Maternal One-Carbon Nutrient Intake and Risk of Being Overweight or Obese in Their Offspring—A Transgenerational Prospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Assessment of Maternal Nutrient Intake during the Period Surrounding Pregnancy
2.3. Assessment of Weight Outcomes among Offspring
2.3.1. Primary Outcome
2.3.2. Secondary Outcomes
2.4. Assessment of Covariates
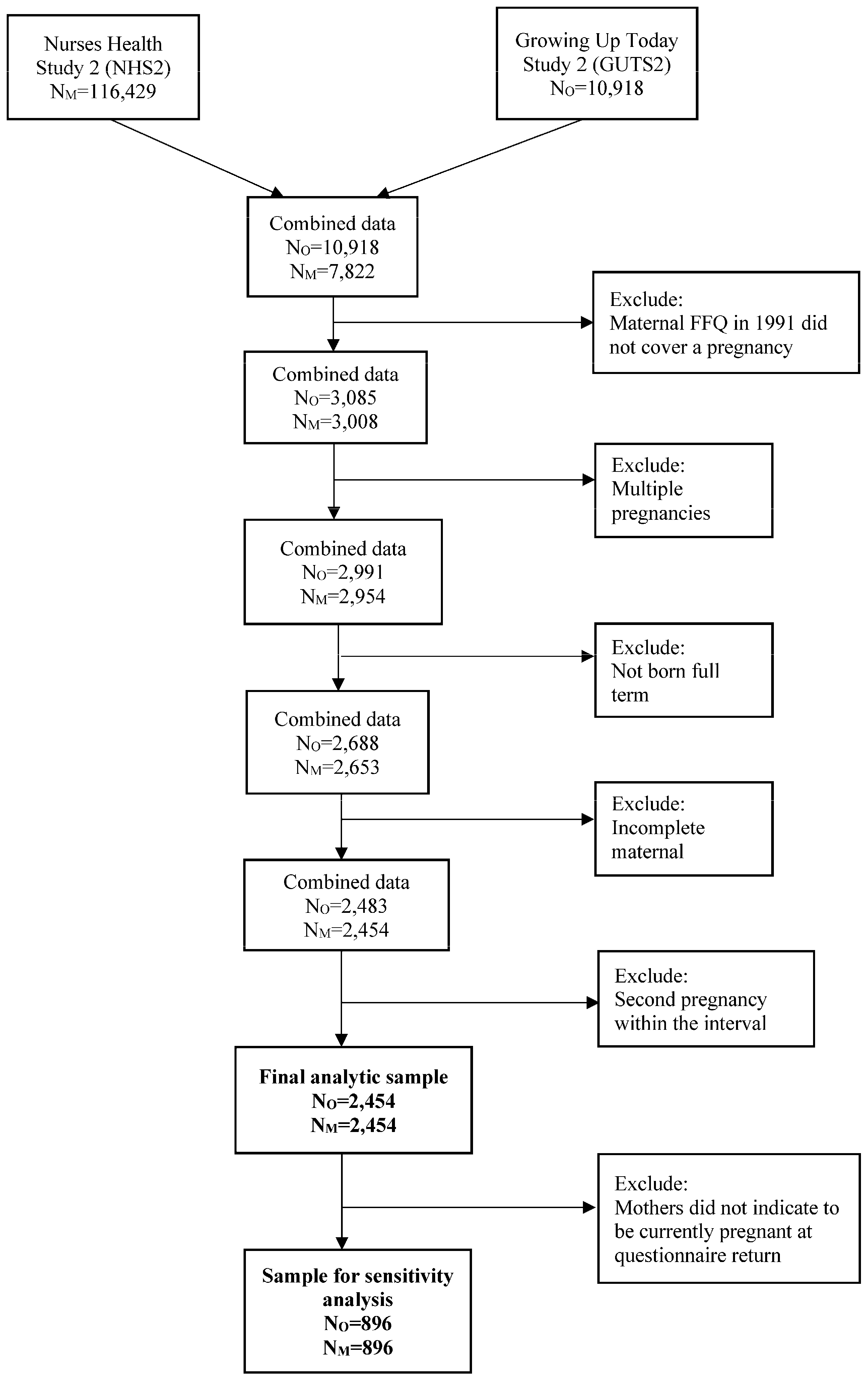
2.5. Final Analytic Samples for the Analyses
2.6. Statistical Analysis
3. Results
| Quintile of Dietary Folate Intake | Quintile of Dietary Phosphatidylcholine Intake | |||||
|---|---|---|---|---|---|---|
| Q1 | Q3 | Q5 | Q1 | Q3 | Q5 | |
| Number of mother–child pairs | 489 | 490 | 491 | 490 | 492 | 490 |
| Maternal characteristics before pregnancy | ||||||
| Body mass index (kg/m2) before pregnancy a | 23.3 ± 4.4 | 22.5 ± 3.8 | 22.7 ± 3.8 | 22.0 ± 3.2 | 22.8 ± 4.2 | 23.5 ± 4.3 |
| Parity before included pregnancy, % b | ||||||
| Nulliparous | 15.4 | 20.9 | 27.5 | 25.8 | 21.6 | 16.6 |
| One previous pregnancy | 30.9 | 34.0 | 30.6 | 30.9 | 29.3 | 33.2 |
| Two previous pregnancies | 28.1 | 24.2 | 22.4 | 22.0 | 29.8 | 23.7 |
| Three previous pregnancies | 25.7 | 20.9 | 19.6 | 21.3 | 19.3 | 26.6 |
| Smoking history before pregnancy, % | ||||||
| Never | 68.7 | 71.9 | 77.3 | 76.2 | 74.8 | 68.4 |
| Past | 21.7 | 22.8 | 18.5 | 18.3 | 17.4 | 24.4 |
| Current | 9.6 | 5.3 | 4.2 | 5.5 | 7.8 | 7.1 |
| Maternal characteristics during pregnancy | ||||||
| Maternal age at birth of the child a, y | 32.5 ± 3.8 | 32.8 ± 3.5 | 33.0 ± 3.7 | 32.9 ± 3.6 | 32.7 ± 3.5 | 33.1 ± 3.8 |
| Partner’s education, % b | ||||||
| High school degree | 39.5 | 31.2 | 31.5 | 28.3 | 34.6 | 33.3 |
| College degree | 29.6 | 34.7 | 33.4 | 32.8 | 34.6 | 34.6 |
| Graduate school degree | 30.9 | 34.1 | 35.1 | 38.9 | 30.7 | 32.0 |
| Married in 1989, % b | 91.8 | 93.7 | 89.8 | 90.4 | 92.9 | 90.4 |
| Total energy intake c (kcal/d) | 1846 (1473; 2225) | 1973 (1706; 2326) | 1717 (1435; 1957) | 1884 (1535; 2728) | 1970 (1665; 2316) | 1864 (1525; 2264) |
| Alcohol c (g/d) | 0.9 (0; 2.9) | 0.9 (0; 2.8) | 0.0 (0; 1.9) | 0.0 (0; 2.6) | 0.9 (0; 2.7) | 0.0 (0; 2.0) |
| Energy-adjusted trans fat c (g/d) | 3.3 (2.6; 4.1) | 2.9 (2.3; 3.6) | 2.8 (2.2; 3.5) | 2.8 (2.2; 3.8) | 2.9 (2.3; 3.7) | 2.9 (2.3; 3.7) |
| Coffee c (cups/d) | 0.4 (0; 2.5) | 0.4 (0; 2.0) | 0.1 (0; 1.0) | 0.1 (0; 1.0) | 0.4 (0; 1.4) | 0.5 (0; 2.0) |
| Sugar-sweetened beverages c (servings/d) | 0.6 (0.1; 1.3) | 0.4 (0.1; 1.0) | 0.2 (0.1; 0.9) | 0.4 (0.1; 1.0) | 0.4 (0.1; 1.0) | 0.5 (0.1; 1.1) |
| Artifically-sweetened beverages c (servings/d) | 0.1 (0; 0.4) | 0.1 (0; 0.5) | 0.1 (0; 0.4) | 0.2 (0.1; 0.9) | 0.1 (0.0; 0.6) | 0.1 (0.0; 0.3) |
| Refined grains c (servings/d) | 1.2 (0.8; 1.8) | 1.3 (0.8; 1.9) | 1.1 (0.7; 1.5) | 1.2 (0.7; 1.9) | 1.2 (0.8; 1.7) | 1.2 (0.8; 1.8) |
| Ratio of polyunsaturated to saturated fat c | 0.5 (0.4; 0.5) | 0.5 (0.4; 0.6) | 0.5 (0.4; 0.6) | 0.5 (0.4; 0.6) | 0.5 (0.4; 0.6) | 0.5 (0.4; 0.6) |
| Physical activity, min/week, % | ||||||
| 0 | 23.7 | 14.2 | 17.4 | 17.7 | 16.3 | 17.3 |
| 1–149 | 43.2 | 40.1 | 39.9 | 39.0 | 43.9 | 44.9 |
| 150–299 | 16.3 | 20.0 | 21.4 | 21.7 | 18.1 | 18.2 |
| ≥300 | 16.8 | 25.7 | 21.2 | 21.5 | 21.6 | 19.6 |
| Offspring characteristics | ||||||
| Offspring gender, % | ||||||
| Male | 47.8 | 45.7 | 46.0 | 47.4 | 45.9 | 45.5 |
| Female | 52.2 | 54.3 | 54.0 | 52.6 | 54.1 | 54.5 |
| Offspring age at GUTSII baseline 2004, y | 13.4 ± 0.6 | 13.2 ± 0.5 | 13.1 ± 0.4 | 13.2 ± 0.5 | 13.2 ± 0.5 | 13.2 ± 0.6 |
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hales, C.M.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Prevalence of Obesity Among Adults and Youth: United States, 2015–2016; NCHS Data Brief; National Center for Health Statistics: Hyattsville, MD, USA, 2017; pp. 1–8.
- Romieu, I.; Dossus, L.; Barquera, S.; Blottière, H.M.; Franks, P.W.; Gunter, M.; Hwalla, N.; Hursting, S.D.; Leitzmann, M.; Margetts, B.; et al. Energy balance and obesity: What are the main drivers? Cancer Causes Control 2017, 28, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, P.D.; Buss, C.; Entringer, S.; Swanson, J.M. Developmental origins of health and disease: Brief history of the approach and current focus on epigenetic mechanisms. Semin. Reprod. Med. 2009, 27, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.; Mai, C.T.; Mulinare, J.; Isenburg, J.; Flood, T.J.; Ethen, M.; Frohnert, B.; Kirby, R.S. Updated estimates of neural tube defects prevented by mandatory folic Acid fortification—United States, 1995–2011. MMWR Morb. Mortal. Wkly Rep. 2015, 64, 1–5. [Google Scholar] [PubMed]
- Anderson, O.S.; Sant, K.E.; Dolinoy, D.C. Nutrition and epigenetics: An interplay of dietary methyl donors, one-carbon metabolism and DNA methylation. J. Nutr. Biochem. 2012, 23, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Tamburini, S.; Shen, N.; Wu, H.C.; Clemente, J.C. The microbiome in early life: Implications for health outcomes. Nat. Med. 2016, 22, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Gurwara, S.; Ajami, N.J.; Jang, A.; Hessel, F.C.; Chen, L.; Plew, S.; Wang, Z.; Graham, D.Y.; Hair, C.; White, D.L.; et al. Dietary Nutrients Involved in One-Carbon Metabolism and Colonic Mucosa-Associated Gut Microbiome in Individuals with an Endoscopically Normal Colon. Nutrients 2019, 11, 613. [Google Scholar] [CrossRef] [PubMed]
- Schugar, R.C.; Shih, D.M.; Warrier, M.; Helsley, R.N.; Burrows, A.; Ferguson, D.; Brown, A.L.; Gromovsky, A.D.; Heine, M.; Chatterjee, A.; et al. The TMAO-Producing Enzyme Flavin-Containing Monooxygenase 3 Regulates Obesity and the Beiging of White Adipose Tissue. Cell Rep. 2017, 19, 2451–2461. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, D.D.; Chiuve, S.E.; Manson, J.E.; Willett, W.C.; Hu, F.B.; Qi, L. Dietary phosphatidylcholine intake and type 2 diabetes in men and women. Diabetes Care 2015, 38, e13–e14. [Google Scholar] [CrossRef] [PubMed]
- Hammoud, R.; Pannia, E.; Liao, C.-S.; Chatterjee, D.; Ho, M.; Yang, N.; Kubant, R.; Pausova, Z.; Anderson, G.H. Maternal Choline Intake Programs Hypothalamic Energy Regulatory Pathways and Long-Term Phenotype in Male Wistar Rat Offspring (OR35-04-19). Curr. Dev. Nutr. 2019, 3 (Suppl. S1), nzz048.OR35-04-19. [Google Scholar] [CrossRef]
- Yang, N.V.; Pannia, E.; Chatterjee, D.; Kubant, R.; Ho, M.; Hammoud, R.; Pausova, Z.; Anderson, G.H. Gestational folic acid content alters the development and function of hypothalamic food intake regulating neurons in Wistar rat offspring post-weaning. Nutr. Neurosci. 2020, 23, 149–160. [Google Scholar] [CrossRef]
- van Lee, L.; Crozier, S.R.; Aris, I.M.; Tint, M.T.; Sadananthan, S.A.; Michael, N.; Quah, P.L.; Robinson, S.M.; Inskip, H.M.; Harvey, N.C.; et al. Prospective associations of maternal choline status with offspring body composition in the first 5 years of life in two large mother-offspring cohorts: The Southampton Women’s Survey cohort and the Growing Up in Singapore Towards healthy Outcomes cohort. Int. J. Epidemiol. 2019, 48, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Yajnik, C.S.; Deshpande, S.S.; Jackson, A.A.; Refsum, H.; Rao, S.; Fisher, D.J.; Bhat, D.S.; Naik, S.S.; Coyaji, K.J.; Joglekar, C.V.; et al. Vitamin B12 and folate concentrations during pregnancy and insulin resistance in the offspring: The Pune Maternal Nutrition Study. Diabetologia 2008, 51, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.-H.; Liu, Y.-J.; Retnakaran, R.; MacFarlane, A.J.; Hamilton, J.; Smith, G.; Walker, M.C.; Wen, S.W. Maternal folate status and obesity/insulin resistance in the offspring: A systematic review. Int. J. Obes. 2016, 40, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Bertoia, M.L.; Lenart, E.B.; Stampfer, M.J.; Willett, W.C.; Speizer, F.E.; Chavarro, J.E. Origin, Methods, and Evolution of the Three Nurses’ Health Studies. Am. J. Public Health 2016, 106, 1573–1581. [Google Scholar] [CrossRef]
- US Department of Agriculture. USDA Database for the Choline Content of Common Foods; U S. Department of Agriculture: Washington, DC, USA, 2004.
- Zeisel, S.H.; Mar, M.-H.; Howe, J.C.; Holden, J.M. Concentrations of choline-containing compounds and betaine in common foods. J. Nutr. 2003, 133, 1302–1307. [Google Scholar] [CrossRef] [PubMed]
- Wiedeman, A.M.; Barr, S.I.; Green, T.J.; Xu, Z.; Innis, S.M.; Kitts, D.D. Dietary Choline Intake: Current State of Knowledge Across the Life Cycle. Nutrients 2018, 10, 1513. [Google Scholar] [CrossRef]
- Yuan, C.; Spiegelman, D.; Rimm, E.B.; Rosner, B.A.; Stampfer, M.J.; Barnett, J.B.; Chavarro, J.E.; Rood, J.C.; Harnack, L.J.; Sampson, L.K.; et al. Relative Validity of Nutrient Intakes Assessed by Questionnaire, 24-Hour Recalls, and Diet Records as Compared with Urinary Recovery and Plasma Concentration Biomarkers: Findings for Women. Am. J. Epidemiol. 2018, 187, 1051–1063. [Google Scholar] [CrossRef]
- Cho, E.; Zeisel, S.H.; Jacques, P.; Selhub, J.; Dougherty, L.; Colditz, G.A.; Willett, W.C. Dietary choline and betaine assessed by food-frequency questionnaire in relation to plasma total homocysteine concentration in the Framingham Offspring Study. Am. J. Clin. Nutr. 2006, 83, 905–911. [Google Scholar] [CrossRef]
- Himes, J.H.; Faricy, A. Validity and reliability of self-reported stature and weight of US adolescents. Am. J. Hum. Biol. 2001, 13, 255–260. [Google Scholar] [CrossRef]
- Cole, T.J.; Bellizzi, M.C.; Flegal, K.M.; Dietz, W.H. Establishing a standard definition for child overweight and obesity worldwide: International survey. BMJ 2000, 320, 1240–1243. [Google Scholar] [CrossRef]
- World Health Organization. Obesity—Preventing and Managing the Global Epidemic. Report on a WHO Consultation; World Health Organization: Geneva, Switzerland, 2000.
- Strohmaier, S.; Bogl, L.H.; Eliassen, A.H.; Massa, J.; Field, A.E.; Chavarro, J.E.; Ding, M.; Tamimi, R.M.; Schernhammer, E. Maternal healthful dietary patterns during peripregnancy and long-term overweight risk in their offspring. Eur. J. Epidemiol. 2020, 35, 283–293. [Google Scholar] [CrossRef]
- Shenkin, S.D.; Zhang, M.G.; Der, G.; Mathur, S.; Mina, T.H.; Reynolds, R.M. Validity of recalled v. recorded birth weight: A systematic review and meta-analysis. J. Dev. Orig. Health Dis. 2017, 8, 137–148. [Google Scholar] [CrossRef]
- Spiegelman, D.; Hertzmark, E. Easy SAS calculations for risk or prevalence ratios and differences. Am. J. Epidemiol. 2005, 162, 199–200. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Y.; Rimm, E.B.; Hu, F.B.; Albert, C.M.; Rexrode, K.M.; Manson, J.E.; Qi, L. Dietary phosphatidylcholine and risk of all-cause and cardiovascular-specific mortality among US women and men. Am. J. Clin. Nutr. 2016, 104, 173–180. [Google Scholar] [CrossRef]
- Stuebe, A.M.; Forman, M.R.; Michels, K.B. Maternal-recalled gestational weight gain, pre-pregnancy body mass index, and obesity in the daughter. Int. J. Obes. 2009, 33, 743–752. [Google Scholar] [CrossRef]
- van Parys, A.; Karlsson, T.; Vinknes, K.J.; Olsen, T.; Øyen, J.; Dierkes, J.; Nygård, O.; Lysne, V. Food Sources Contributing to Intake of Choline and Individual Choline Forms in a Norwegian Cohort of Patients with Stable Angina Pectoris. Front. Nutr. 2021, 8, 676026. [Google Scholar] [CrossRef]
- Zeisel, S.H.; Da Costa, K.A.; Franklin, P.D.; Alexander, E.A.; Lamont, J.T.; Sheard, N.F.; Beiser, A. Choline, an essential nutrient for humans. FASEB J. 1991, 5, 2093–2098. [Google Scholar] [CrossRef]
- Tayebati, S.K.; Amenta, F. Choline-containing phospholipids: Relevance to brain functional pathways. Clin. Chem. Lab. Med. 2013, 51, 513–521. [Google Scholar] [CrossRef]
- McCann, J.C.; Hudes, M.; Ames, B.N. An overview of evidence for a causal relationship between dietary availability of choline during development and cognitive function in offspring. Neurosci. Biobehav. Rev. 2006, 30, 696–712. [Google Scholar] [CrossRef]
- Caudill, M.A.; Strupp, B.J.; Muscalu, L.; Nevins, J.E.H.; Canfield, R.L. Maternal choline supplementation during the third trimester of pregnancy improves infant information processing speed: A randomized, double-blind, controlled feeding study. FASEB J. 2018, 32, 2172–2180. [Google Scholar] [CrossRef]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.-M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.W.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Malinowska, A.M.; Szwengiel, A.; Chmurzynska, A. Dietary, anthropometric, and biochemical factors influencing plasma choline, carnitine, trimethylamine, and trimethylamine-N-oxide concentrations. Int. J. Food Sci. Nutr. 2017, 68, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, Y.; Zhou, R.; Chen, X.; Wang, C.; Tan, X.; Wang, L.; Zheng, R.; Zhang, H.; Ling, W.; et al. Associations of gut-flora-dependent metabolite trimethylamine-N-oxide, betaine and choline with non-alcoholic fatty liver disease in adults. Sci. Rep. 2016, 6, 19076. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, J.K.; Tuomainen, T.-P.; Voutilainen, S. Dietary intake of choline and phosphatidylcholine and risk of type 2 diabetes in men: The Kuopio Ischaemic Heart Disease Risk Factor Study. Eur. J. Nutr. 2020, 59, 3857–3861. [Google Scholar] [CrossRef] [PubMed]
- Watkins, A.J.; Rubini, E.; Hosier, E.D.; Morgan, H.L. Paternal programming of offspring health. Early Hum. Dev. 2020, 150, 105185. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, S.; Truijen, I.; Ghosh, M.; Duca, R.C.; Langie, S.A.S.; Bekaert, B.; Freson, K.; Huybrechts, I.; Koppen, G.; Devlieger, R.; et al. The effect of paternal methyl-group donor intake on offspring DNA methylation and birth weight. J. Dev. Orig. Health Dis. 2017, 8, 311–321. [Google Scholar] [CrossRef]
- Relton, C.L.; Pearce, M.S.; Parker, L. The influence of erythrocyte folate and serum vitamin B12 status on birth weight. Br. J. Nutr. 2005, 93, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, R.M.; Vollset, S.E.; Monsen, A.L.B.; Ulvik, A.; Haugen, M.; Meltzer, H.M.; Magnus, P.; Ueland, P.M. Infant birth size is not associated with maternal intake and status of folate during the second trimester in Norwegian pregnant women. J. Nutr. 2010, 140, 572–579. [Google Scholar] [CrossRef]
- Lewis, S.J.; Leary, S.; Davey Smith, G.; Ness, A. Body composition at age 9 years, maternal folate intake during pregnancy and methyltetrahydrofolate reductase (MTHFR) C677T genotype. Br. J. Nutr. 2009, 102, 493–496. [Google Scholar] [CrossRef]
- Wang, G.; Hu, F.B.; Mistry, K.B.; Zhang, C.; Ren, F.; Huo, Y.; Paige, D.; Bartell, T.; Hong, X.; Caruso, D.; et al. Association Between Maternal Prepregnancy Body Mass Index and Plasma Folate Concentrations with Child Metabolic Health. JAMA Pediatr. 2016, 170, e160845. [Google Scholar] [CrossRef] [PubMed]
- Crozier, S.R.; Robinson, S.M.; Godfrey, K.M.; Cooper, C.; Inskip, H.M. Women’s dietary patterns change little from before to during pregnancy. J. Nutr. 2009, 139, 1956–1963. [Google Scholar] [CrossRef] [PubMed]
| Quintile of Maternal One-Carbon Nutrient Intake | p for Trend | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Total folate | ||||||
| Median intake (µg/d) | 275 | 439 | 708 | 1010 | 1355 | |
| Cases/participants | 231/483 | 235/489 | 224/488 | 222/487 | 223/488 | |
| Basic model | 1 (ref) | 1.02 (0.90, 1.16) | 0.96 (0.84, 1.10) | 0.95 (0.83, 1.09) | 0.96 (0.84, 1.10) | 0.34 |
| Multivariate model | 1 (ref) | 1.09 (0.96, 1.24) | 1.06 (0.93, 1.21) | 1.04 (0.91, 1.20) | 1.03 (0.90, 1.18) | 0.96 |
| Total vitamin B12 | ||||||
| Median intake (µg/d) | 5 | 8 | 11 | 14 | 20 | |
| Cases/participants | 250/534 | 246/525 | 213/459 | 207/449 | 219/468 | |
| Basic model | 1 (ref) | 1.01 (0.89, 1.14) | 1.00 (0.87, 1.14) | 0.98 (0.86, 1.12) | 1.00 (0.88, 1.15) | 0.97 |
| Multivariate model | 1 (ref) | 1.04 (0.92, 1.17) | 1.07 (0.94, 1.22) | 1.03 (0.90, 1.17) | 1.04 (0.91, 1.19) | 0.65 |
| Total vitamin B6 | ||||||
| Median intake (mg/d) | 2.0 | 3.0 | 4.3 | 6.4 | 12.6 | |
| Cases/participants | 228/485 | 248/505 | 219/478 | 224/482 | 216/485 | |
| Basic model | 1 (ref) | 1.06 (0.93, 1.20) | 0.97 (0.85, 1.11) | 0.99 (0.86, 1.13) | 0.96 (0.83, 1.10) | 0.29 |
| Multivariate model | 1 (ref) | 1.13 (0.99, 1.28) | 1.04 (0.91, 1.19) | 1.05 (0.92, 1.20) | 1.02 (0.89, 1.17) | 0.60 |
| Total vitamin B2 | ||||||
| Median intake (mg/d) | 1.8 | 2.6 | 3.6 | 4.4 | 5.9 | |
| Cases/participants | 233/482 | 225/487 | 229/492 | 223/484 | 225/490 | |
| Basic model | 1 (ref) | 0.96 (0.84, 1.09) | 0.95 (0.84, 1.09) | 0.95 (0.84, 1.09) | 0.95 (0.83, 1.08) | 0.46 |
| Multivariate model | 1 (ref) | 0.98 (0.86, 1.11) | 1.03 (0.91, 1.17) | 1.04 (0.91, 1.19) | 1.01 (0.88, 1.15) | 0.69 |
| Total methionine | ||||||
| Median intake (g/d) | 1.6 | 1.9 | 2.1 | 2.2 | 2.5 | |
| Cases/participants | 211/488 | 213/474 | 242/495 | 237/497 | 232/481 | |
| Basic model | 1 (ref) | 1.05 (0.91, 1.21) | 1.13 (0.99, 1.30) | 1.11 (0.97, 1.27) | 1.11 (0.97, 1.28) | 0.09 |
| Multivariate model | 1 (ref) | 1.05 (0.92, 1.21) | 1.12 (0.99, 1.28) | 1.10 (0.96, 1.25) | 1.07 (0.93, 1.23) | 0.27 |
| Total choline | ||||||
| Median intake (mg/d) | 269 | 311 | 338 | 366 | 407 | |
| Cases/participants | 200/484 | 227/490 | 251/486 | 231/486 | 226/488 | |
| Basic model | 1 (ref) | 1.15 (1.00, 1.32) | 1.25 (1.09, 1.43) | 1.16 (1.01, 1.34) | 1.14 (0.99, 1.32) | 0.08 |
| Multivariate model | 1 (ref) | 1.16 (1.01, 1.34) | 1.26 (1.10, 1.44) | 1.16 (1.01, 1.34) | 1.16 (1.01, 1.34) | 0.06 |
| Phosphatidylcholine | ||||||
| Median intake (mg/d) | 115 | 139 | 158 | 176 | 206 | |
| Cases/participants | 204/487 | 205/486 | 225/490 | 248/487 | 253/485 | |
| Basic model | 1 (ref) | 1.00 (0.87, 1.16) | 1.10 (0.95, 1.26) | 1.23 (1.07, 1.41) | 1.26 (1.10, 1.44) | <0.0001 |
| Multivariate model | 1 (ref) | 0.98 (0.85, 1.14) | 1.06 (0.92, 1.22) | 1.18 (1.03, 1.35) | 1.16 (1.01, 1.33) | 0.003 |
| Total betaine | ||||||
| Median intake (mg/d) | 72 | 91 | 108 | 129 | 174 | |
| Cases/participants | 241/484 | 225/489 | 235/488 | 233/487 | 201/486 | |
| Basic model | 1 (ref) | 0.94 (0.82, 1.07) | 0.97 (0.86, 1.11) | 0.97 (0.86, 1.11) | 0.84 (0.73, 0.97) | 0.03 |
| Multivariate model | 1 (ref) | 0.97 (0.86, 1.10) | 1.04 (0.91, 1.18) | 1.06 (0.93, 1.20) | 0.93 (0.81, 1.07) | 0.46 |
| Quintiles of Maternal Phosphatidylcholine Intake | p for Trend | p for Interaction | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||
| Egg | |||||||
| Lower egg consumers | |||||||
| Median intake (mg/d) | 113 | 139 | 156 | 174 | 204 | ||
| Cases/Offspring | 129/312 | 92/211 | 73/155 | 70/136 | 37/78 | ||
| Basic model | 1 (ref) | 1.04 (0.85, 1.28) | 1.14 (0.92, 1.41) | 1.22 (0.99, 1.50) | 1.15 (0.88, 1.49) | 0.07 | |
| Multivariable model | 1 (ref) | 1.02 (0.84, 1.25) | 1.12 (0.91, 1.38) | 1.19 (0.96, 1.46) | 1.05 (0.80, 1.38) | 0.25 | |
| Higher egg consumers | |||||||
| Median intake (mg/d) | 120 | 140 | 158 | 176 | 207 | ||
| Cases/Offspring | 75/175 | 113/275 | 152/335 | 178/351 | 216/407 | ||
| Basic model | 1 (ref) | 0.96 (0.77, 1.19) | 1.06 (0.86, 1.30) | 1.18 (0.97, 1.45) | 1.24 (1.02, 1.51) | 0.0007 | |
| Multivariable model | 1 (ref) | 0.94 (0.76, 1.16) | 1.02 (0.83, 1.25) | 1.15 (0.95, 1.39) | 1.14 (0.95, 1.38) | 0.014 | 0.87 |
| Fish | |||||||
| Lower fish consumers | |||||||
| Median intake (mg/d) | 114 | 139 | 157 | 176 | 206 | ||
| Cases/Offspring | 128/303 | 104/240 | 104/223 | 107/202 | 107/204 | ||
| Basic model | 1 (ref) | 1.03 (0.84, 1.25) | 1.11 (0.92, 1.35) | 1.26 (1.05, 1.51) | 1.26 (1.04, 1.51) | 0.003 | |
| Multivariable model | 1 (ref) | 1.03 (0.85, 1.25) | 1.09 (0.90, 1.32) | 1.24 (1.03, 1.49) | 1.18 (0.98, 1.43) | 0.02 | |
| Higher fish consumers | |||||||
| Median intake (mg/d) | 116 | 140 | 158 | 176 | 207 | ||
| Cases/Offspring | 76/184 | 101/246 | 121/267 | 141/285 | 146/281 | ||
| Basic model | 1 (ref) | 0.99 (0.78, 1.24) | 1.09 (0.87, 1.35) | 1.19 (0.97, 1.46) | 1.25 (1.02, 1.54) | 0.004 | |
| Multivariable model | 1 (ref) | 0.94 (0.75, 1.17) | 1.02 (0.82, 1.26) | 1.09 (0.89, 1.33) | 1.10 (0.90, 1.35) | 0.11 | 0.97 |
| Red meat | |||||||
| Lower red meat consumers | |||||||
| Median intake (mg/d) | 113 | 139 | 157 | 176 | 204 | ||
| Cases/Offspring | 167/377 | 114/267 | 107/250 | 98/209 | 88/179 | ||
| Basic model | 1 (ref) | 0.96 (0.80, 1.15) | 0.97 (0.81, 1.16) | 1.05 (0.88, 1.26) | 1.11 (0.92, 1.34) | 0.23 | |
| Multivariable model | 1 (ref) | 0.94 (0.78, 1.12) | 0.94 (0.79, 1.13) | 1.05 (0.88, 1.26) | 1.05 (0.87, 1.27) | 0.46 | |
| Higher red meat consumers | |||||||
| Median intake (mg/d) | 120 | 140 | 158 | 176 | 207 | ||
| Cases/Offspring | 37/110 | 91/219 | 118/240 | 150/278 | 165/306 | ||
| Basic model | 1 (ref) | 1.24 (0.91, 1.68) | 1.47 (1.10, 1.97) | 1.61 (1.21, 2.14) | 1.62 (1.22, 2.15) | <0.0001 | |
| Multivariable model | 1 (ref) | 1.23 (0.92, 1.65) | 1.45 (1.10, 1.92) | 1.58 (1.21, 2.08) | 1.50 (1.14, 1.98) | 0.001 | 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogl, L.H.; Strohmaier, S.; Hu, F.B.; Willett, W.C.; Eliassen, A.H.; Hart, J.E.; Sun, Q.; Chavarro, J.E.; Field, A.E.; Schernhammer, E.S. Maternal One-Carbon Nutrient Intake and Risk of Being Overweight or Obese in Their Offspring—A Transgenerational Prospective Cohort Study. Nutrients 2024, 16, 1210. https://doi.org/10.3390/nu16081210
Bogl LH, Strohmaier S, Hu FB, Willett WC, Eliassen AH, Hart JE, Sun Q, Chavarro JE, Field AE, Schernhammer ES. Maternal One-Carbon Nutrient Intake and Risk of Being Overweight or Obese in Their Offspring—A Transgenerational Prospective Cohort Study. Nutrients. 2024; 16(8):1210. https://doi.org/10.3390/nu16081210
Chicago/Turabian StyleBogl, Leonie H., Susanne Strohmaier, Frank B. Hu, Walter C. Willett, A. Heather Eliassen, Jaime E. Hart, Qi Sun, Jorge E. Chavarro, Alison E. Field, and Eva S. Schernhammer. 2024. "Maternal One-Carbon Nutrient Intake and Risk of Being Overweight or Obese in Their Offspring—A Transgenerational Prospective Cohort Study" Nutrients 16, no. 8: 1210. https://doi.org/10.3390/nu16081210
APA StyleBogl, L. H., Strohmaier, S., Hu, F. B., Willett, W. C., Eliassen, A. H., Hart, J. E., Sun, Q., Chavarro, J. E., Field, A. E., & Schernhammer, E. S. (2024). Maternal One-Carbon Nutrient Intake and Risk of Being Overweight or Obese in Their Offspring—A Transgenerational Prospective Cohort Study. Nutrients, 16(8), 1210. https://doi.org/10.3390/nu16081210










