Effects of Lactobacillus salivarius ssp. salicinius SA-03 Supplementation on Reversing Phthalate-Induced Asthma in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Measurement of Animal Body Weight and Dietary Intake
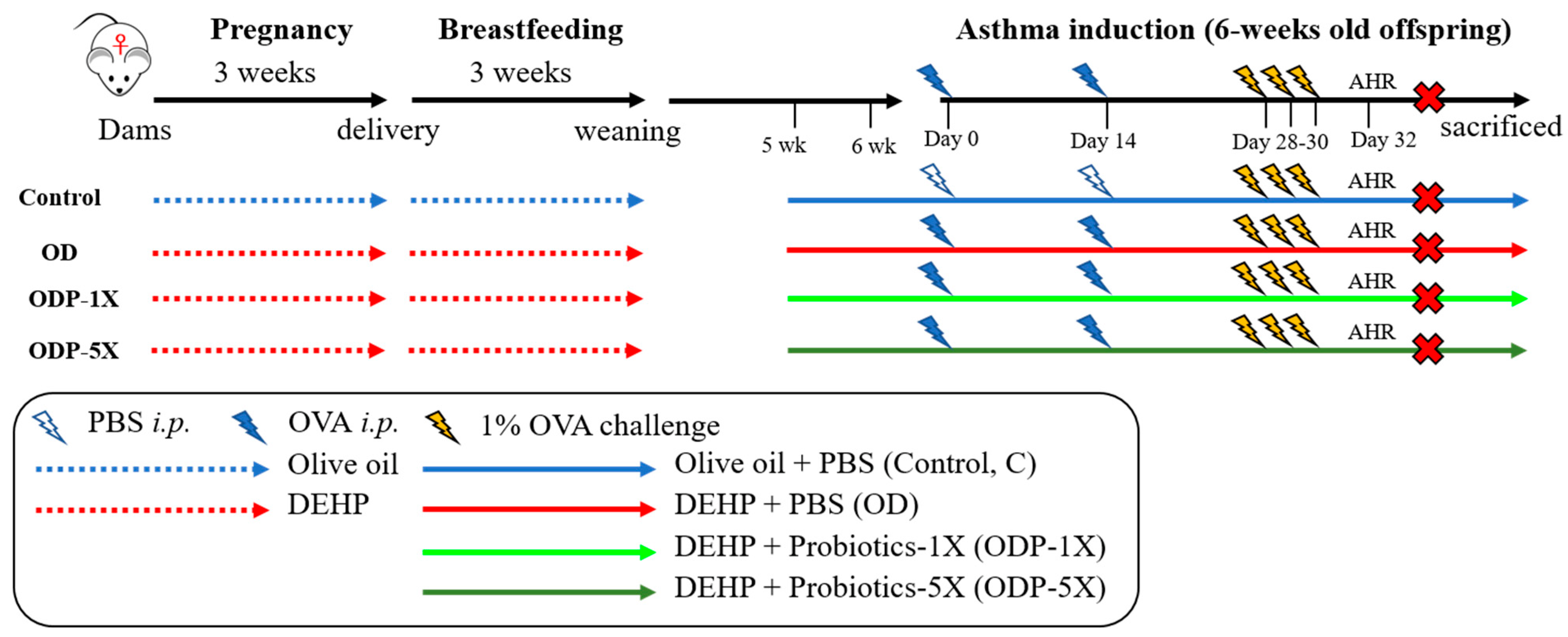
2.3. Establishment of the DEHP Causes of Allergic Asthma in the Pediatric Animal Model
2.4. Sample Preparation
2.5. Asthmatic Animal Model
2.6. Group of Experimental Animals
2.7. Airway Hyper-Responsiveness (AHR)
2.8. Hematology Analysis
2.9. Serum OVA-Specific IgE and IgG1 Measurements
2.10. Collection of Bronchoalveolar Lavage Fluid (BALF)
2.11. Measurement of Cytokines
2.12. Eosinophil Smear
2.13. Flow Cytometry Analysis
2.14. Histopathological Evaluation
2.15. Statistical Analysis
3. Results
3.1. Body Weight and Dietary Intake
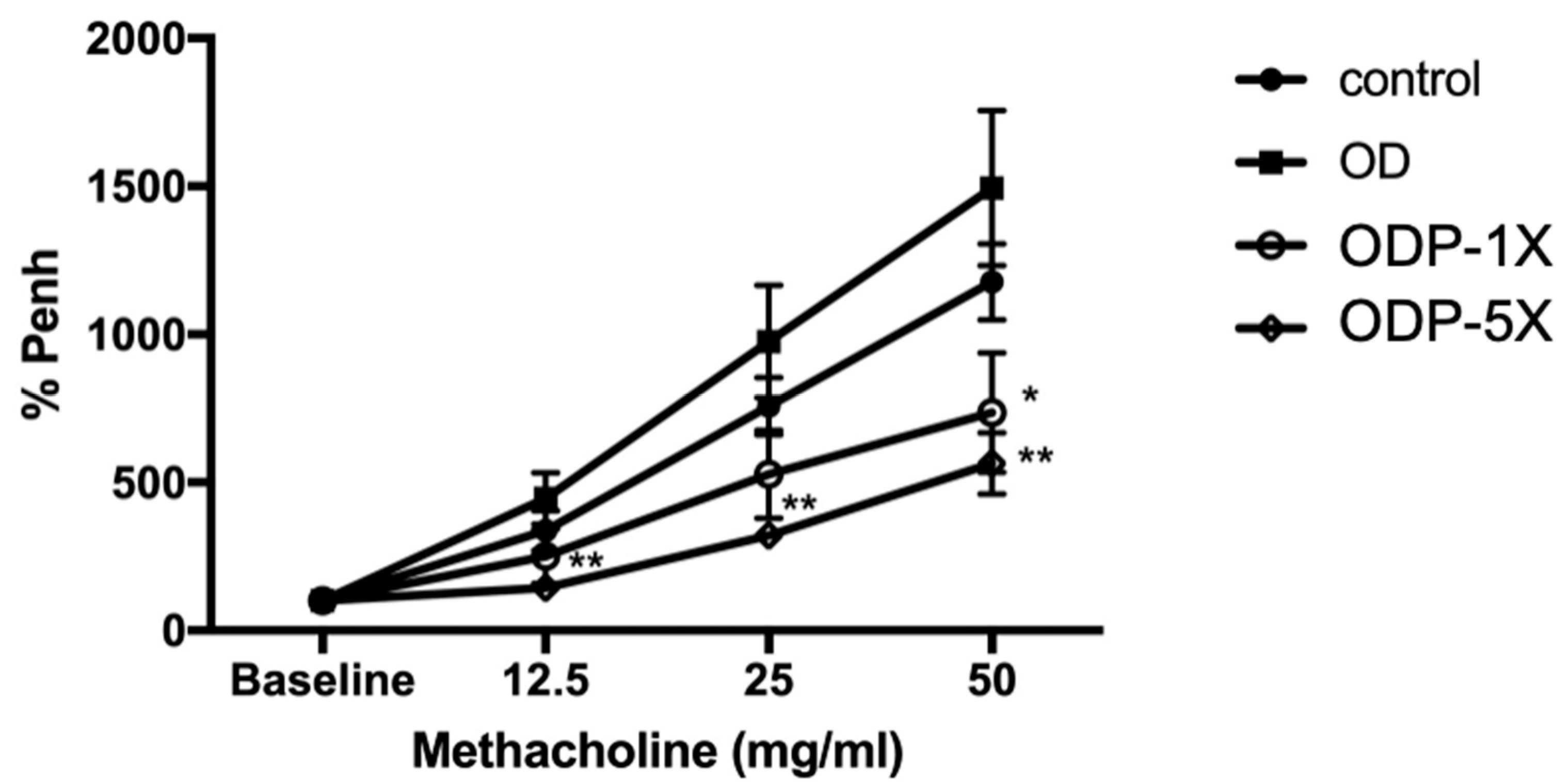
3.2. Lactobacillus salivarius ssp. salicinius SA-03 Reduces the Airway Hypersensitivity Caused by OVA/DEHP
3.3. Lactobacillus salivarius ssp. salicinius SA-03 Reduces the OVA-Specific IgE Level in Serum and OVA-Specific IgG1 Level in Lung Lavage Fluid
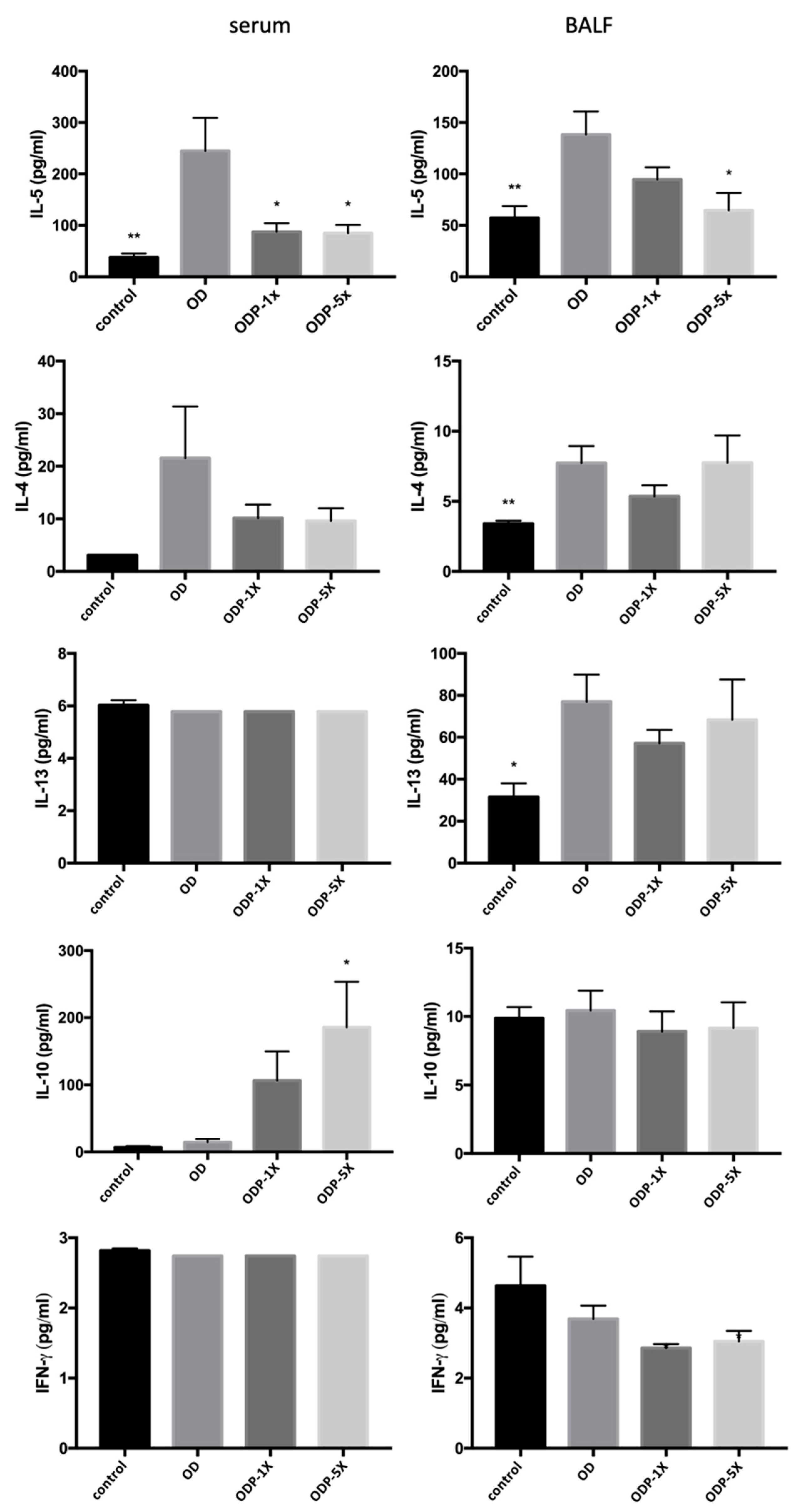
3.4. Effects of DEHP and Lactobacillus salivarius ssp. salicinius SA-03 on Asthma-Related Cytokines in Serum and BALF
3.5. Effects of DEHP and Lactobacillus salivarius ssp. salicinius SA-03 on Asthma-Related Eosinophils in Blood and BALF
3.6. Effects of Plasticizers and Lactobacillus salivarius ssp. salicinius SA-03 on Various Immune Cells
3.7. Lactobacillus salivarius ssp. salicinius SA-03 Improves OVA/DEHP-Induced Lung Tissue Lesions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, C.F.; Wang, I.J. Changes in Urinary Phthalate Metabolite Levels before and after the Phthalate Contamination Event and Identification of Exposure Sources in a Cohort of Taiwanese Children. Int. J. Environ. Res. Public Health 2017, 14, 935. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.J.; Lin, C.C.; Lin, Y.J.; Hsieh, W.S.; Chen, P.C. Early life phthalate exposure and atopic disorders in children: A prospective birth cohort study. Environ. Int. 2014, 62, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.J.; Karmaus, W.J.; Chen, S.L.; Holloway, J.W.; Ewart, S. Effects of phthalate exposure on asthma may be mediated through alterations in DNA methylation. Clin. Epigenetics 2015, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.J.; Karmaus, W.J. Oxidative Stress-Related Genetic Variants May Modify Associations of Phthalate Exposures with Asthma. Int. J. Environ. Res. Public. Health 2017, 14, 162. [Google Scholar] [CrossRef] [PubMed]
- Bølling, A.K.; Sripada, K.; Becher, R.; Bekö, G. Phthalate exposure and allergic diseases: Review of epidemiological and experimental evidence. Environ. Int. 2020, 139, 105706. [Google Scholar] [CrossRef] [PubMed]
- Nuzzi, G.; Di Cicco, M.E.; Peroni, D.G. Breastfeeding and Allergic Diseases: What’s New? Children 2021, 8, 330. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, F.; Dong, J.; You, M.; Fu, Y.; Li, C.; Lu, Y.; Chen, J. Maternal exposure to environmental DEHP exacerbated OVA-induced asthmatic responses in rat offspring. Sci. Total Environ. 2018, 615, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Santamarina, A.; Gonzalez, E.G.; Lamas, A.; Mondragon, A.D.C.; Regal, P.; Miranda, J.M. Probiotics as a Possible Strategy for the Prevention and Treatment of Allergies. A Narrative Review. Foods 2021, 10, 701. [Google Scholar] [CrossRef] [PubMed]
- Spacova, I.; Van Beeck, W.; Seys, S.; Devos, F.; Vanoirbeek, J.; Vanderleyden, J.; Ceuppens, J.; Petrova, M.; Lebeer, S. Lactobacillus rhamnosus probiotic prevents airway function deterioration and promotes gut microbiome resilience in a murine asthma model. Gut Microbes 2020, 11, 1729–1744. [Google Scholar] [CrossRef]
- Lan, H.; Gui, Z.; Zeng, Z.; Li, D.; Qian, B.; Qin, L.Y.; Dai, L.; Song, J.L. Oral administration of Lactobacillus plantarum CQPC11 attenuated the airway inflammation in an ovalbumin (OVA)-induced Balb/c mouse model of asthma. J. Food Biochem. 2022, 46, e14036. [Google Scholar] [CrossRef]
- Mennini, M.; Dahdah, L.; Artesani, M.C.; Fiocchi, A.; Martelli, A. Probiotics in Asthma and Allergy Prevention. Front. Pediatr. 2017, 5, 165. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Song, X.; Wang, G.; Xia, Y.; Xiong, Z.; Ai, L. Understanding Ligilactobacillus salivarius from Probiotic Properties to Omics Technology: A Review. Foods 2024, 13, 895. [Google Scholar] [CrossRef] [PubMed]
- Messaoudi, S.; Manai, M.; Kergourlay, G.; Prévost, H.; Connil, N.; Chobert, J.M.; Dousset, X. Lactobacillus salivarius: Bacteriocin and probiotic activity. Food Microbiol. 2013, 36, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Drago, L.; De Vecchi, E.; Gabrieli, A.; De Grandi, R.; Toscano, M. Immunomodulatory Effects of Lactobacillus salivarius LS01 and Bifidobacterium breve BR03, Alone and in Combination, on Peripheral Blood Mononuclear Cells of Allergic Asthmatics. Allergy Asthma Immunol. Res. 2015, 7, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Dec, M.; Stępień-Pyśniak, D.; Puchalski, A.; Hauschild, T.; Pietras-Ożga, D.; Ignaciuk, S.; Urban-Chmiel, R. Biodiversity of Ligilactobacillus salivarius Strains from Poultry and Domestic Pigeons. Animals 2021, 11, 972. [Google Scholar] [CrossRef]
- Neville, B.A.; O’Toole, P.W. Probiotic properties of Lactobacillus salivarius and closely related Lactobacillus species. Future Microbiol. 2010, 5, 759–774. [Google Scholar]
- Krupa-Kotara, K.; Gwioździk, W.; Nandzik, S.; Grajek, M. The Role of Microbiota Pattern in Anxiety and Stress Disorders—A Review of the State of Knowledge. Psych 2023, 5, 602–618. [Google Scholar] [CrossRef]
- Sohail, M.U.; Hedin, L.; Al-Asmakh, M. Dysbiosis of the Salivary Microbiome is Associated with Hypertension and Correlated with Metabolic Syndrome Biomarkers. Diabetes Metab. Syndr. Obes. 2021, 14, 4641–4653. [Google Scholar] [CrossRef]
- Jahreis, S.; Trump, S.; Bauer, M.; Bauer, T.; Thürmann, L.; Feltens, R.; Wang, Q.; Gu, L.; Grützmann, K.; Röder, S.; et al. Maternal phthalate exposure promotes allergic airway inflammation over 2 generations through epigenetic modifications. J. Allergy Clin. Immunol. 2018, 141, 741–753. [Google Scholar] [CrossRef]
- Lee, M.C.; Hsu, Y.J.; Ho, H.H.; Hsieh, S.H.; Kuo, Y.W.; Sung, H.C.; Huang, C.C. Lactobacillus salivarius Subspecies salicinius SA-03 is a New Probiotic Capable of Enhancing Exercise Performance and Decreasing Fatigue. Microorganisms 2020, 8, 545. [Google Scholar] [CrossRef]
- Paveljšek, D.; Juvan, P.; Košir, R.; Rozman, D.; Hacin, B.; Ivičak-Kocjan, K.; Rogelj, I. Lactobacillus fermentum L930BB and Bifidobacterium animalis subsp. animalis IM386 initiate signalling pathways involved in intestinal epithelial barrier protection. Benef. Microbes 2018, 9, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Wittke, A.; Weaver, V.; Mahon, B.D.; August, A.; Cantorna, M.T. Vitamin D receptor-deficient mice fail to develop experimental allergic asthma. J. Immun. 2004, 173, 3432–3436. [Google Scholar] [CrossRef]
- Li, C.Y.; Lin, H.C.; Hsueh, K.C.; Wu, S.F.; Fang, S.H. Oral administration of Lactobacillus salivarius inhibits the allergic airway response in mice. Can. J. Microbiol. 2010, 56, 373–379. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kay, A.B.; Ying, S.; Durham, S.R. Phenotype of cells positive for interleukin-4 and interleukin-5 mRNA in allergic tissue reactions. Int. Arch. Allergy Immunol. 1995, 107, 208–210. [Google Scholar] [CrossRef] [PubMed]
- Borish, L.; Aarons, A.; Rumbyrt, J.; Cvietusa, P.; Negri, J.; Wenzel, S. Interleukin-10 regulation in normal subjects and patients with asthma. J. Allergy Clin. Immunol. 1996, 97, 1288–1296. [Google Scholar] [CrossRef] [PubMed]
- Hawrylowicz, C.M.; O’Garra, A. Potential role of interleukin-10-secreting regulatory T cells in allergy and asthma. Nat. Rev. Immunol. 2005, 5, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Greenfeder, S.; Umland, S.P.; Cuss, F.M.; Chapman, R.W.; Egan, R.W. Th2 cytokines and asthma. The role of interleukin-5 in allergic eosinophilic disease. Respir. Res. 2001, 2, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Assa'ad, A.H.; Gupta, S.K.; Collins, M.H.; Thomson, M.; Heath, A.T.; Smith, D.A.; Perschy, T.L.; Jurgensen, C.H.; Ortega, H.G.; Aceves, S.S. An antibody against IL-5 reduces numbers of esophageal intraepithelial eosinophils in children with eosinophilic esophagitis. Gastroenterology 2011, 141, 1593–1604. [Google Scholar] [CrossRef]
- Eum, S.Y.; Hailé, S.; Lefort, J.; Huerre, M.; Vargaftig, B.B. Eosinophil recruitment into the respiratory epithelium following antigenic challenge in hyper-IgE mice is accompanied by interleukin 5-dependent bronchial hyperresponsiveness. Proc. Natl. Acad. Sci. USA 1995, 92, 12290–12294. [Google Scholar] [CrossRef]
- Tesciuba, A.G.; Subudhi, S.; Rother, R.P.; Faas, S.J.; Frantz, A.M.; Elliot, D.; Weinstock, J.; Matis, L.A.; Bluestone, J.A.; Sperling, A.I. Inducible costimulator regulates Th2-mediated inflammation, but not Th2 differentiation, in a model of allergic airway disease. J. Immunol. 2001, 167, 1996–2003. [Google Scholar] [CrossRef]
- Redmond, W.L.; Ruby, C.E.; Weinberg, A.D. The role of OX40-mediated co-stimulation in T-cell activation and survival. Crit. Rev. Immunol. 2009, 29, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Mäkelä, M.J.; Kanehiro, A.; Borish, L.; Dakhama, A.; Loader, J.; Joetham, A.; Xing, Z.; Jordana, M.; Larsen, G.L.; Gelfand, E.W. IL-10 is necessary for the expression of airway hyperresponsiveness but not pulmonary inflammation after allergic sensitization. Proc. Natl. Acad. Sci. USA 2000, 97, 6007–6012. [Google Scholar] [CrossRef] [PubMed]
- Kips, J.C. Cytokines in asthma. Eur. Respir. J. Suppl. 2001, 34, 24s–33s. [Google Scholar] [CrossRef] [PubMed]
- Bickert, T.; Trujillo-Vargas, C.M.; Duechs, M.; Wohlleben, G.; Polte, T.; Hansen, G.; Oelschlaeger, T.A.; Erb, K.J. Probiotic Escherichia coli Nissle 1917 suppresses allergen-induced Th2 responses in the airways. Int. Arch. Allergy Immunol. 2009, 149, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Nogales, A.; Algieri, F.; Garrido-Mesa, J.; Vezza, T.; Utrilla, M.P.; Chueca, N.; Fernández-Caballero, J.A.; García, F.; Rodríguez-Cabezas, M.E.; Gálvez, J. The Administration of Escherichia coli Nissle 1917 Ameliorates Development of DSS-Induced Colitis in Mice. Front. Pharmacol. 2018, 9, 468. [Google Scholar] [CrossRef] [PubMed]
- Yun, X.; Shang, Y.; Li, M. Effect of Lactobacillus salivarius on Th1/Th2 cytokines and the number of spleen CD4⁺ CD25⁺ Foxp3⁺ Treg in asthma Balb/c mouse. Int. J. Clin. Exp. Pathol. 2015, 8, 7661–7674. [Google Scholar] [PubMed]
- Thorsen, J.; Rasmussen, M.A.; Waage, J.; Mortensen, M.; Brejnrod, A.; Bønnelykke, K.; Chawes, B.L.; Brix, S.; Sørensen, S.J.; Stokholm, J.; et al. Infant airway microbiota and topical immune perturbations in the origins of childhood asthma. Nat. Commun. 2019, 10, 5001. [Google Scholar] [CrossRef]
- Teo, S.M.; Tang, H.H.F.; Mok, D.; Judd, L.M.; Watts, S.C.; Pham, K.; Holt, B.J.; Kusel, M.; Serralha, M.; Troy, N.; et al. Airway Microbiota Dynamics Uncover a Critical Window for Interplay of Pathogenic Bacteria and Allergy in Childhood Respiratory Disease. Cell Host Microbe 2018, 24, 341–352. [Google Scholar] [CrossRef]
- Chiu, C.J.; Huang, M.T. Asthma in the Precision Medicine Era: Biologics and Probiotics. Int. J. Mol. Sci. 2021, 22, 4528. [Google Scholar] [CrossRef]
- Zubeldia-Varela, E.; Barker-Tejeda, T.C.; Obeso, D.; Villaseñor, A.; Barber, D.; Pérez-Gordo, M. Microbiome and Allergy: New Insights and Perspectives. J. Investig. Allergol. Clin. Immunol. 2022, 32, 327–344. [Google Scholar] [CrossRef]
- Arrieta, M.C.; Stiemsma, L.T.; Dimitriu, P.A.; Thorson, L.; Russell, S.; Yurist-Doutsch, S.; Kuzeljevic, B.; Gold, M.J.; Britton, H.M.; Lefebvre, D.L.; et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci. Transl. Med. 2015, 7, 307ra152. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsson, T.R.; Jakobsson, H.E.; Andersson, A.F.; Björkstén, B.; Engstrand, L.; Jenmalm, M.C. Low gut microbiota diversity in early infancy precedes asthma at school age. Clin. Exp. Allergy 2014, 44, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Hufnagl, K.; Pali-Schöll, I.; Roth-Walter, F.; Jensen-Jarolim, E. Dysbiosis of the gut and lung microbiome has a role in asthma. Semin. Immunopathol. 2020, 42, 75–93. [Google Scholar] [CrossRef]
- Kim, Y.H.; Park, M.R.; Kim, S.Y.; Kim, M.Y.; Kim, K.W.; Sohn, M.H. Respiratory microbiome profiles are associated with distinct inflammatory phenotype and lung function in children with asthma. J. Investig. Allergol. Clin. Immunol. 2023. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, T.-J.; Huang, C.-C.; Lee, M.-C.; Lee, Y.-P.; Huang, W.-C.; Chuang, H.-L.; Wang, I.-J. Effects of Lactobacillus salivarius ssp. salicinius SA-03 Supplementation on Reversing Phthalate-Induced Asthma in Mice. Nutrients 2024, 16, 1160. https://doi.org/10.3390/nu16081160
Lin T-J, Huang C-C, Lee M-C, Lee Y-P, Huang W-C, Chuang H-L, Wang I-J. Effects of Lactobacillus salivarius ssp. salicinius SA-03 Supplementation on Reversing Phthalate-Induced Asthma in Mice. Nutrients. 2024; 16(8):1160. https://doi.org/10.3390/nu16081160
Chicago/Turabian StyleLin, Tien-Jen, Chi-Chang Huang, Mon-Chien Lee, Yen-Peng Lee, Wen-Chung Huang, Hsiao-Li Chuang, and I-Jen Wang. 2024. "Effects of Lactobacillus salivarius ssp. salicinius SA-03 Supplementation on Reversing Phthalate-Induced Asthma in Mice" Nutrients 16, no. 8: 1160. https://doi.org/10.3390/nu16081160
APA StyleLin, T.-J., Huang, C.-C., Lee, M.-C., Lee, Y.-P., Huang, W.-C., Chuang, H.-L., & Wang, I.-J. (2024). Effects of Lactobacillus salivarius ssp. salicinius SA-03 Supplementation on Reversing Phthalate-Induced Asthma in Mice. Nutrients, 16(8), 1160. https://doi.org/10.3390/nu16081160








