4075.620 (3759.22) | 3697.980 (4342.15) | p = 0.877 |
| SCF | 107.500 (60.20) | 85.215 (79.39) | 110.870 (54.70) | 120.470 (61.12) | p = 0.194 |
| sTIE-2 | 2836.785 (3001.54) | 3153.260 (2941.08) | 2642.790 (3197.91) | 4077.990 (2826.10) | p = 0.306 |
| sVEGFR-1 | 66.865 (170.09) | 50.355 (128.63) | 70.170 (139.73) | 90.050 (726.83) | p = 0.249 |
| sVEGFR-2 | 641.525 (489.23) | 619.960 (416.69) | 636.410 (512.71) | 751.930 (655.89) | p = 0.394 |
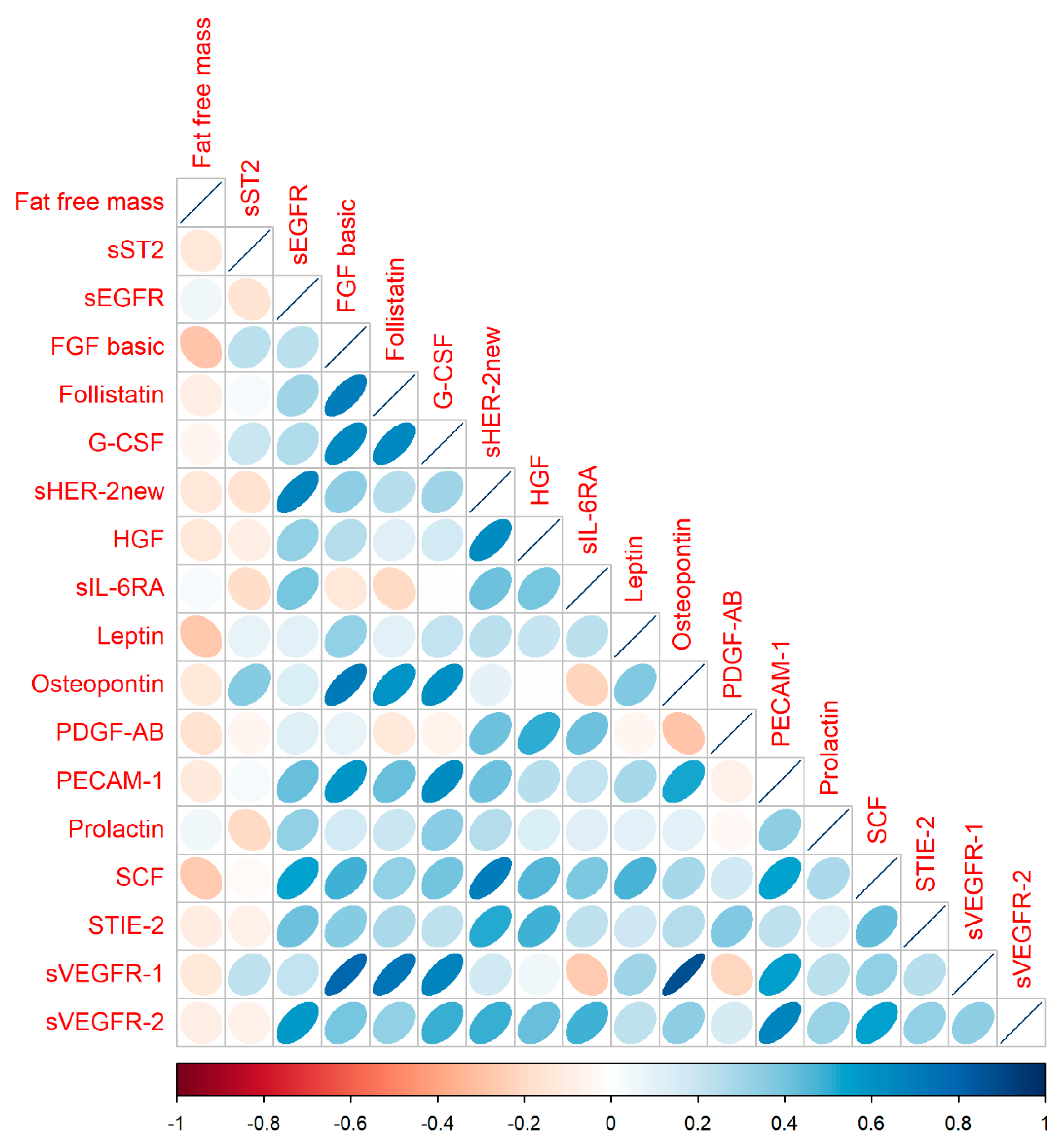
| Fat Free Mass | sST2 | sEGFR | FGF Basic | Follistatin | G-CSF | sHER-2new | HGF | sIL-6RA | Leptin | Osteopontin | PDGF-AB | PECAM-1 | Prolactin | SCF | S-TIE2 | sVEGFR-1 | sVEGFR-2 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fat-free mass | ||||||||||||||||||
| sST2 | −0.13 | |||||||||||||||||
| sEGFR | 0.05 | −0.16 | ||||||||||||||||
| FGF basic | −0.28 | 0.23 | 0.24 | |||||||||||||||
| Follistatin | −0.09 | 0.03 | 0.31 | 0.69 | ||||||||||||||
| G-CSF | −0.04 | 0.20 | 0.27 | 0.65 | 0.64 | |||||||||||||
| sHER-2new | −0.13 | −0.16 | 0.67 | 0.35 | 0.24 | 0.30 | ||||||||||||
| HGF | −0.14 | −0.09 | 0.33 | 0.26 | 0.11 | 0.16 | 0.64 | |||||||||||
| sIL-6RA | 0.04 | −0.18 | 0.39 | −0.13 | −0.21 | 0.00 | 0.42 | 0.39 | ||||||||||
| Leptin | −0.27 | 0.09 | 0.10 | 0.33 | 0.10 | 0.21 | 0.23 | 0.20 | 0.23 | |||||||||
| Osteopontin | −0.12 | 0.37 | 0.13 | 0.70 | 0.60 | 0.61 | 0.10 | 0.00 | −0.22 | 0.38 | ||||||||
| PDGF-AB | −0.16 | −0.05 | 0.13 | 0.09 | −0.12 | −0.07 | 0.42 | 0.51 | 0.42 | −0.04 | −0.28 | |||||||
| PECAM-1 | −0.11 | 0.03 | 0.42 | 0.58 | 0.42 | 0.64 | 0.42 | 0.25 | 0.21 | 0.28 | 0.53 | −0.07 | ||||||
| Prolactin | 0.05 | −0.20 | 0.34 | 0.17 | 0.19 | 0.35 | 0.26 | 0.13 | 0.12 | 0.11 | 0.10 | −0.04 | 0.35 | |||||
| SCF | −0.26 | −0.02 | 0.54 | 0.48 | 0.33 | 0.41 | 0.69 | 0.45 | 0.38 | 0.48 | 0.29 | 0.17 | 0.54 | 0.27 | ||||
| S—TIE2 | −0.10 | −0.07 | 0.42 | 0.37 | 0.28 | 0.23 | 0.52 | 0.48 | 0.23 | 0.18 | 0.25 | 0.37 | 0.23 | 0.13 | 0.43 | |||
| sVEGFR-1 | −0.12 | 0.23 | 0.22 | 0.80 | 0.73 | 0.67 | 0.17 | 0.06 | −0.27 | 0.31 | 0.88 | −0.22 | 0.55 | 0.24 | 0.34 | 0.25 | ||
| sVEGFR-2 | −0.09 | −0.08 | 0.57 | 0.40 | 0.33 | 0.50 | 0.49 | 0.42 | 0.48 | 0.23 | 0.35 | 0.15 | 0.67 | 0.31 | 0.55 | 0.34 | 0.34 |
| Group/Subgroup of Patients | Adjusted Variable | Predicting Variables | Β | t | p |
|---|---|---|---|---|---|
| All patients | FGF basic | −0.38 | −2.39 | 0.019 | |
| Osteopontin | 0.27 | 1.87 | 0.065 | ||
| SCF | −0.19 | −1.11 | 0.271 | ||
| sST2 | −0.16 | −1.44 | 0.153 | ||
| sEGFR | 0.22 | 1.57 | 0.120 | ||
| sHER-2new | −0.21 | −1.20 | 0.235 | ||
| Regression summary | Multiple R = 0.48 Multiple R2 = 0.24 Corrected R2 = 0.17 F(6.71) = 3.59 p < 0.01 | ||||
| Adjusted for sex | Male | FGF basic | −0.49 | −2.77 | 0.008 |
| Osteopontin | 0.40 | 2.29 | 0.027 | ||
| Regression summary | Multiple R = 0.39 Multiple R2 = 0.15 Corrected R2 = 0.11 F(2.46) = 4.07 p < 0.05 | ||||
| Adjusted for sex | Female | Follistatin | −0.58 | −2.44 | 0.024 |
| Prolactin | 0.30 | 1.831 | 0.081 | ||
| sIL-6RA | −0.69 | −3.26 | 0.004 | ||
| PECAM-1 | 0.38 | 1.74 | 0.096 | ||
| sVEGFR-1 | −0.31 | −1.12 | 0.251 | ||
| Leptin | 0.31 | 1.47 | 0.155 | ||
| sTIE-2 | −0.22 | −1.32 | 0.203 | ||
| Regression summary | Multiple R = 0.73 Multiple R2 = 0.53 Corrected R2 = 0.37 F(7.21) = 3.39 p < 0.05 | ||||
| Adjusted for diabetes | No diabetes | SCF | −0.26 | −1.13 | 0.265 |
| sST2 | −0.35 | −2.34 | 0.024 | ||
| Osteopontin | 0.55 | 2.92 | 0.006 | ||
| FGF basic | −0.42 | −2.12 | 0.04 | ||
| sHER-2new | −0.26 | −1.14 | 0.262 | ||
| Regression summary | Multiple R = 0.58 Multiple R2 = 0.33 Corrected R2 = 0.25 F(5.39) = 3.88 p < 0.01 | ||||
| Adjusted for diabetes | Diabetes | FGF basic | −0.45 | −2.02 | 0.053 |
| sEGFR | 0.28 | 1.72 | 0.097 | ||
| Leptin | −0.28 | −1.53 | 0.137 | ||
| G-CSF | 0.23 | 1.06 | 0.296 | ||
| Regression summary | Multiple R = 0.54 Multiple R2 = 0.3 Corrected R2 = 0.2 F(4.28) = 2.94 p < 0.05 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korzonek-Szlacheta, I.; Hudzik, B.; Zubelewicz-Szkodzińska, B.; Czuba, Z.P.; Szlacheta, P.; Tomasik, A. The Association between Circulating Cytokines and Body Composition in Frail Patients with Cardiovascular Disease. Nutrients 2024, 16, 1227. https://doi.org/10.3390/nu16081227
Korzonek-Szlacheta I, Hudzik B, Zubelewicz-Szkodzińska B, Czuba ZP, Szlacheta P, Tomasik A. The Association between Circulating Cytokines and Body Composition in Frail Patients with Cardiovascular Disease. Nutrients. 2024; 16(8):1227. https://doi.org/10.3390/nu16081227
Chicago/Turabian StyleKorzonek-Szlacheta, Ilona, Bartosz Hudzik, Barbara Zubelewicz-Szkodzińska, Zenon P. Czuba, Patryk Szlacheta, and Andrzej Tomasik. 2024. "The Association between Circulating Cytokines and Body Composition in Frail Patients with Cardiovascular Disease" Nutrients 16, no. 8: 1227. https://doi.org/10.3390/nu16081227
APA StyleKorzonek-Szlacheta, I., Hudzik, B., Zubelewicz-Szkodzińska, B., Czuba, Z. P., Szlacheta, P., & Tomasik, A. (2024). The Association between Circulating Cytokines and Body Composition in Frail Patients with Cardiovascular Disease. Nutrients, 16(8), 1227. https://doi.org/10.3390/nu16081227







