A 12-Week, Single-Centre, Randomised, Double-Blind, Placebo-Controlled, Parallel-Design Clinical Trial for the Evaluation of the Efficacy and Safety of Lactiplantibacillus plantarum SKO-001 in Reducing Body Fat
Abstract
1. Introduction
2. Materials and Methods
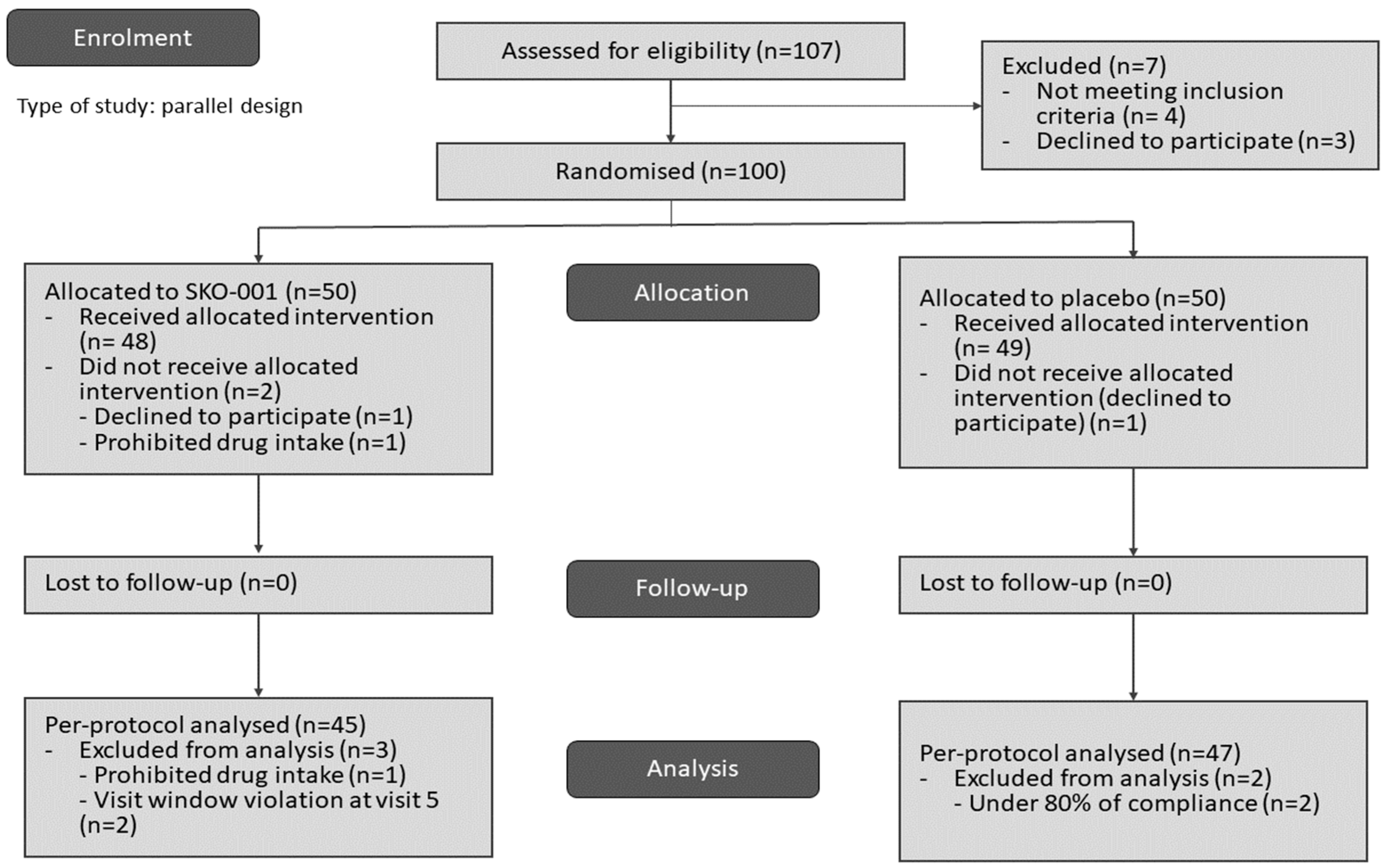
2.1. Study Design and Participants
2.2. Participant Selection Criteria
2.3. Contraindicated Drugs/Foods
2.4. Intervention
2.5. Randomisation and Blinding
2.6. Endpoints
2.7. Safety
2.8. Sample Size Calculation
2.9. Statistical Analyses
3. Results
3.1. Participant Characteristics
3.2. Study Endpoints
3.3. Safety
3.4. Physical Activity and Diet
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dai, H.; Alsalhe, T.A.; Chalghaf, N.; Riccò, M.; Bragazzi, N.L.; Wu, J. The global burden of disease attributable to high body mass index in 195 countries and territories, 1990-2017: An analysis of the Global Burden of Disease Study. PLoS Med. 2020, 17, e1003198. [Google Scholar] [CrossRef]
- Zeng, Q.; Li, D.; He, Y.; Li, Y.; Yang, Z.; Zhao, X.; Liu, Y.; Wang, Y.; Sun, J.; Feng, X.; et al. Discrepant gut microbiota markers for the classification of obesity-related metabolic abnormalities. Sci. Rep. 2019, 9, 13424. [Google Scholar] [CrossRef]
- Perna, S.; Ilyas, Z.; Giacosa, A.; Gasparri, C.; Peroni, G.; Faliva, M.A.; Rigon, C.; Naso, M.; Riva, A.; Petrangolini, G.; et al. Is probiotic supplementation useful for the management of body weight and other anthropometric measures in adults affected by overweight and obesity with metabolic related diseases? A systematic review and meta-analysis. Nutrients 2021, 13, 666. [Google Scholar] [CrossRef] [PubMed]
- Ilhan, Z.E.; DiBaise, J.K.; Dautel, S.E.; Isern, N.G.; Kim, Y.M.; Hoyt, D.W.; Schepmoes, A.A.; Brewer, H.M.; Weitz, K.K.; Metz, T.O.; et al. Temporospatial shifts in the human gut microbiome and metabolome after gastric bypass surgery. NPJ Biofilms Microbiomes 2020, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Florent, V.; Dennetiere, S.; Gaudrat, B.; Andrieux, S.; Mulliez, E.; Norberciak, L.; Jacquez, K. Prospective monitoring of small intestinal bacterial overgrowth after gastric bypass: Clinical, biological, and gas chromatographic aspects. Obes. Surg. 2024, 34, 947–958. [Google Scholar] [CrossRef]
- Zhou, Y.D.; Liang, F.X.; Tian, H.R.; Luo, D.; Wang, Y.Y.; Yang, S.R. Mechanisms of gut microbiota-immune-host interaction on glucose regulation in type 2 diabetes. Front. Microbiol. 2023, 14, 1121695. [Google Scholar] [CrossRef]
- Wiciński, M.; Gębalski, J.; Gołębiewski, J.; Malinowski, B. Probiotics for the treatment of overweight and obesity in humans—A review of clinical trials. Microorganisms 2020, 8, 1148. [Google Scholar] [CrossRef] [PubMed]
- Puebla-Barragan, S.; Reid, G. Probiotics in cosmetic and personal care products: Trends and challenges. Molecules 2021, 26, 1249. [Google Scholar] [CrossRef] [PubMed]
- Esteve, E.; Ricart, W.; Fernández-Real, J.M. Gut microbiota interactions with obesity, insulin resistance and type 2 diabetes. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 483–490. [Google Scholar] [CrossRef]
- Cox, T.O.; Lundgren, P.; Nath, K.; Thaiss, C.A. Metabolic control by the microbiome. Genome Med. 2022, 14, 80. [Google Scholar] [CrossRef] [PubMed]
- Mekkes, M.C.; Weenen, T.; Brummer, R.J.; Claassen, E. The development of probiotic treatment in obesity: A review. Benef. Microbes 2014, 5, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Fei, Y.; Han, X.; Liu, G.; Fang, J. Lactobacillus plantarum alleviates obesity by altering the composition of the gut microbiota in high-fat diet-fed mice. Front. Nutr. 2022, 9, 947367. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.J.; Yu, H.; Kim, J.I.; Seo, H.W.; Kim, J.G.; Kim, S.; Lee, H.S.; Cheon, H.G. Anti-obesity effects of Lactiplantibacillus plantarum SKO-001 in high-fat diet-induced obese mice. Eur. J. Nutr. 2023, 62, 1611–1622. [Google Scholar] [CrossRef]
- Sohn, M.; Na, G.Y.; Chu, J.; Joung, H.; Kim, B.K.; Lim, S. Efficacy and Safety of Lactobacillus plantarum K50 on Lipids in Koreans With Obesity: A Randomized, Double-Blind Controlled Clinical Trial. Front. Endocrinol. 2022, 12, 790046. [Google Scholar] [CrossRef]
- Mo, S.J.; Lee, K.; Hong, H.J.; Hong, D.K.; Jung, S.H.; Park, S.D.; Shim, J.J.; Lee, J.L. Effects of Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032 on Overweight and the Gut Microbiota in Humans: Randomized, Double-Blinded, Placebo-Controlled Clinical Trial. Nutrients 2022, 14, 2484. [Google Scholar] [CrossRef]
- Sohn, M.; Jung, H.; Lee, W.S.; Kim, T.H.; Lim, S. Effect of Lactobacillus plantarum LMT1-48 on Body Fat in Overweight Subjects: A Randomized, Double-Blind, Placebo-Controlled Trial. Diabetes Metab. J. 2023, 47, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-G.; Jung, J.; Kang, J.-H.; Kwon, J.S.; Yu, S.P.; Baik, T.G. Effect of a herbal extract powder (YY-312) from Imperata cylindrica Beauvois, Citrus unshiu Markovich, and Evodia officinalis Dode on body fat mass in overweight adults: A 12-week, randomized, double-blind, placebo-controlled, parallel-group clinical trial. BMC Complement. Altern. Med. 2017, 17, 275. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.K.; Haam, J.H.; Kim, B.T.; Kim, E.M.; Park, J.H.; Rhee, S.Y.; Jeon, E.; Kang, E.; Nam, G.E.; Koo, H.Y.; et al. Evaluation and treatment of obesity and its comorbidities: 2022 update of clinical practice guidelines for obesity by the Korean Society for the Study of Obesity. J. Obes. Metab. Syndr. 2023, 32, 1–24. [Google Scholar] [CrossRef]
- Ministry of Health and Welfare, South Korea: 2020 Community Integrated Health Promotion Project. Available online: https://www.mohw.go.kr/board.es?mid=a10107010100&bid=0046&act=view&list_no=372345&tag=&cg_code=&list_depth=1 (accessed on 1 January 2024).
- Štšepetova, J.; Rätsep, M.; Gerulis, O.; Jõesaar, A.; Mikelsaar, M.; Songisepp, E. Impact of Lactiplantibacillus plantarum inducia on metabolic and antioxidative response in cholesterol and BMI variable indices: Randomised, double-blind, placebo-controlled trials. Benef. Microbes 2023, 14, 1–15. [Google Scholar] [CrossRef]
- Tang, C.; Kong, L.; Shan, M.; Lu, Z.; Lu, Y. Protective and ameliorating effects of probiotics against diet-induced obesity: A review. Food Res. Int. 2021, 147, 110490. [Google Scholar] [CrossRef] [PubMed]
- Arita, Y.; Kihara, S.; Ouchi, N.; Takahashi, M.; Maeda, K.; Miyagawa, J.; Hotta, K.; Shimomura, I.; Nakamura, T.; Miyaoka, K.; et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem. Biophys. Res. Commun. 1999, 257, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Okubo, K.; Shimomura, I.; Funahashi, T.; Matsuzawa, Y.; Matsubara, K. CDNA Cloning and expression of a novel adipose specific collagen-like factor, APM1 (AdiposeMost Abundant Gene Transcript 1). Biochem. Biophys. Res. Commun. 1996, 221, 286–289. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-G.; Park, K.S.; Kim, M.; Kim, H.-S.; Suh, Y.S.; Ahn, J.D.; Park, K.; Chang, Y.; Lee, I. Relationship between serum adiponectin and leptin concentrations and body fat distribution. Diabetes Res. Clin. Pract. 2004, 63, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Riezu-Boj, J.I.; Barajas, M.; Pérez-Sánchez, T.; Pajares, M.J.; Araña, M.; Milagro, F.I.; Urtasun, R. Lactiplantibacillus plantarum DSM20174 attenuates the progression of non-alcoholic fatty liver disease by modulating gut microbiota, improving metabolic risk factors, and attenuating adipose inflammation. Nutrients 2022, 14, 5212. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, G.; Apovian, C.M. Future pharmacotherapy for obesity: New anti-obesity drugs on the horizon. Curr. Obes. Rep. 2018, 7, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Petruzzelli, M.; Schweiger, M.; Schreiber, R.; Campos-Olivas, R.; Tsoli, M.; Allen, J.D.; Swarbrick, M.M.; Rose-John, S.; Rincón, M.; Robertson, G.; et al. A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell Metab. 2014, 20, 433–447. [Google Scholar] [CrossRef]
- Kang, J.H.; Yun, S.I.; Park, M.H.; Park, J.; Jeong, S.Y.; Park, H.O. Anti-obesity effect of Lactobacillus gasseri BNR17 in high-sucrose diet-induced obese mice. PLoS ONE 2013, 8, e54617. [Google Scholar] [CrossRef] [PubMed]
- Abdulan, I.M.; Popescu, G.; Maștaleru, A.; Oancea, A.; Costache, A.D.; Cojocaru, D.-C.; Cumpăt, C.-M.; Ciuntu, B.M.; Rusu, B.; Leon, M.M. Winter holidays and their impact on eating behavior—A systematic review. Nutrients 2023, 15, 4201. [Google Scholar] [CrossRef]
- Fujihira, K.; Takahashi, M.; Wang, C.; Hayashi, N. Factors explaining seasonal variation in energy intake: A review. Front. Nutr. 2023, 10, 1192223. [Google Scholar] [CrossRef]
- Tanaka, N.; Okuda, T.; Shinohara, H.; Yamasaki, R.S.; Hirano, N.; Kang, J.; Ogawa, M.; Nishi, N.N. Relationship between seasonal changes in food intake and energy metabolism, physical activity, and body composition in young Japanese women. Nutrients 2022, 14, 506. [Google Scholar] [CrossRef]
- Pérez-Sánchez, T.; Ruiz-Zarzuela, I.; de Blas, I.; Balcázar, J.L. Probiotics in aquaculture: A current assessment. Rev. Aquacult. 2014, 6, 133–146. [Google Scholar] [CrossRef]
- Kadooka, Y.; Sato, M.; Imaizumi, K.; Ogawa, A.; Ikuyama, K.; Akai, Y.; Okano, M.; Kagoshima, M.; Tsuchida, T. Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. Eur. J. Clin. Nutr. 2010, 64, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Osterberg, K.L.; Boutagy, N.E.; McMillan, R.P.; Stevens, J.R.; Frisard, M.I.; Kavanaugh, J.W.; Davy, B.M.; Davy, K.P.; Hulver, M.W. Probiotic supplementation attenuates increases in body mass and fat mass during high-fat diet in healthy young adults. Obesity 2015, 23, 2364–2370. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.K.; Youn, S.J.; Choi, Y.; Eun, S.W.; Shin, S.M. Body fat reduction effect of Bifidobacterium breve B-3: A randomized, double-blind, placebo comparative clinical trial. Nutrients 2023, 15, 28. [Google Scholar] [CrossRef] [PubMed]
- Ferre, P.; Foufelle, F. SREBP-1c transcription factor and lipid homeostasis: Clinical perspective. Horm. Res. 2007, 68, 72–82. [Google Scholar] [CrossRef]
- Kubota, N.; Terauchi, Y.; Miki, H.; Tamemoto, H.; Yamauchi, T.; Komeda, K.; Satoh, S.; Nakano, R.; Ishii, C.; Sugiyama, T. PPAR gamma mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol. Cell 1999, 4, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.R.; Barrick, C.; Kim, K.A.; Lindner, J.; Blondeau, B.; Fujimoto, Y.; Shiota, M.; Kesterson, R.A.; Kahn, B.B.; Magnuson, M.A. Deletion of PPARgamma in adipose tissues of mice protects against high fat diet-induced obesity and insulin resistance. Proc. Natl. Acad. Sci. USA 2005, 102, 6207–6212. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, T.A.; Bereshchenki, O.; Garcia-Silva, S.; Ermakova, O.; Kurz, E.; Mandrup, S.; Porce, B.T.; Nerlov, C. Distinct C/EBPa motifs regulate lipogenic and gluconeogenic gene expression in vivo. EMBO J. 2007, 26, 1081–1093. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.G.; Vos, P.D.; Guerre-Millo, M.; Wong, E.; Herman, T.; Staels, B.; Briggs, M.R.; Auwerx, J. The adipocyte specific transcription factor C/EBPa modulates human ob gene expression. Proc. Natl. Acad. Sci. USA 1996, 93, 5507–5511. [Google Scholar] [CrossRef] [PubMed]
- Dixon, J.B.; le Roux, C.W.; Rubino, F.; Zimmet, P. Bariatric surgery for type 2 diabetes. Lancet 2012, 379, 2300–2311. [Google Scholar] [CrossRef] [PubMed]



| SKO-001 Group (N = 50) | Placebo Group (N = 50) | p-Value | ||
|---|---|---|---|---|
| Sex | Men | 9 | 6 | 0.575 1 |
| Women | 41 | 44 | ||
| Age, years | 48.90 ± 10.60 | 47.14 ± 9.67 | 0.248 2 | |
| Height, cm | 161.13 ± 7.67 | 161.19 ± 7.26 | 0.971 2 | |
| Weight, kg | 71.19 ± 8.25 | 71.64 ± 7.67 | 0.781 3 | |
| Body mass index, kg/m2 | 27.34 ± 1.49 | 27.51 ± 1.40 | 0.539 2 | |
| Parameter | Treatment | Baseline | 12 Weeks | p-Value | p-Value |
|---|---|---|---|---|---|
| Total cholesterol (mg/dL) | SKO-001 | 216.44 ± 36.95 | 204.67 ± 35.78 | 0.003 ** | 0.158 |
| Placebo | 208.17 ± 35.47 | 203.62 ± 34.22 | 0.194 | ||
| p-value | 0.277 | 0.886 | |||
| TG (mg/dL) | SKO-001 | 115.42 ± 40.43 | 126.33 ± 62.87 | 0.221 | 0.055 |
| Placebo | 125.13 ± 77.42 | 111.19 ± 62.13 | 0.079 | ||
| p-value | 0.77 | 0.248 | |||
| HDL-C (mg/dL) | SKO-001 | 60.33 ± 11.89 | 60.62 ± 12.17 | 0.953 | 0.191 |
| Placebo | 58.23 ± 12.43 | 60.06 ± 13.47 | 0.079 | ||
| p-value | 0.239 | 0.705 | |||
| LDL-C (mg/dL) | SKO-001 | 136.91 ± 30.78 | 130.60 ± 32.12 | 0.039 * | 0.025 * |
| Placebo | 131.38 ± 32.98 | 134.62 ± 33.11 | 0.282 | ||
| p-value | 0.408 | 0.556 | |||
| Adiponectin (ng/mL) | SKO-001 | 10,197.11 ± 5733.93 | 10,651.47 ± 6458.85 | 0.548 | 0.023 * |
| Placebo | 10,224.15 ± 5248.66 | 9595.95 ± 5120.76 | 0.015 * | ||
| p-value | 0.828 | 0.581 | |||
| Leptin (ng/mL) | SKO-001 | 26.68 ± 13.76 | 22.65 ± 12.84 | 0.014 * | 0.542 |
| Placebo | 26.61 ± 13.20 | 23.03 ± 12.88 | 0.093 | ||
| p-value | 0.95 | 0.96 | |||
| Insulin (mIU/L) | SKO-001 | 7.84 ± 6.13 | 6.34 ± 5.55 | 0.084 | 0.687 |
| Placebo | 5.83 ± 2.80 | 6.17 ± 10.01 | 0.063 | ||
| p-value | 0.337 | 0.617 |
| Parameter | Treatment | Baseline | 12 Weeks | p-Value | p-Value |
|---|---|---|---|---|---|
| Visceral fat (cm2) | SKO-001 | 125.91 ± 34.33 | 126.57 ± 48.96 | 0.247 | 0.081 |
| Placebo | 122.98 ± 39.29 | 126.45 ± 41.33 | 0.196 | ||
| p-value | 0.703 | 0.685 | |||
| Subcutaneous fat (cm2) | SKO-001 | 223.23 ± 64.91 | 208.09 ± 62.63 | 0.008 ** | 0.995 |
| Placebo | 231.83 ± 48.19 | 216.64 ± 55.87 | 0.002 ** | ||
| p-value | 0.196 | 0.327 | |||
| Abdominal fat (cm2) | SKO-001 | 349.14 ± 75.90 | 334.66 ± 85.46 | 0.059 | 0.762 |
| Placebo | 354.81 ± 56.64 | 343.09 ± 67.57 | 0.028 | ||
| p-value | 0.687 | 0.602 | |||
| VSR | SKO | 0.59 ± 0.22 | 0.64 ± 0.27 | 0.073 | 0.41 |
| Placebo | 0.55 ± 0.25 | 0.62 ± 0.30 | 0.001 ** | ||
| p-value | 0.309 | 0.652 |
| SKO-001 | Placebo | Total | p-Value & | ||
|---|---|---|---|---|---|
| Severity | Mild | 46 | 44 | 90 | 0.495 |
| Moderate | 0 | 1 | 1 | ||
| Severe | 0 | 0 | 0 | ||
| SAE (non-fatal) | 0 | 1 | 1 | ||
| SAE (fatal) | 0 | 0 | 0 | ||
| Results | Complete healing (no aftereffects) | 45 | 40 | 85 | 0.111 |
| Healing (with sequelae) | 0 | 0 | 0 | ||
| In progress | 1 | 6 | 7 | ||
| Permanent damage | 0 | 0 | 0 | ||
| Death | 0 | 0 | 0 | ||
| Relevance | Definitely related | 0 | 0 | 0 | 1.000 |
| Probably related | 0 | 0 | 0 | ||
| Possibly related | 0 | 0 | 0 | ||
| Possibly not related | 1 | 0 | 1 | ||
| Definitely not related | 45 | 46 | 91 | ||
| UK, unassessable | 0 | 0 | 0 | ||
| Interventional formulation-related actions | None | 6 | 10 | 16 | 0.283 |
| Dose change/pause | 0 | 0 | 0 | ||
| Interruption of intake | 0 | 0 | 0 | ||
| Combination drug intake | 40 | 35 | 75 | ||
| Non-drug treatment | 0 | 1 | 1 | ||
| Increased length of hospitalisation | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, S.M.; Park, J.-S.; Kim, S.B.; Cho, Y.H.; Seo, H.; Lee, H.S. A 12-Week, Single-Centre, Randomised, Double-Blind, Placebo-Controlled, Parallel-Design Clinical Trial for the Evaluation of the Efficacy and Safety of Lactiplantibacillus plantarum SKO-001 in Reducing Body Fat. Nutrients 2024, 16, 1137. https://doi.org/10.3390/nu16081137
Shin SM, Park J-S, Kim SB, Cho YH, Seo H, Lee HS. A 12-Week, Single-Centre, Randomised, Double-Blind, Placebo-Controlled, Parallel-Design Clinical Trial for the Evaluation of the Efficacy and Safety of Lactiplantibacillus plantarum SKO-001 in Reducing Body Fat. Nutrients. 2024; 16(8):1137. https://doi.org/10.3390/nu16081137
Chicago/Turabian StyleShin, Seon Mi, Jeong-Su Park, Sang Back Kim, Young Hee Cho, Hee Seo, and Hak Sung Lee. 2024. "A 12-Week, Single-Centre, Randomised, Double-Blind, Placebo-Controlled, Parallel-Design Clinical Trial for the Evaluation of the Efficacy and Safety of Lactiplantibacillus plantarum SKO-001 in Reducing Body Fat" Nutrients 16, no. 8: 1137. https://doi.org/10.3390/nu16081137
APA StyleShin, S. M., Park, J.-S., Kim, S. B., Cho, Y. H., Seo, H., & Lee, H. S. (2024). A 12-Week, Single-Centre, Randomised, Double-Blind, Placebo-Controlled, Parallel-Design Clinical Trial for the Evaluation of the Efficacy and Safety of Lactiplantibacillus plantarum SKO-001 in Reducing Body Fat. Nutrients, 16(8), 1137. https://doi.org/10.3390/nu16081137






