Diabetic Rats Induced Using a High-Fat Diet and Low-Dose Streptozotocin Treatment Exhibit Gut Microbiota Dysbiosis and Osteoporotic Bone Pathologies
Abstract
1. Introduction
2. Materials and Method
2.1. Experimental Animals
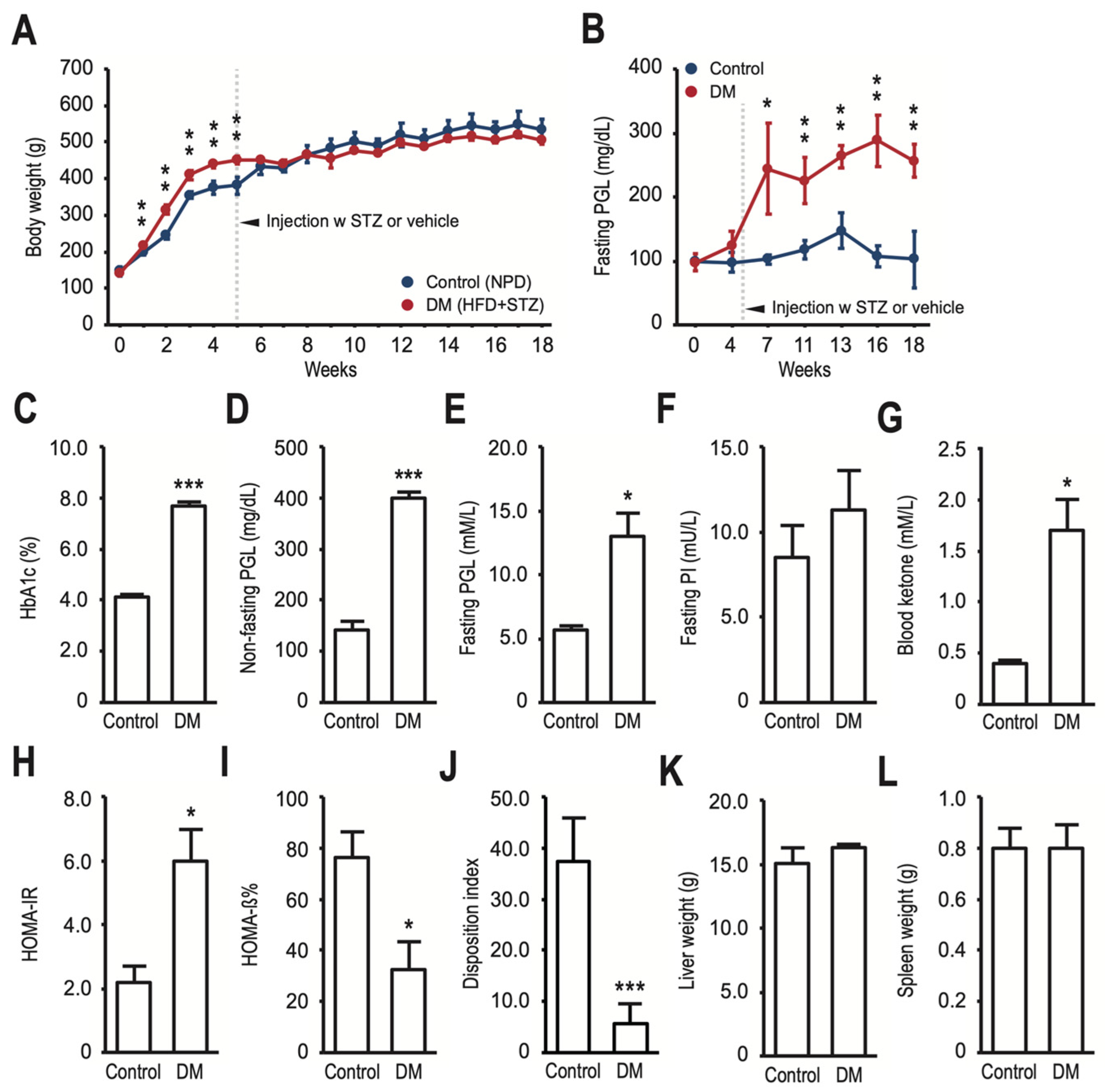
2.2. Study Design and Development of HFD-Fed and Low-Dose STZ-Treated T2DM Rats (Figure S1)
2.3. Blood Collection and Analytical Methods
2.4. Micro-Computed Tomography Analysis
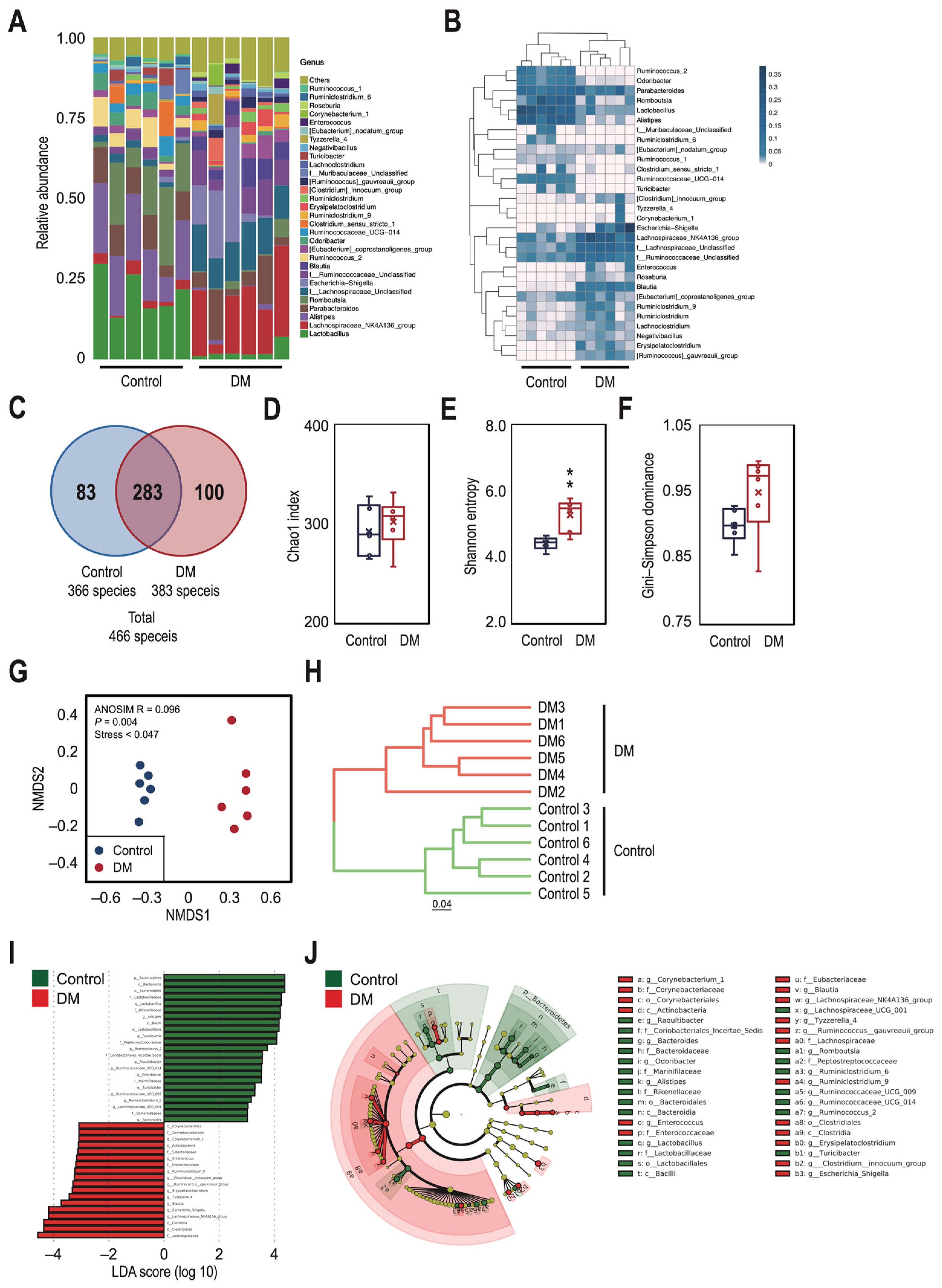
2.5. Compositional Analysis of the Gut Microbiota Using Pyrosequencing and Data Analysis
2.6. Statistical Analysis
3. Results
3.1. HFD-Fed and Low-Dose STZ-Induced T2DM Rats Have Decreased Bone Density, Reduced Bone Turnover, and Altered Gut Microbiota
3.2. There Were Significant Differences in the Taxonomic Profile of Gut Microbiota between the Control and T2DM Rats
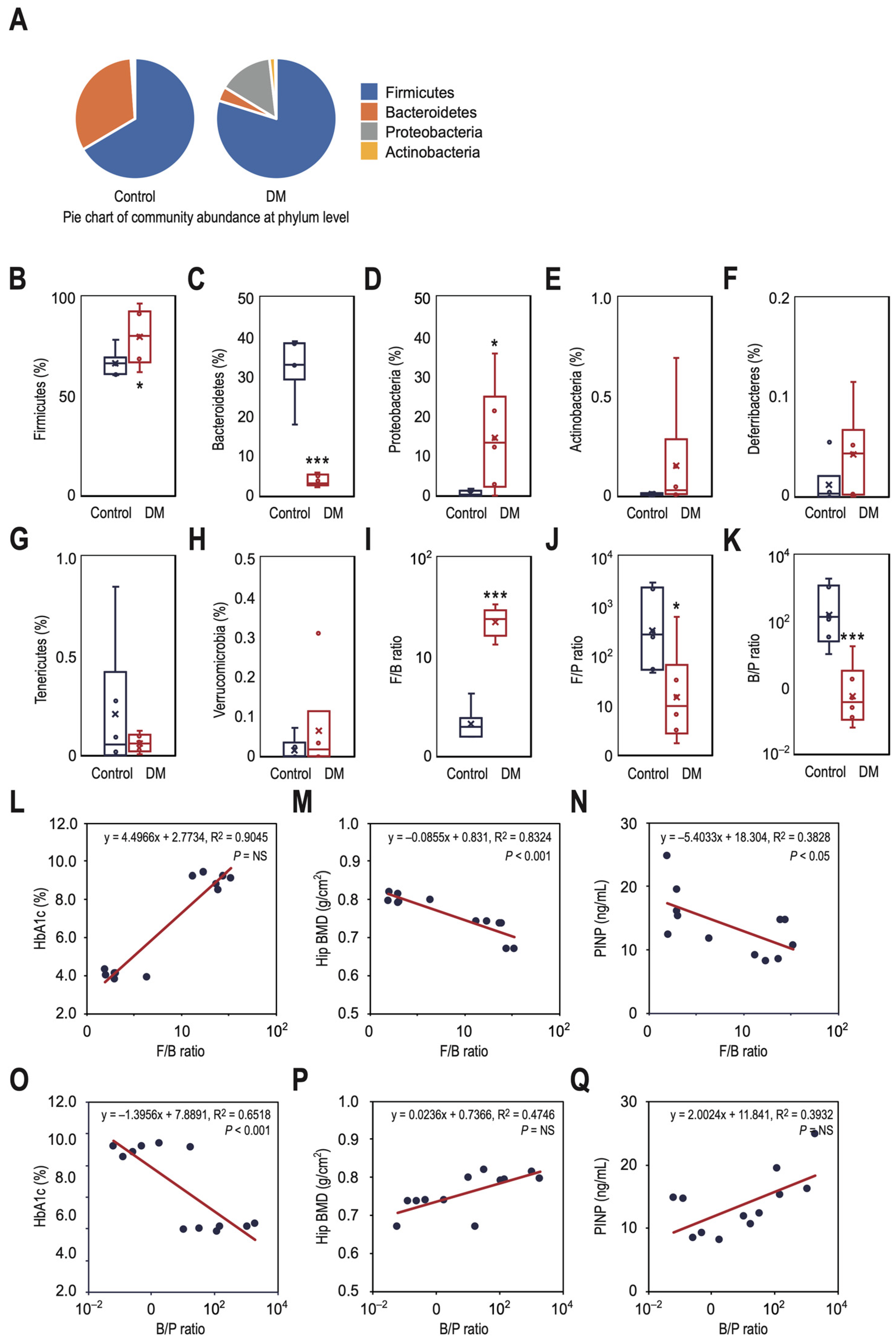
3.3. T2DM Rats Have a Higher Ratio of Firmicutes to Bacteroidetes in Their Gut Microbiota, Which Correlates with Decreased Bone Density and Reduced Bone Turnover
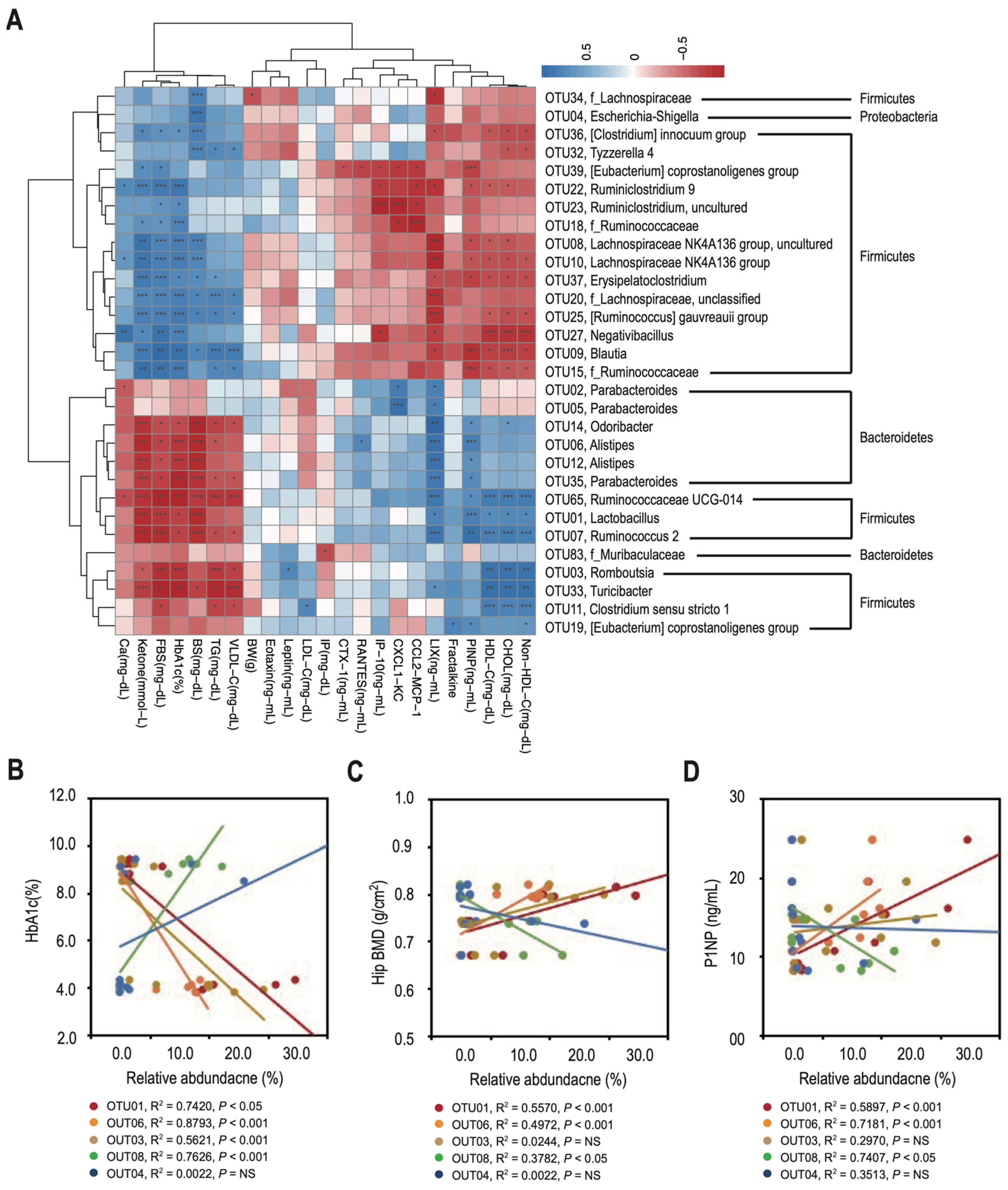
3.4. Changes in the Relative Abundance of Predominant Genera Correlate with Decreased Bone Density and Reduced Bone Turnover in T2DM Rats
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hofbauer, L.C.; Brueck, C.C.; Singh, S.K.; Dobnig, H. Osteoporosis in patients with diabetes mellitus. J. Bone Min. Res. 2007, 22, 1317–1328. [Google Scholar] [CrossRef] [PubMed]
- Thrailkill, K.M.; Lumpkin, C.K., Jr.; Bunn, R.C.; Kemp, S.F.; Fowlkes, J.L. Is insulin an anabolic agent in bone? Dissecting the diabetic bone for clues. Am. J. Physiol. Endocrinol. Metab. 2005, 289, E735–E745. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Zhong, Y.; Huang, G.; Chen, Y.; Shi, H.; Zhang, Z. The relationship between insulin resistance and osteoporosis in elderly male type 2 diabetes mellitus and diabetic nephropathy. Ann. Endocrinol. 2012, 73, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.C.; Chuang, P.Y.; Yang, T.Y.; Huang, T.W.; Chang, S.F. Hyperglycemia inhibits osteoblastogenesis of rat bone marrow stromal cells via activation of the Notch2 signaling pathway. Int. J. Med. Sci. 2019, 16, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Fu, Z.; Wang, X.; Zhou, P.; Yang, Q.; Jiang, Y.; Zhu, D. A narrative review of diabetic bone disease: Characteristics, pathogenesis, and treatment. Front. Endocrinol. 2022, 13, 1052592. [Google Scholar] [CrossRef] [PubMed]
- de Paula, F.J.; Horowitz, M.C.; Rosen, C.J. Novel insights into the relationship between diabetes and osteoporosis. Diabetes Metab. Res. Rev. 2010, 26, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Dou, J.; Wang, J.; Zhang, Q. Differences in the roles of types 1 and 2 diabetes in the susceptibility to the risk of fracture: A systematic review and meta-analysis. Diabetol. Metab. Syndr. 2021, 13, 84. [Google Scholar] [CrossRef]
- Koromani, F.; Ghatan, S.; van Hoek, M.; Zillikens, M.C.; Oei, E.H.G.; Rivadeneira, F.; Oei, L. Type 2 Diabetes Mellitus and Vertebral Fracture Risk. Curr. Osteoporos. Rep. 2021, 19, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Tuomilehto, J.; Lindström, J.; Eriksson, J.G.; Valle, T.T.; Hämäläinen, H.; Ilanne-Parikka, P.; Keinänen-Kiukaanniemi, S.; Laakso, M.; Louheranta, A.; Rastas, M.; et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N. Engl. J. Med. 2001, 344, 1343–1350. [Google Scholar] [CrossRef]
- Kopp, W. How Western Diet And Lifestyle Drive The Pandemic of Obesity and Civilization Diseases. Diabetes Metab. Syndr. Obes. 2019, 12, 2221–2236. [Google Scholar] [CrossRef]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef]
- Dabke, K.; Hendrick, G.; Devkota, S. The gut microbiome and metabolic syndrome. J. Clin. Investig. 2019, 129, 4050–4057. [Google Scholar] [CrossRef]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xiao, Y.; Li, D.; Zhang, S.; Wu, Y.; Zhang, Q.; Bai, W. New insights into the mechanisms of high-fat diet mediated gut microbiota in chronic diseases. iMeta 2023, 2, e69. [Google Scholar] [CrossRef]
- Hernandez, C.J.; Guss, J.D.; Luna, M.; Goldring, S.R. Links Between the Microbiome and Bone. J. Bone Min. Res. 2016, 31, 1638–1646. [Google Scholar] [CrossRef] [PubMed]
- Pacifici, R. Bone Remodeling and the Microbiome. Cold Spring Harb. Perspect. Med. 2018, 8, a031203. [Google Scholar] [CrossRef] [PubMed]
- King, A.J. The use of animal models in diabetes research. Br. J. Pharmacol. 2012, 166, 877–894. [Google Scholar] [CrossRef] [PubMed]
- Robinson, N.B.; Krieger, K.; Khan, F.M.; Huffman, W.; Chang, M.; Naik, A.; Yongle, R.; Hameed, I.; Krieger, K.; Girardi, L.N.; et al. The current state of animal models in research: A review. Int. J. Surg. 2019, 72, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, K.; Viswanad, B.; Asrat, L.; Kaul, C.L.; Ramarao, P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: A model for type 2 diabetes and pharmacological screening. Pharmacol. Res. 2005, 52, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Sanada, H.; Yokokawa, H.; Yoneda, M.; Yatabe, J.; Sasaki Yatabe, M.; Williams, S.M.; Felder, R.A.; Jose, P.A. High body mass index is an important risk factor for the development of type 2 diabetes. Intern. Med. 2012, 51, 1821–1826. [Google Scholar] [CrossRef]
- Czech, M.P. Insulin action and resistance in obesity and type 2 diabetes. Nat. Med. 2017, 23, 804–814. [Google Scholar] [CrossRef]
- Rerup, C.C. Drugs producing diabetes through damage of the insulin secreting cells. Pharmacol. Rev. 1970, 22, 485–518. [Google Scholar] [PubMed]
- Furman, B.L. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. Pharmacol. 2015, 70, 5.47.1–5.47.20. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, A.; Jeddi, S. Streptozotocin as a tool for induction of rat models of diabetes: A practical guide. Excli J. 2023, 22, 274–294. [Google Scholar]
- Trandafir, A.I.; Sima, O.C.; Gheorghe, A.M.; Ciuche, A.; Cucu, A.P.; Nistor, C.; Carsote, M. Trabecular Bone Score (TBS) in Individuals with Type 2 Diabetes Mellitus: An Updated Review. J. Clin. Med. 2023, 12, 7399. [Google Scholar] [CrossRef] [PubMed]
- Tomasiuk, J.M.; Nowakowska-Płaza, A.; Wisłowska, M.; Głuszko, P. Osteoporosis and diabetes—Possible links and diagnostic difficulties. Reumatologia 2023, 61, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Rajan, R.; Cherian, K.E.; Kapoor, N.; Paul, T.V. Trabecular Bone Score-An Emerging Tool in the Management of Osteoporosis. Indian J. Endocrinol. Metab. 2020, 24, 237–243. [Google Scholar] [PubMed]
- Ahuja, V.; Kadowaki, T.; Evans, R.W.; Kadota, A.; Okamura, T.; El Khoudary, S.R.; Fujiyoshi, A.; Barinas-Mitchell, E.J.; Hisamatsu, T.; Vishnu, A.; et al. Comparison of HOMA-IR, HOMA-β% and disposition index between US white men and Japanese men in Japan: The ERA JUMP study. Diabetologia 2015, 58, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.; Knight, R. UniFrac: A new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 2005, 71, 8228–8235. [Google Scholar] [CrossRef]
- Xia, Y.; Sun, J. Hypothesis Testing and Statistical Analysis of Microbiome. Genes. Dis. 2017, 4, 138–148. [Google Scholar] [CrossRef]
- Sokal, R.R.; Michener, C.D. A statistical method for evaluating systematic relationships. Univ. Kans. Sci. Bull. 1958, 38, 1409–1438. [Google Scholar]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Szafranski, S.P.; Wos-Oxley, M.L.; Vilchez-Vargas, R.; Jáuregui, R.; Plumeier, I.; Klawonn, F.; Tomasch, J.; Meisinger, C.; Kühnisch, J.; Sztajer, H.; et al. High-resolution taxonomic profiling of the subgingival microbiome for biomarker discovery and periodontitis diagnosis. Appl. Environ. Microbiol. 2015, 81, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Azen, R.; Sass, D.A. Comparing the squared multiple correlation coefficients of non-nested models: An examination of confidence intervals and hypothesis testing. Br. J. Math. Stat. Psychol. 2008, 61, 163–178. [Google Scholar] [CrossRef] [PubMed]
- McGarry, J.D. Banting lecture 2001: Dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes 2002, 51, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.C.; Chan, J.C. Type 2 diabetes in East Asians: Similarities and differences with populations in Europe and the United States. Ann. N. Y. Acad. Sci. 2013, 1281, 64–91. [Google Scholar] [CrossRef]
- Weir, G.C.; Bonner-Weir, S. Five stages of evolving beta-cell dysfunction during progression to diabetes. Diabetes 2004, 53 (Suppl. S3), S16–S21. [Google Scholar] [CrossRef]
- Winzell, M.S.; Ahrén, B. The high-fat diet-fed mouse: A model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes 2004, 53 (Suppl. S3), S215–S219. [Google Scholar] [CrossRef]
- Hereu, M.; Ramos-Romero, S.; Busquets, C.; Atienza, L.; Amézqueta, S.; Miralles-Pérez, B.; Nogués, M.R.; Méndez, L.; Medina, I.; Torres, J.L. Effects of combined D-fagomine and omega-3 PUFAs on gut microbiota subpopulations and diabetes risk factors in rats fed a high-fat diet. Sci. Rep. 2019, 9, 16628. [Google Scholar] [CrossRef]
- Tebé, C.; Martínez-Laguna, D.; Carbonell-Abella, C.; Reyes, C.; Moreno, V.; Diez-Perez, A.; Collins, G.S.; Prieto-Alhambra, D. The association between type 2 diabetes mellitus, hip fracture, and post-hip fracture mortality: A multi-state cohort analysis. Osteoporos. Int. 2019, 30, 2407–2415. [Google Scholar] [CrossRef] [PubMed]
- Thong, E.P.; Milat, F.; Enticott, J.C.; Joham, A.E.; Ebeling, P.R.; Mishra, G.D.; Teede, H.J. The diabetes-fracture association in women with type 1 and type 2 diabetes is partially mediated by falls: A 15-year longitudinal study. Osteoporos. Int. 2021, 32, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.J.; Chan, D.D.; Lee, J.K.; Tabu, I.; Alpuerto, B.B. The global burden of fragility fractures—What are the differences, and where are the gaps. Best. Pr. Res. Clin. Rheumatol. 2022, 36, 101777. [Google Scholar] [CrossRef] [PubMed]
- Bommer, C.; Sagalova, V.; Heesemann, E.; Manne-Goehler, J.; Atun, R.; Bärnighausen, T.; Davies, J.; Vollmer, S. Global Economic Burden of Diabetes in Adults: Projections From 2015 to 2030. Diabetes Care 2018, 41, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, J.K.; Leutscher, P.; Sørensen, S. Gut Microbiota in Bone Health and Diabetes. Curr. Osteoporos. Rep. 2021, 19, 462–479. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yang, K.; Fan, H.; Wei, M.; Xiong, Q. Targeting the gut microbiota and its metabolites for type 2 diabetes mellitus. Front. Endocrinol. 2023, 14, 1114424. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.; Mitchell, A.L.; Boland, M.; Forster, S.C.; Gloor, G.B.; Tarkowska, A.; Lawley, T.D.; Finn, R.D. A new genomic blueprint of the human gut microbiota. Nature 2019, 568, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Sikalidis, A.K.; Maykish, A. The Gut Microbiome and Type 2 Diabetes Mellitus: Discussing a Complex Relationship. Biomedicines 2020, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Cai, L.; Guo, W. New Advances in Improving Bone Health Based on Specific Gut Microbiota. Front. Cell Infect. Microbiol. 2022, 12, 821429. [Google Scholar] [CrossRef]
- Lyu, Z.; Hu, Y.; Guo, Y.; Liu, D. Modulation of bone remodeling by the gut microbiota: A new therapy for osteoporosis. Bone Res. 2023, 11, 31. [Google Scholar] [CrossRef]
- Zhang, S.; Cai, Y.; Meng, C.; Ding, X.; Huang, J.; Luo, X.; Cao, Y.; Gao, F.; Zou, M. The role of the microbiome in diabetes mellitus. Diabetes Res. Clin. Pr. 2021, 172, 108645. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Li, N.; Shi, J.; Li, H.; Yue, Y.; Jiao, W.; Wang, N.; Song, Y.; Huo, G.; Li, B. Lactobacillus acidophilus alleviates type 2 diabetes by regulating hepatic glucose, lipid metabolism and gut microbiota in mice. Food Funct. 2019, 10, 5804–5815. [Google Scholar] [CrossRef] [PubMed]
- Sapra, L.; Dar, H.Y.; Bhardwaj, A.; Pandey, A.; Kumari, S.; Azam, Z.; Upmanyu, V.; Anwar, A.; Shukla, P.; Mishra, P.K.; et al. Lactobacillus rhamnosus attenuates bone loss and maintains bone health by skewing Treg-Th17 cell balance in Ovx mice. Sci. Rep. 2021, 11, 1807. [Google Scholar] [CrossRef] [PubMed]
- Parker, B.J.; Wearsch, P.A.; Veloo, A.C.M.; Rodriguez-Palacios, A. The Genus Alistipes: Gut Bacteria with Emerging Implications to Inflammation, Cancer, and Mental Health. Front. Immunol. 2020, 11, 906. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, S.; Mei, F.; Zhao, M.; Xia, G.; Shen, X. Tilapia nilotica Head Lipids Improved Bone Loss by Regulating Inflammation and Serum Metabolism Through Gut Microbiota in Ovariectomized Rats. Front. Nutr. 2021, 8, 792793. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; You, Y.; Huang, L.; Long, S.; Zhang, J.; Guo, C.; Zhang, N.; Wu, X.; Xiao, Y.; Tan, H. Alterations in Gut Microbiota of Gestational Diabetes Patients during the First Trimester of Pregnancy. Front. Cell Infect. Microbiol. 2020, 10, 58. [Google Scholar] [CrossRef] [PubMed]
- Martiniakova, M.; Babikova, M.; Mondockova, V.; Blahova, J.; Kovacova, V.; Omelka, R. The Role of Macronutrients, Micronutrients and Flavonoid Polyphenols in the Prevention and Treatment of Osteoporosis. Nutrients 2022, 14, 523. [Google Scholar] [CrossRef] [PubMed]
- Lippi, L.; Uberti, F.; Folli, A.; Turco, A.; Curci, C.; d’Abrosca, F.; de Sire, A.; Invernizzi, M. Impact of nutraceuticals and dietary supplements on mitochondria modifications in healthy aging: A systematic review of randomized controlled trials. Aging Clin. Exp. Res. 2022, 34, 2659. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.; Hridayanka, K.S.N.; Duttaroy, A.K. Bioactives and their roles in bone metabolism of osteoarthritis: Evidence and mechanisms on gut-bone axis. Front. Immunol. 2024, 14, 1323233. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.J.; Jung, S.; Jang, J.S.; Mo, S.; Kwon, J.O.; Kim, M.K.; Kim, H.H. PARK2 Induces Osteoclastogenesis through Activation of the NF-κB Pathway. Mol. Cells 2022, 45, 749. [Google Scholar] [CrossRef]
- Yao, Y.; Cai, X.; Ren, F.; Ye, Y.; Wang, F.; Zheng, C.; Qian, Y.; Zhang, M. The Macrophage-Osteoclast Axis in Osteoimmunity and Osteo-Related Diseases. Front. Immunol. 2021, 12, 664871. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Lee, H.; Chanda, D.; Thoudam, T.; Kang, H.J.; Harris, R.A.; Lee, I.K. The Role of Pyruvate Metabolism in Mitochondrial Quality Control and Inflammation. Mol. Cells 2023, 46, 259. [Google Scholar] [CrossRef] [PubMed]
- Agostini, D.; Gervasi, M.; Ferrini, F.; Bartolacci, A.; Stranieri, A.; Piccoli, G.; Barbieri, E.; Sestili, P.; Patti, A.; Stocchi, V.; et al. An Integrated Approach to Skeletal Muscle Health in Aging. Nutrients 2023, 15, 1802. [Google Scholar] [CrossRef] [PubMed]

| Class Description | Ingredient | Grams |
|---|---|---|
| Protein | Casein, Lactic, 30 Mesh | 200.00 g |
| Protein | Cystine, L | 3.00 g |
| Carbohydrate | Lodex 10 | 125.00 g |
| Carbohydrate | Sucrose, Fine Granulated | 72.80 g |
| Fiber | Solka Floc, FCC200 | 50.00 g |
| Fat | Lard | 245.00 g |
| Fat | Soybean Oil, USP | 25.00 g |
| Mineral | S10026B | 50.00 g |
| Vitamin | Choline Bitartate | 2.00 g |
| Vitamin | V10001C | 1.00 g |
| Dye | Dye, Blue FD&C #1, Alum. Lake 35–42% | 0.05 g |
| Parameters | NPD (n = 8) | HFD (n = 8) | HFD + STZ (n = 8) |
|---|---|---|---|
| Body weight (g) | 374.6 ± 19.8 | 429.3 ± 8.8 ** | 419.5 ± 9.6 * |
| Fasting PGL (mM/L) | 5.44 ± 0.48 | 5.84 ± 0.47 | 13.00 ± 1.78 *, ## |
| Fasting PI (mU/L) | 3.45 ± 0.72 | 7.80 ± 0.83 * | 11.30 ± 2.26 * |
| HOMA-IR | 0.86 ± 0.24 | 2.16 ± 0.38 * | 5.96 ± 0.99 **, ## |
| Leptin (ng/mL) | 4687.0 ± 902.6 | 13339.5 ± 1723.9 ** | 13073.0 ± 3112.0 * |
| RANTES (CCL5) (ng/mL) | 6830.0 ± 787.8 | 10413.0 ± 1379.1 * | 13043.0 ± 1420.4 * |
| Eotaxin (CCL) (ng/mL) | 26.5 ± 4.3 | 25.4 ± 3.0 | 36.3 ± 4.2 |
| IP-10 (CXCL10) (ng/mL) | 219.6 ± 23.7 | 271.9 ± 23.8 | 365.6 ± 37.1 *, # |
| Fractalkine (CX3CL1) (ng/mL) | 33.6 ± 1.3 | 60.7 ± 8.5 * | 36.7 ± 4.8 # |
| LIX (CXCL5) (ng/mL) | 4489.0 ± 461.7 | 5822.6 ± 503.6 | 4747.1 ± 348.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, K.-C.; Chuang, P.-Y.; Yang, T.-Y.; Tsai, Y.-H.; Li, Y.-Y.; Chang, S.-F. Diabetic Rats Induced Using a High-Fat Diet and Low-Dose Streptozotocin Treatment Exhibit Gut Microbiota Dysbiosis and Osteoporotic Bone Pathologies. Nutrients 2024, 16, 1220. https://doi.org/10.3390/nu16081220
Huang K-C, Chuang P-Y, Yang T-Y, Tsai Y-H, Li Y-Y, Chang S-F. Diabetic Rats Induced Using a High-Fat Diet and Low-Dose Streptozotocin Treatment Exhibit Gut Microbiota Dysbiosis and Osteoporotic Bone Pathologies. Nutrients. 2024; 16(8):1220. https://doi.org/10.3390/nu16081220
Chicago/Turabian StyleHuang, Kuo-Chin, Po-Yao Chuang, Tien-Yu Yang, Yao-Hung Tsai, Yen-Yao Li, and Shun-Fu Chang. 2024. "Diabetic Rats Induced Using a High-Fat Diet and Low-Dose Streptozotocin Treatment Exhibit Gut Microbiota Dysbiosis and Osteoporotic Bone Pathologies" Nutrients 16, no. 8: 1220. https://doi.org/10.3390/nu16081220
APA StyleHuang, K.-C., Chuang, P.-Y., Yang, T.-Y., Tsai, Y.-H., Li, Y.-Y., & Chang, S.-F. (2024). Diabetic Rats Induced Using a High-Fat Diet and Low-Dose Streptozotocin Treatment Exhibit Gut Microbiota Dysbiosis and Osteoporotic Bone Pathologies. Nutrients, 16(8), 1220. https://doi.org/10.3390/nu16081220






