IL-33 Reduces Saturated Fatty Acid Accumulation in Mouse Atherosclerotic Foci
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Blood Pressure Assessment
2.3. Hematological Analysis
2.4. Measurement of Arteriosclerotic Lesion Area
2.5. Isolation of Mononuclear Cells from Aortas in Mice
2.6. Tissue Preparation and Flow Cytometry
2.7. Quantification of Free Fatty Acids in the Aorta and Sera
2.8. Quantitative Real-Time Polymerase Chain Reaction of the Aorta and Jejunum
2.9. Cells and Treatment
2.10. qRT-PCR
2.11. Histological Examination
2.12. Statistical Analyses
3. Results
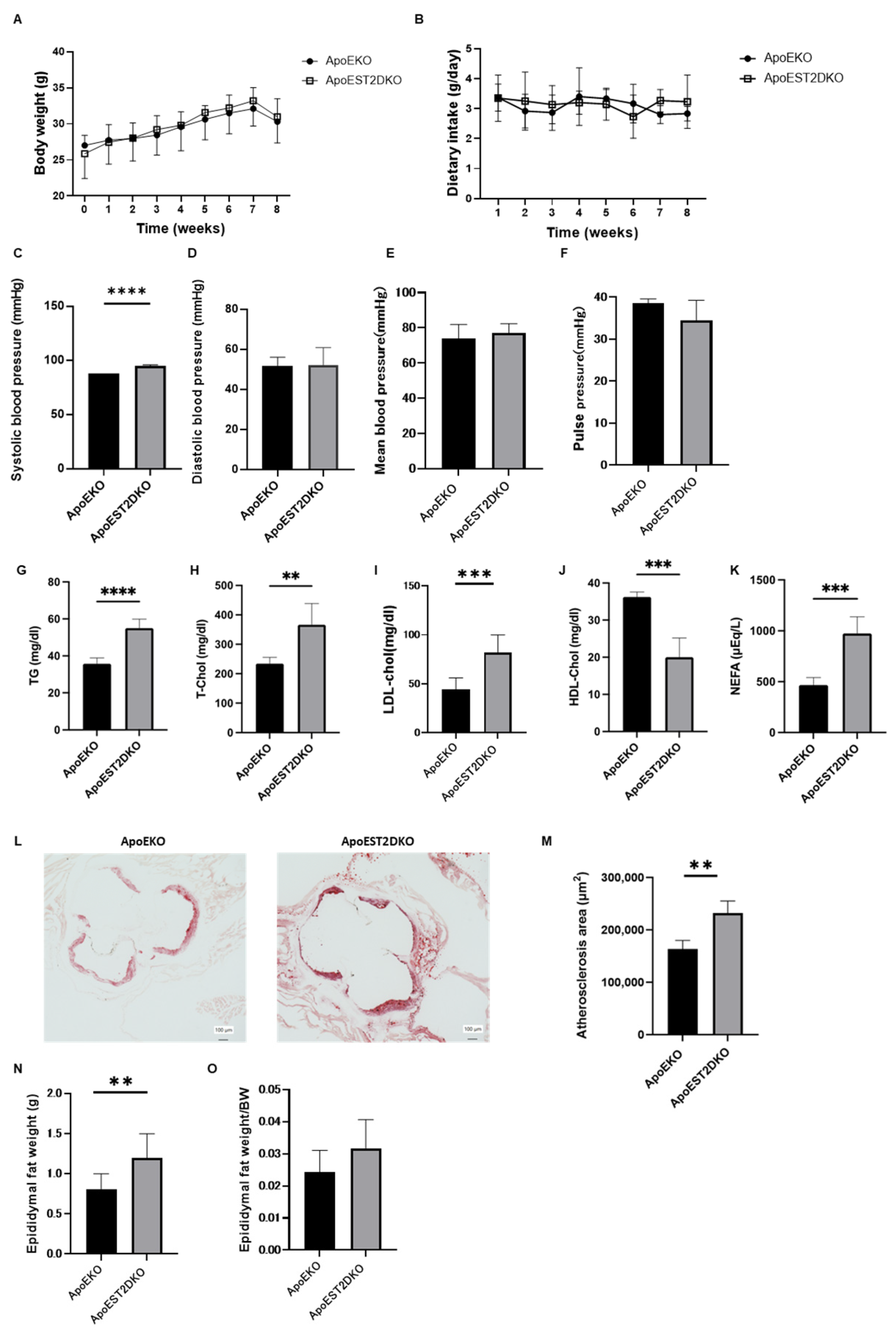
3.1. Comparison of Body Weight and Serum Lipid Levels between ApoEKO and ApoEST2DKO Mice
3.2. Comparison of Atherosclerotic Foci in the Aortic Rings of ApoEKO and ApoEST2DKO Mice Treated with an HFHSD
3.3. Comparison of Visceral Fat Mass Weight in ApoEKO and ApoEST2DKO Mice Treated with an HFHSD
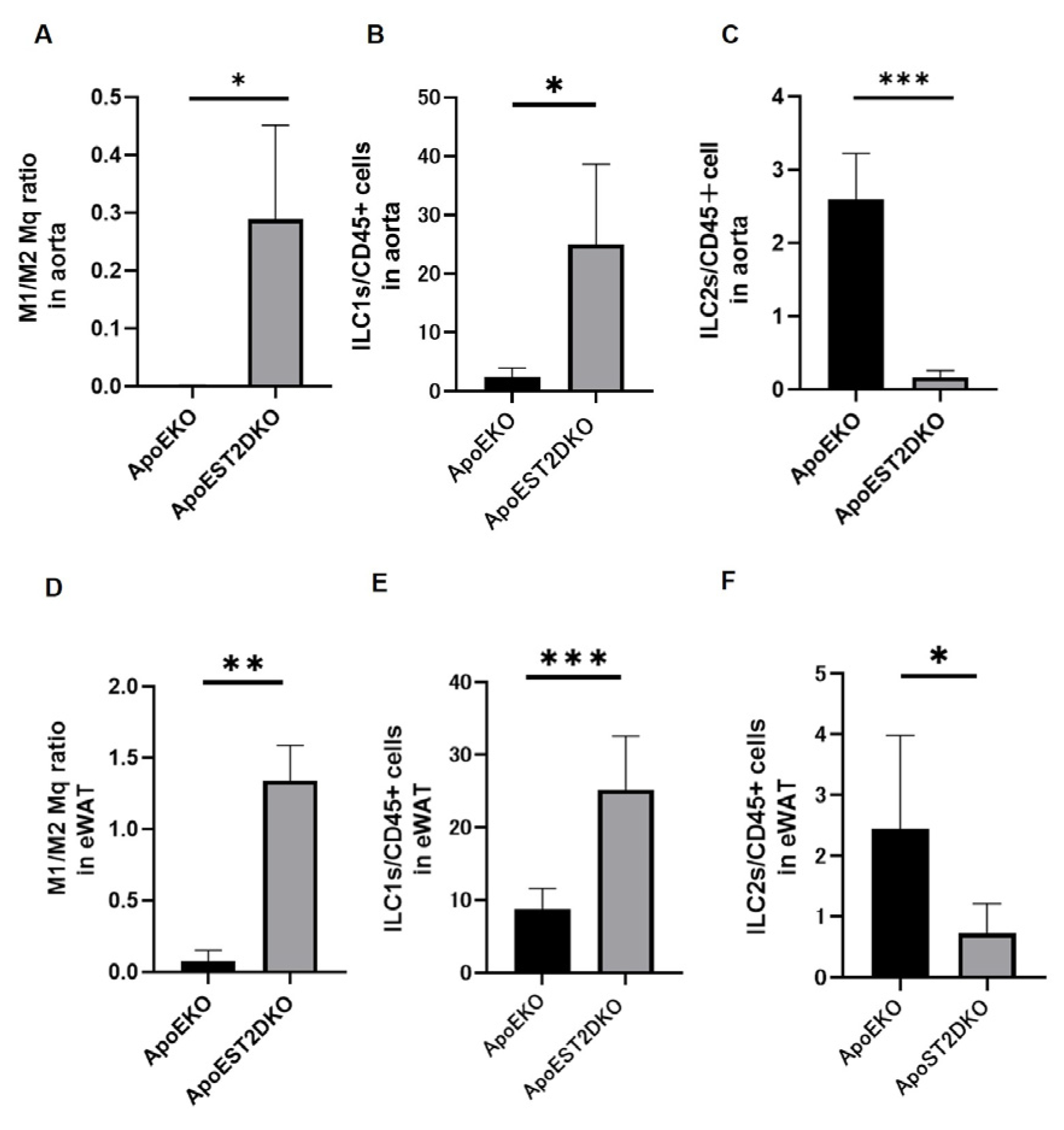
3.4. Aortic Inflammation and Anti-Inflammatory Cell Populations
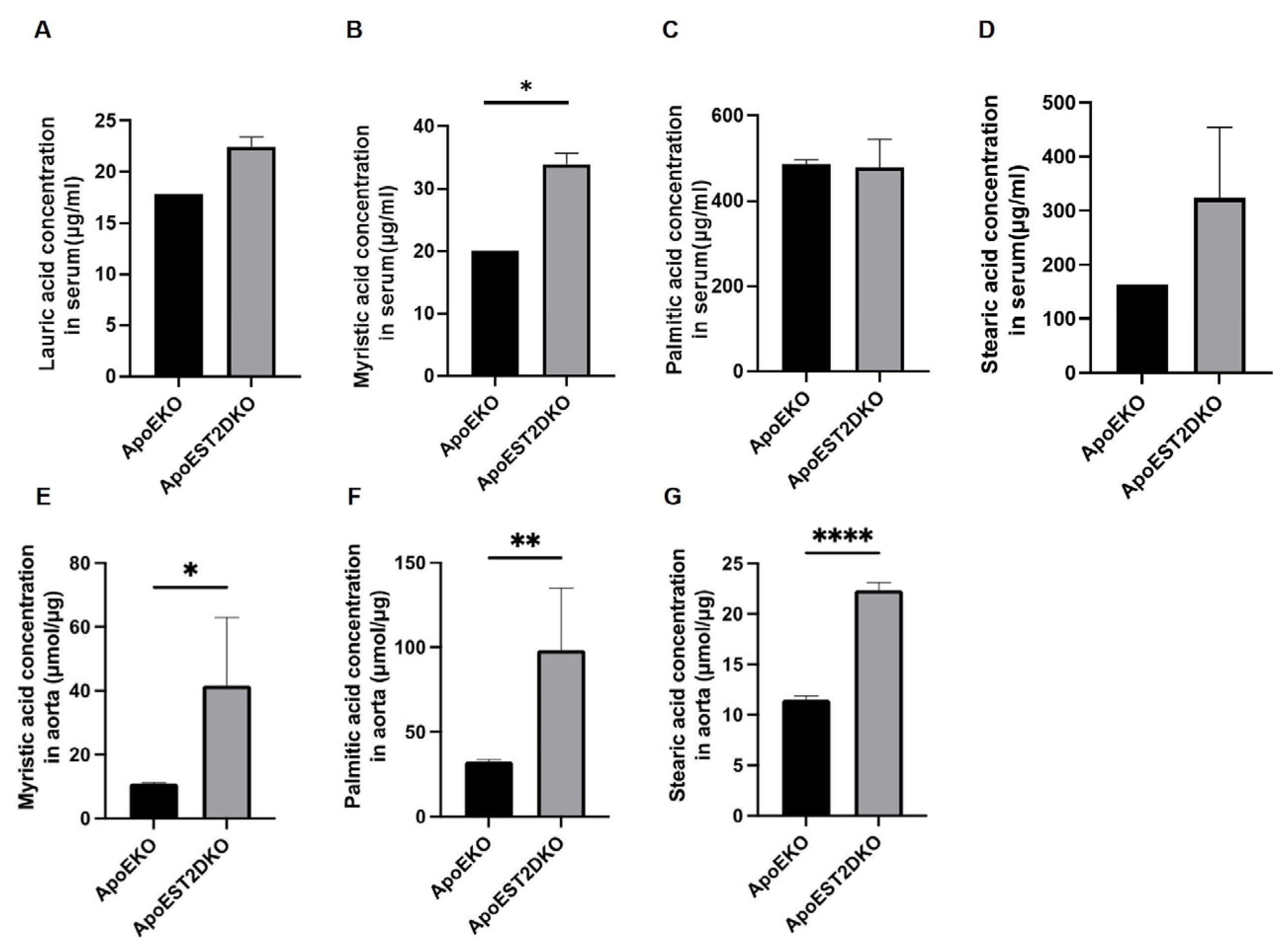
3.5. Saturated Fatty Acids Present in the Aorta and Serum
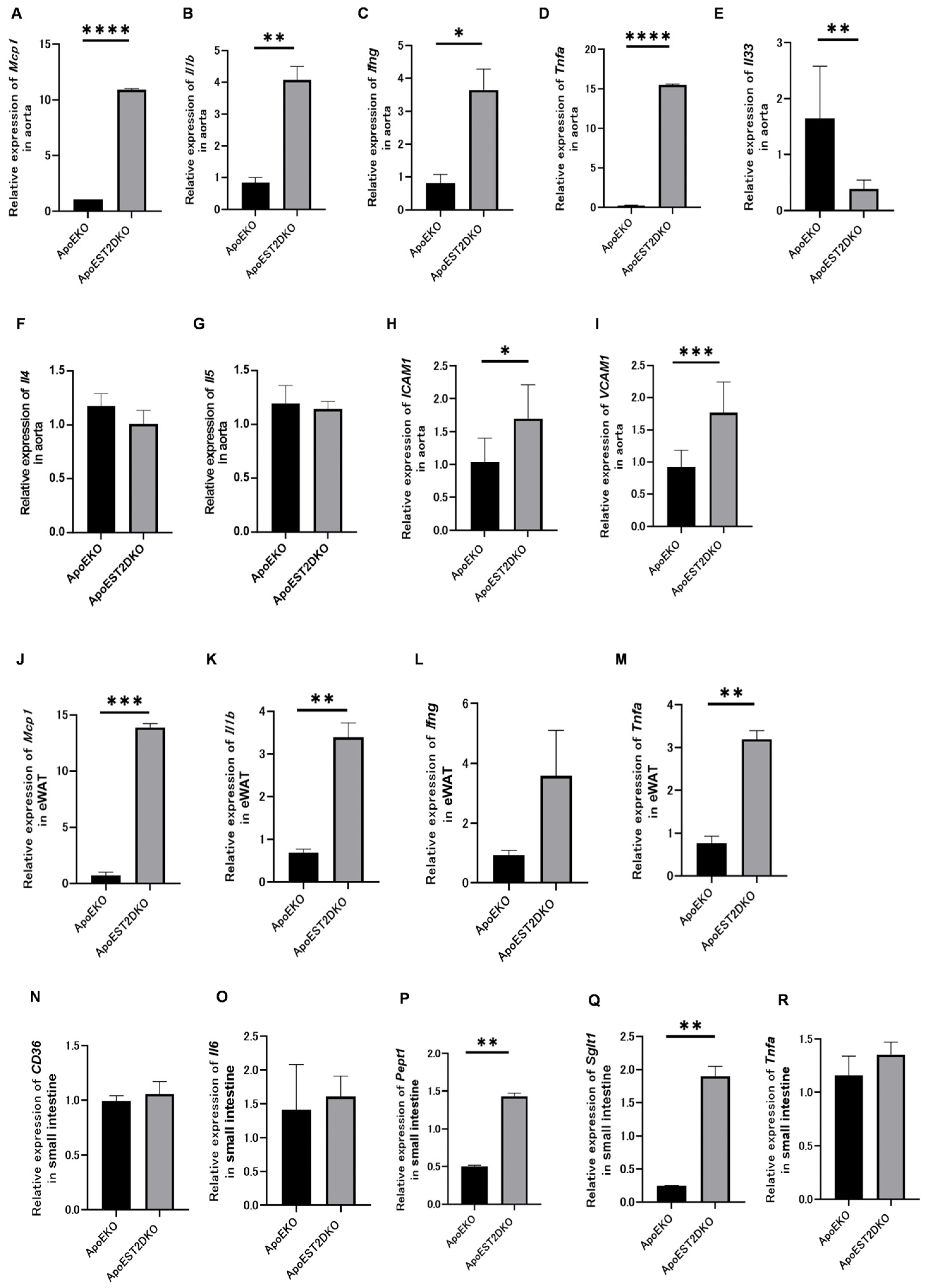
3.6. Expression of Genes Related to Inflammatory and Anti-Inflammatory Cytokines and Cell Adhesion Factors
3.7. eWAT Expression of Inflammatory Cytokine-Related Genes
3.8. Expression of Fatty Acid Transporters and Genes Related to Protein Metabolism, Sugar Metabolism, and Inflammatory Cytokines in the Jejunum
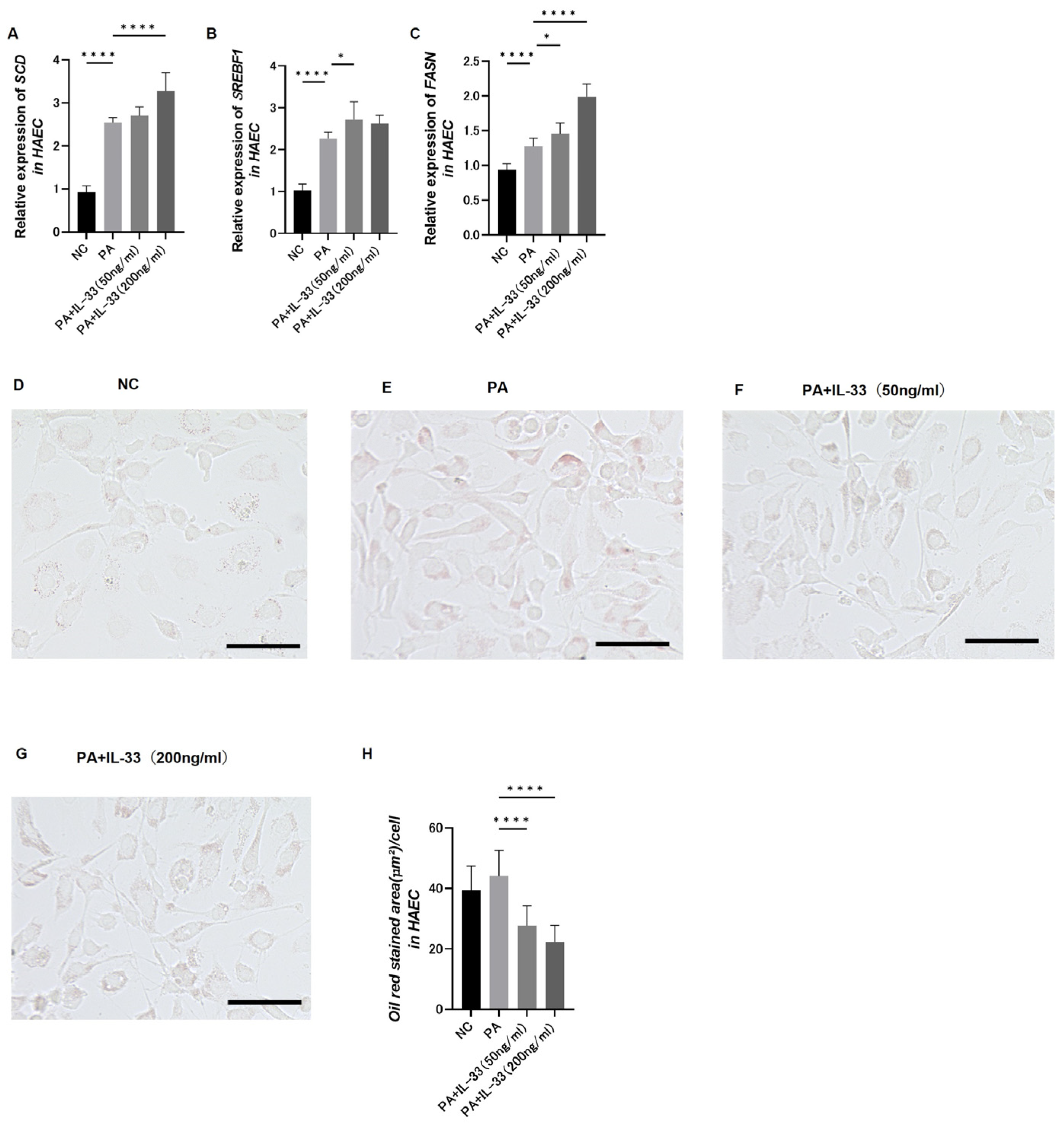
3.9. Evaluation of Fatty Acid Metabolism in Atherosclerosis Using Primary Normal HAECs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ross, R. Atherosclerosis—An inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Vozenilek, A.E.; Navratil, A.R.; Green, J.M.; Coleman, D.T.; Blackburn, C.M.; Finney, A.C.; Pearson, B.H.; Chrast, R.; Finck, B.N.; Klein, R.L.; et al. Macrophage-associated Lipin-1 enzymatic activity contributes to modified low-density lipoprotein-induced proinflammatory signaling and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Kanter, J.E.; Kramer, F.; Barnhart, S.; Averill, M.M.; Vivekanandan-Giri, A.; Vickery, T.; Li, L.O.; Becker, L.; Yuan, W.; Chait, A.; et al. Diabetes promotes an inflammatory macrophage phenotype and atherosclerosis through acyl-CoA synthetase 1. Proc. Natl. Acad. Sci. USA 2012, 109, E715–E724. [Google Scholar] [CrossRef]
- Brestoff, J.R.; Kim, B.S.; Saenz, S.A.; Stine, R.R.; Monticelli, L.A.; Sonnenberg, G.F.; Thome, J.J.; Farber, D.L.; Lutfy, K.; Seale, P.; et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature 2015, 519, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Painter, J.D.; Akbari, O. Type 2 innate lymphoid cells: Protectors in Type 2 diabetes. Front. Immunol. 2021, 12, 727008. [Google Scholar] [CrossRef] [PubMed]
- Boonpiyathad, T.; Sözener, Z.C.; Satitsuksanoa, P.; Akdis, C.A. Immunologic mechanisms in asthma. Semin. Immunol. 2019, 46, 101333. [Google Scholar] [CrossRef] [PubMed]
- Clottu, A.S.; Humbel, M.; Fluder, N.; Karampetsou, M.P.; Comte, D. Innate lymphoid cells in autoimmune diseases. Front. Immunol. 2021, 12, 789788. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, J.; Owyang, A.; Oldham, E.; Song, Y.; Murphy, E.; McClanahan, T.K.; Zurawski, G.; Moshrefi, M.; Qin, J.; Li, X.; et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 2005, 23, 479–490. [Google Scholar] [CrossRef]
- Mantani, P.T.; Dunér, P.; Ljungcrantz, I.; Nilsson, J.; Björkbacka, H.; Fredrikson, G.N. ILC2 transfers to apolipoprotein E deficient mice reduce the lipid content of atherosclerotic lesions. BMC Immunol. 2019, 20, 47. [Google Scholar] [CrossRef]
- Fernández-Gallego, N.; Castillo-González, R.; Méndez-Barbero, N.; López-Sanz, C.; Obeso, D.; Villaseñor, A.; Escribese, M.M.; López-Melgar, B.; Salamanca, J.; Benedicto-Buendía, A.; et al. The impact of type 2 immunity and allergic diseases in atherosclerosis. Allergy 2022, 77, 3249–3266. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.M.; Xu, D.; Asquith, D.L.; Denby, L.; Li, Y.; Sattar, N.; Baker, A.H.; McInnes, I.B.; Liew, F.Y. IL-33 reduces the development of atherosclerosis. J. Exp. Med. 2008, 205, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Okamura, T.; Hashimoto, Y.; Mori, J.; Yamaguchi, M.; Majima, S.; Senmaru, T.; Ushigome, E.; Nakanishi, N.; Asano, M.; Yamazaki, M.; et al. ILC2s improve glucose metabolism through the control of saturated fatty acid absorption within visceral fat. Front. Immunol. 2021, 12, 669629. [Google Scholar] [CrossRef]
- Buckley, M.L.; Williams, J.O.; Chan, Y.-H.; Laubertová, L.; Gallagher, H.; Moss, J.W.E.; Ramji, D.P. The Interleukin-33-Mediated Inhibition of Expression of Two Key Genes Implicated in Atherosclerosis in Human Macrophages Requires MAP Kinase, Phosphoinositide 3-Kinase and Nuclear Factor-ΚB Signaling Pathways. Sci. Rep. 2019, 9, 11317. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Schilperoort, M.; Cao, Y.; Shi, J.; Tabas, I.; Tao, W. Macrophage-targeted nanomedicine for the diagnosis and treatment of atherosclerosis. Nat. Rev. Cardiol. 2022, 19, 228–249. [Google Scholar] [CrossRef] [PubMed]
- Kirihara, Y.; Takechi, M.; Kurosaki, K.; Kobayashi, Y.; Kurosawa, T. Anesthetic effects of a mixture of medetomidine, midazolam and butorphanol in two strains of mice. Exp. Anim. 2013, 62, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Wilde, E.; Aubdool, A.A.; Thakore, P.; Baldissera, L., Jr.; Alawi, K.M.; Keeble, J.; Nandi, M.; Brain, S.D. Tail-Cuff Technique and Its Influence on Central Blood Pressure in the Mouse. J. Am. Heart Assoc. 2017, 6, e005204. [Google Scholar] [CrossRef]
- Rehman, S.; Hashmi, M.F.; Nelson, V.L. Blood pressure measurement. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK482189/ (accessed on 28 December 2022).
- Yuan, Z.; Kishimoto, C.; Sano, H.; Shioji, K.; Xu, Y.; Yokode, M. Immunoglobulin treatment suppresses atherosclerosis in apolipoprotein E-deficient mice via the Fc portion. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H899–H906. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Yin, C.; Mohanta, S.; Weber, C.; Habenicht, A. Preparation of Single Cell Suspensions from Mouse Aorta. Bio-Protocol 2016, 6, e1832. [Google Scholar] [CrossRef] [PubMed]
- Molofsky, A.B.; Nussbaum, J.C.; Liang, H.E.; Dyken, S.J.V.; Cheng, L.E.; Mohapatra, A.; Chawla, A.; Locksley, R.M. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J. Exp. Med. 2013, 210, 535–549. [Google Scholar] [CrossRef]
- Wang, S.; Li, J.; Wu, S.; Cheng, L.; Shen, Y.; Ma, W.; She, W.; Yang, C.; Wang, J.; Jiang, W. Type 3 innate lymphoid cell: A new player in liver fibrosis progression. Clin. Sci. 2018, 132, 2565–2582. [Google Scholar] [CrossRef]
- Ono, Y.; Nagai, M.; Yoshino, O.; Koga, K.; Nawaz, A.; Hatta, H.; Nishizono, H.; Izumi, G.; Nakashima, A.; Imura, J.; et al. CD11c+ M1-like macrophages (MΦs) but not CD206+ M2-like MΦ are involved in folliculogenesis in mice ovary. Sci. Rep. 2018, 8, 8171. [Google Scholar] [CrossRef]
- Okamura, T.; Hamaguchi, M.; Bamba, R.; Nakajima, H.; Yoshimura, Y.; Kimura, T.; Nishida, K.; Hashimoto, Y.; Fukuda, T.; Senmaru, T.; et al. Immune modulating effects of additional supplementation of estradiol combined with testosterone in murine testosterone-deficient NAFLD model. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, G989–G999. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.Q.; Xiu, C.K.; Yang, J.; Fang, J.Y.; Lei, Y. Ginseng-Sanqi-Chuanxiong (GSC) extracts ameliorate diabetes-induced endothelial cell senescence through regulating mitophagy via the AMPK pathway. Oxid. Med. Cell. Longev. 2020, 2020, 7151946. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Cai, Y. Concurrent exercise improves insulin resistance and nonalcoholic fatty liver disease by upregulating PPAR-γ and genes involved in the beta-oxidation of fatty acids in ApoE-KO mice fed a high-fat diet. Lipids Health Dis. 2019, 18, 6. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Clemente, M.; Ferré, N.; González-Périz, A.; López-Parra, M.; Horrillo, R.; Titos, E.; Morán-Salvador, E.; Miquel, R.; Arroyo, V.; Funk, C.D.; et al. 5-lipoxygenase deficiency reduces hepatic inflammation and tumor necrosis factor alpha-induced hepatocyte damage in hyperlipidemia-prone ApoE-null mice. Hepatology 2010, 51, 817–827. [Google Scholar] [CrossRef] [PubMed]
- Schierwagen, R.; Maybüchen, L.; Zimmer, S.; Hittatiya, K.; Bäck, C.; Klein, S.; Uschner, F.E.; Reul, W.; Boor, P.; Nickenig, G.; et al. Seven weeks of Western diet in apolipoprotein-E-deficient mice induce metabolic syndrome and non-alcoholic steatohepatitis with liver fibrosis. Sci. Rep. 2015, 5, 12931. [Google Scholar] [CrossRef] [PubMed]
- Pusceddu, I.; Dieplinger, B.; Mueller, T. ST2 and the ST2/IL-33 signalling pathway-biochemistry and pathophysiology in animal models and humans. Clin. Chim. Acta 2019, 495, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, W.; Shah, P.K.; Song, L.; Yang, M.; Sharifi, B.G. Deletion of tenascin-C gene exacerbates atherosclerosis and induces intraplaque hemorrhage in Apo-E-deficient mice. Cardiovasc. Pathol. 2012, 21, 398–413. [Google Scholar] [CrossRef] [PubMed]
- Weinstock, A.; Rahman, K.; Yaacov, O.; Nishi, H.; Menon, P.; Nikain, C.A.; Garabedian, M.L.; Pena, S.; Akbar, N.; Sansbury, B.E.; et al. Wnt signaling enhances macrophage responses to IL-4 and promotes resolution of atherosclerosis. eLife 2021, 10, e67932. [Google Scholar] [CrossRef]
- Binder, C.J.; Hartvigsen, K.; Chang, M.K.; Miller, M.; Broide, D.; Palinski, W.; Curtiss, L.K.; Corr, M.; Witztum, J.L. IL-5 links adaptive and natural immunity specific for epitopes of oxidized LDL and protects from atherosclerosis. J. Clin. Investig. 2004, 114, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Lin, Y.; Wu, Y.; Zhou, J.; Cao, L.; Chen, J.; Li, Y.; Tan, N.; Zhong, S. Bacteroides fragilis supplementation deteriorated metabolic dysfunction, inflammation, and aorta atherosclerosis by inducing gut microbiota dysbiosis in animal model. Nutrients 2022, 14, 2199. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wu, D.; Zeng, W.; Chen, Y.; Guo, M.; Lu, B.; Li, H.; Sun, C.; Yang, L.; Jiang, X.; et al. The role of intestinal dysbacteriosis-induced arachidonic acid metabolism disorder in inflammaging in atherosclerosis. Front. Cell. Infect. Microbiol. 2021, 11, 618265. [Google Scholar] [CrossRef] [PubMed]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A relevant marker of gut dysbiosis in obese patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Febbraio, M.; Reddy, S.P.; Yu, D.Y.; Yamamoto, M.; Silverstein, R.L. CD36 participates in a signaling pathway that regulates ROS formation in murine VSMCs. J. Clin. Investig. 2010, 120, 3996–4006. [Google Scholar] [CrossRef] [PubMed]
- Kiyan, Y.; Tkachuk, S.; Hilfiker-Kleiner, D.; Haller, H.; Fuhrman, B.; Dumler, I. oxLDL induces inflammatory responses in vascular smooth muscle cells via urokinase receptor association with CD36 and TLR4. J. Mol. Cell. Cardiol. 2014, 66, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, J.; Cui, W.; Silverstein, R.L. CD36, a signaling receptor and fatty acid transporter that regulates immune cell metabolism and fate. J. Exp. Med. 2022, 219, e20211314. [Google Scholar] [CrossRef] [PubMed]
- Satoh, N. The influence of saturated fatty acid and unsaturated fatty acid on obesity and atherosclerosis. Oleoscience 2010, 10, 365–370. [Google Scholar] [CrossRef]
- Hooper, L.; Martin, N.; Abdelhamid, A.; Davey Smith, G. Reduction in saturated fat intake for cardiovascular disease. Cochrane Database Syst. Rev. 2020, 5, CD011737. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.; Williams, P.T.; Krauss, R.M. Effects of a very high saturated fat diet on LDL particles in adults with atherogenic dyslipidemia: A randomized controlled trial. PLoS ONE 2017, 12, e0170664. [Google Scholar] [CrossRef]
- Brandsma, E.; Kloosterhuis, N.J.; Koster, M.; Dekker, D.C.; Gijbels, M.J.J.; van der Velden, S.V.D.; Ríos-Morales, M.; Van Faassen, M.J.; Loreti, M.G.; De Bruin, A.; et al. A proinflammatory gut microbiota increases systemic inflammation and accelerates atherosclerosis. Circ. Res. 2019, 124, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.M.; Liew, F.Y. The IL-33/ST2 pathway—A new therapeutic target in cardiovascular disease. Pharmacol. Ther. 2011, 131, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Ascenzi, F.; De Vitis, C.D.; Maugeri-Saccà, M.; Napoli, C.; Ciliberto, G.; Mancini, R. SCD1, autophagy and cancer: Implications for therapy. J. Exp. Clin. Cancer Res. 2021, 40, 265. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Park, J.S.; Lee, Y.S.; Han, J.; Lee, D.K.; Kwon, S.W.; Han, D.H.; Lee, Y.H.; Bae, S.H. SQSTM1/p62 activates NFE2L2/NRF2 via ULK1-mediated autophagic KEAP1 degradation and protects mouse liver from lipotoxicity. Autophagy 2020, 16, 1949–1973. [Google Scholar] [CrossRef] [PubMed]
- Hannah, V.C.; Ou, J.; Luong, A.; Goldstein, J.L.; Brown, M.S. Unsaturated fatty acids down-regulate srebp isoforms 1a and 1c by two mechanisms in HEK-293 cells. J. Biol. Chem. 2001, 276, 4365–4372. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hosomi, Y.; Okamura, T.; Sakai, K.; Yuge, H.; Yoshimura, T.; Majima, S.; Okada, H.; Senmaru, T.; Ushigome, E.; Nakanishi, N.; et al. IL-33 Reduces Saturated Fatty Acid Accumulation in Mouse Atherosclerotic Foci. Nutrients 2024, 16, 1195. https://doi.org/10.3390/nu16081195
Hosomi Y, Okamura T, Sakai K, Yuge H, Yoshimura T, Majima S, Okada H, Senmaru T, Ushigome E, Nakanishi N, et al. IL-33 Reduces Saturated Fatty Acid Accumulation in Mouse Atherosclerotic Foci. Nutrients. 2024; 16(8):1195. https://doi.org/10.3390/nu16081195
Chicago/Turabian StyleHosomi, Yukako, Takuro Okamura, Kimiko Sakai, Hiroki Yuge, Takashi Yoshimura, Saori Majima, Hiroshi Okada, Takafumi Senmaru, Emi Ushigome, Naoko Nakanishi, and et al. 2024. "IL-33 Reduces Saturated Fatty Acid Accumulation in Mouse Atherosclerotic Foci" Nutrients 16, no. 8: 1195. https://doi.org/10.3390/nu16081195
APA StyleHosomi, Y., Okamura, T., Sakai, K., Yuge, H., Yoshimura, T., Majima, S., Okada, H., Senmaru, T., Ushigome, E., Nakanishi, N., Satoh, T., Akira, S., Hamaguchi, M., & Fukui, M. (2024). IL-33 Reduces Saturated Fatty Acid Accumulation in Mouse Atherosclerotic Foci. Nutrients, 16(8), 1195. https://doi.org/10.3390/nu16081195








