Daily Duration of Eating for Children and Adolescents: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.1.1. Population
2.1.2. Condition
2.1.3. Context
2.1.4. Study Design
2.2. Search Strategy
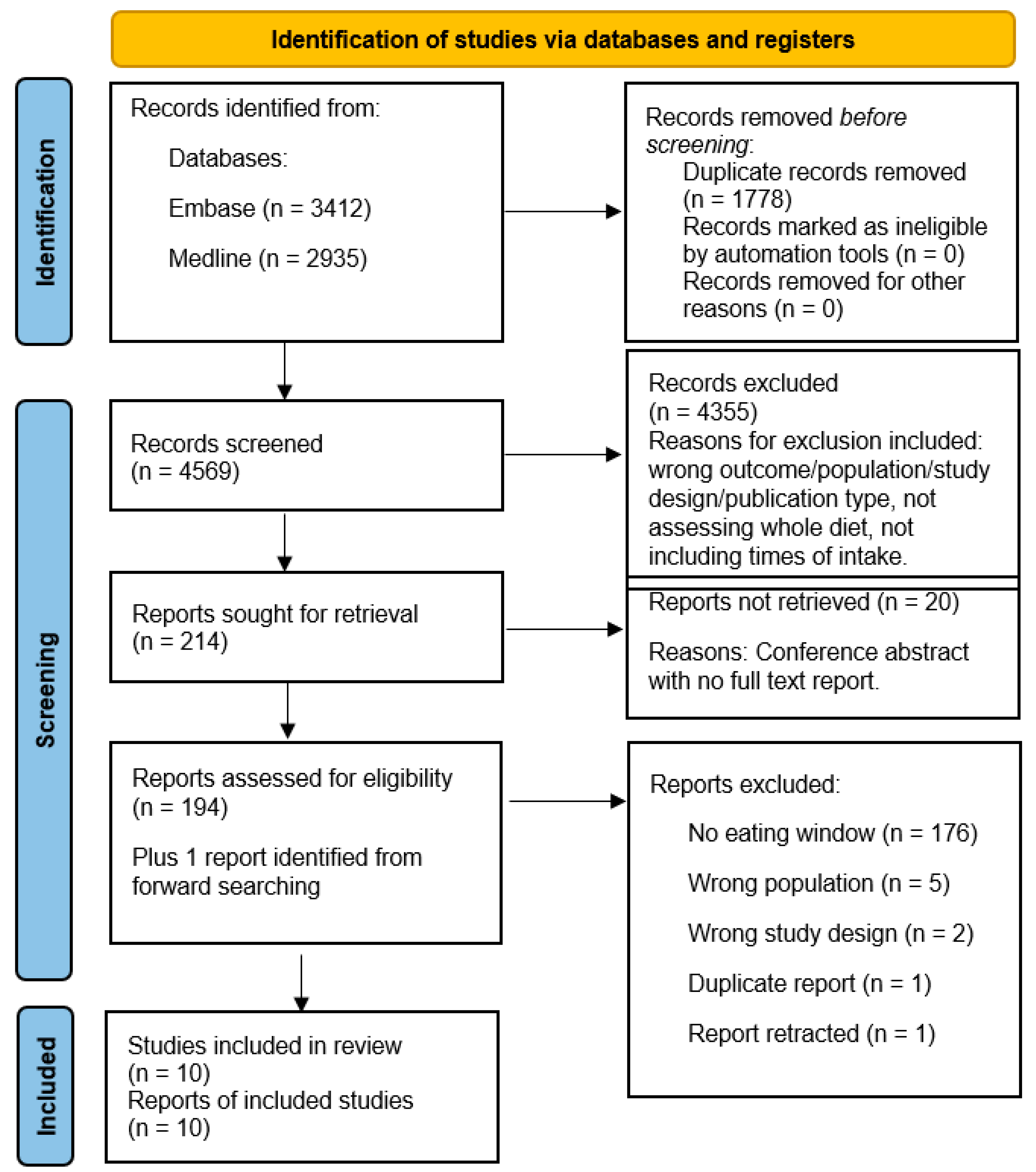
2.3. Selection and Extraction
2.4. Critical Appraisal Assessment
2.5. Data Synthesis
2.6. Sensitivity Analysis
3. Results
3.1. Study Characteristics
3.2. Dietary Assessment Methods
3.3. First and Last Calorie Intakes
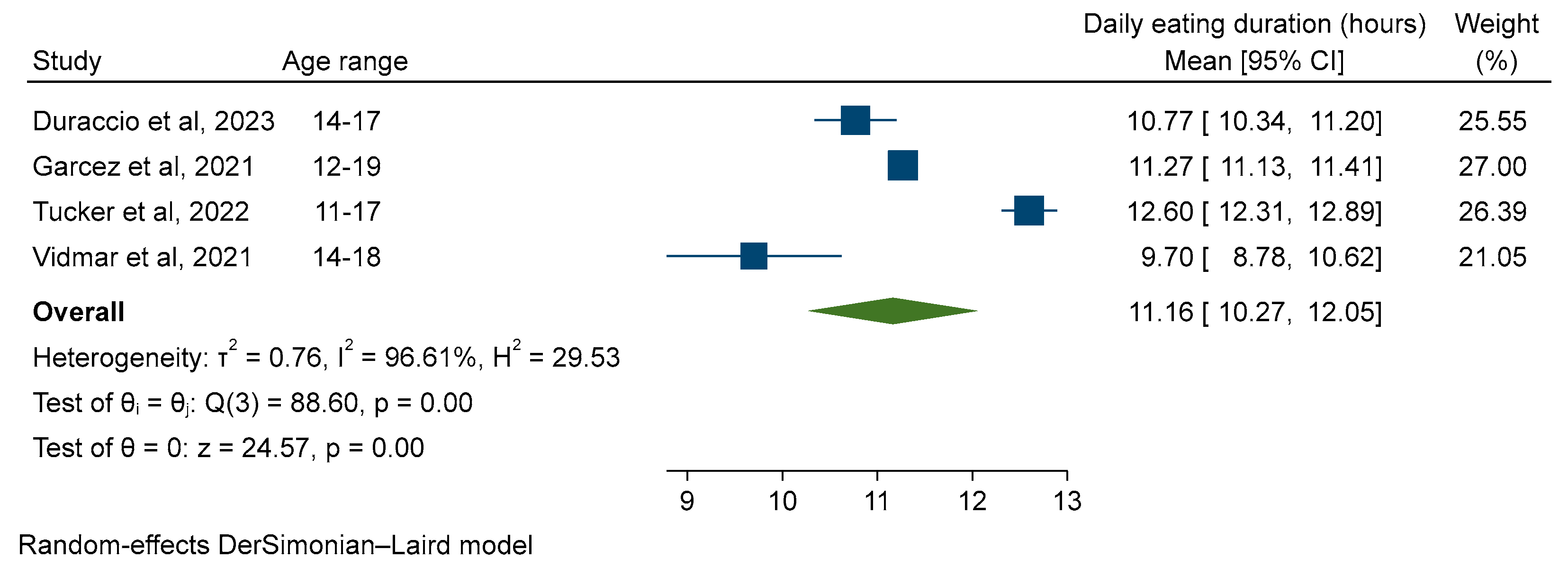
3.4. Duration of Daily Eating
| Study | Dietary Assessment Method | Mean (SD) First and Last Eating Time (hh:mm) | Daily Eating Window (Hours) (Mean (95%CI)) |
|---|---|---|---|
| Berendsen et al. (2020) [32] | Self-reported questionnaire (23 items to assess chrono-nutrition) | Weekdays first: 07:45 (0:51) | Weekdays: 12.93 a |
| Weekdays last: 20:41 (1:15) | |||
| Weekends first: 09:12 (0:47) | Weekends: 12.33 a | ||
| Weekends last: 21:32 (1:11) | |||
| Duraccio et al. (2023) [33] | 3 × 24-h recall (weekdays only) | First: 08:52 (1:44) | 10.77 (10.34, 11.20) d,e |
| Last: 19:39 (1:18) | |||
| Garcez et al. (2021) [34] | Single 24-h recall (day of week not specified) | Data not reported | 11.27 (11.14, 11.40) d,e |
| Intemann et al. (2024) [35] | 3 × 24-h recall (only achieved by 23% of participants) (days of week not specified) | Data not reported | 11.50 (11.47, 11.53) d |
| Jain Gupta and Khare (2020) [36] | Photos taken via mobile phone camera for 21 days. | Data not reported | High school (13–15 years): 14.23 b Higher secondary (16–18 years): 16.36 b |
| Roßbach et al. (2017) [28] | 3-day weighed dietary records (days of week not specified) | Data not reported | Boys 6–10 years: 10.97 (10.22, 11.72) c |
| Boys 11–13 years: 11.50 (10.43, 12.30) c | |||
| Boys 14–18 years: 11.88 (10.50, 13.0) c | |||
| Girls 6–10 years: 11.0 (10.17, 11.72) c | |||
| Girls 11–13 years: 11.42 (10.33, 12.33) c | |||
| Girls 14–18 years: 11.43 (10.13, 12.60) c | |||
| Roßbach et al. (2018) [29] | 3-day weighed dietary records (days of week not specified) | Data not reported | Chronotype T1: 11.12 (10.4, 11.95) c Chronotype T2: 10.88 (10.10, 12.25) c Chronotype T3: 10.85 (9.75, 12.37) c |
| Spaeth et al. (2019) [37] | 3 × 24-h recall (2 weekdays and 1 weekend) | First: 08:26 (1:07) | 11.45 a |
| Last: 19:56 (1:05) | |||
| Tucker et al. (2022) [15] | Self-reported questionnaire (to assess meal and snack patterns, sleep habits, and acceptability of time-limited eating). | Whole sample First: 07:36 (1:30) Last: 20:06 (1:18) Ages 8–10: Not reported Ages 11–17: Not reported | Whole sample: 12.5 (12.24, 12.76) d Ages 8–10: 12.1 (11.69, 12.51) Ages 11–17: 12.6 (12.30, 12.90) e |
| Vidmar et al. (2021) [16] | 2 × 24-h recall (1 weekday and one weekend) | Data not reported | 9.7 (8.79, 10.61) d,e |
3.5. Subgroup Analysis—Age
3.6. Sensitivity Analysis
3.7. Quality Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 5 April 2023).
- NHS Digital. National Childhood Measurement Programme, England, 2022/23 School Year. Available online: https://digital.nhs.uk/data-and-information/publications/statistical/national-child-measurement-programme/2022-23-school-year (accessed on 13 November 2023).
- NHS Digital. Health Survey for England 2019. Available online: https://digital.nhs.uk/data-and-information/publications/statistical/health-survey-for-england/2019/main-findings (accessed on 4 April 2023).
- Sahoo, K.; Sahoo, B.; Choudhury, A.K.; Sofi, N.Y.; Kumar, R.; Bhadoria, A.S. Childhood obesity: Causes and consequences. J. Fam. Med. Prim. Care 2015, 4, 187–192. [Google Scholar] [CrossRef]
- Kelishadi, R.; Heidari-Beni, M. Prevention and Control of Childhood Obesity: The Backbone in Prevention of Non Communicable Disease. Adv. Exp. Med. Biol. 2019, 1121, 61–66. [Google Scholar] [CrossRef]
- Pi-Sunyer, X. The medical risks of obesity. Postgrad. Med. 2009, 121, 21–33. [Google Scholar] [CrossRef]
- Campbell, M.K. Biological, environmental, and social influences on childhood obesity. Pediatr. Res. 2016, 79, 205–211. [Google Scholar] [CrossRef]
- Chaix, A.; Manoogian, E.N.C.; Melkani, G.C.; Panda, S. Time-Restricted Eating to Prevent and Manage Chronic Metabolic Diseases. Annu. Rev. Nutr. 2019, 39, 291–315. [Google Scholar] [CrossRef]
- Panda, S. Circadian physiology of metabolism. Science 2016, 354, 1008–1015. [Google Scholar] [CrossRef]
- Longo, V.D.; Panda, S. Fasting, Circadian Rhythms, and Time-Restricted Feeding in Healthy Lifespan. Cell Metab. 2016, 23, 1048–1059. [Google Scholar] [CrossRef]
- Adafer, R.; Messaadi, W.; Meddahi, M.; Patey, A.; Haderbache, A.; Bayen, S.; Messaadi, N. Food Timing, Circadian Rhythm and Chrononutrition: A Systematic Review of Time-Restricted Eating’s Effects on Human Health. Nutrients 2020, 12, 3770. [Google Scholar] [CrossRef]
- Rothschild, J.; Hoddy, K.K.; Jambazian, P.; Varady, K.A. Time-restricted feeding and risk of metabolic disease: A review of human and animal studies. Nutr. Rev. 2014, 72, 308–318. [Google Scholar] [CrossRef]
- Moon, S.; Kang, J.; Kim, S.H.; Chung, H.S.; Kim, Y.J.; Yu, J.M.; Cho, S.T.; Oh, C.-M.; Kim, T. Beneficial Effects of Time-Restricted Eating on Metabolic Diseases: A Systemic Review and Meta-Analysis. Nutrients 2020, 12, 1267. [Google Scholar] [CrossRef]
- Naguib, M.N.; Hegedus, E.; Raymond, J.K.; Goran, M.I.; Salvy, S.J.; Wee, C.P.; Durazo-Arvizu, R.; Moss, L.; Vidmar, A.P. Continuous Glucose Monitoring in Adolescents With Obesity: Monitoring of Glucose Profiles, Glycemic Excursions, and Adherence to Time Restricted Eating Programs. Front. Endocrinol. 2022, 13, 841838. [Google Scholar] [CrossRef]
- Tucker, J.M.; Siegel, R.; Murray, P.J.; Han, J.C.; Boyer, K.; Reed, N.; Allenby, T.; Novick, M. Acceptability of Time-Limited Eating in Pediatric Weight Management. Front. Endocrinol. 2022, 13, 811489. [Google Scholar] [CrossRef]
- Vidmar, A.P.; Naguib, M.; Raymond, J.K.; Salvy, S.J.; Hegedus, E.; Wee, C.P.; Goran, M.I. Time-Limited Eating and Continuous Glucose Monitoring in Adolescents with Obesity: A Pilot Study. Nutrients 2021, 13, 3697. [Google Scholar] [CrossRef]
- Kant, A.K. Eating patterns of US adults: Meals, snacks, and time of eating. Physiol. Behav. 2018, 193, 270–278. [Google Scholar] [CrossRef]
- Manoogian, E.N.C.; Zadourian, A.; Lo, H.C.; Gutierrez, N.R.; Shoghi, A.; Rosander, A.; Pazargadi, A.; Ormiston, C.K.; Wang, X.; Sui, J.; et al. Feasibility of time-restricted eating and impacts on cardiometabolic health in 24-h shift workers: The Healthy Heroes randomized control trial. Cell Metab. 2022, 34, 1442–1456.e1447. [Google Scholar] [CrossRef]
- Gill, S.; Panda, S. A Smartphone App Reveals Erratic Diurnal Eating Patterns in Humans that Can Be Modulated for Health Benefits. Cell Metab. 2015, 22, 789–798. [Google Scholar] [CrossRef]
- Wilkinson, M.J.; Manoogian, E.N.C.; Zadourian, A.; Lo, H.; Fakhouri, S.; Shoghi, A.; Wang, X.; Fleischer, J.G.; Navlakha, S.; Panda, S.; et al. Ten-Hour Time-Restricted Eating Reduces Weight, Blood Pressure, and Atherogenic Lipids in Patients with Metabolic Syndrome. Cell Metab. 2020, 31, 92–104.e5. [Google Scholar] [CrossRef]
- Vidmar, A.P.; Jones, R.B.; Wee, C.P.; Berger, P.K.; Plows, J.F.; Claudia Rios, R.D.; Raymond, J.K.; Goran, M.I. Timing of food consumption in Hispanic adolescents with obesity. Pediatr. Obes. 2021, 16, e12764. [Google Scholar] [CrossRef]
- Huseinovic, E.; Winkvist, A.; Slimani, N.; Park, M.K.; Freisling, H.; Boeing, H.; Buckland, G.; Schwingshackl, L.; Weiderpass, E.; Rostgaard-Hansen, A.L.; et al. Meal patterns across ten European countries—Results from the European Prospective Investigation into Cancer and Nutrition (EPIC) calibration study. Public. Health Nutr. 2016, 19, 2769–2780. [Google Scholar] [CrossRef]
- Park, M.K.; Freisling, H.; Huseinovic, E.; Winkvist, A.; Huybrechts, I.; Crispim, S.P.; de Vries, J.H.M.; Geelen, A.; Niekerk, M.; van Rossum, C.; et al. Comparison of meal patterns across five European countries using standardized 24-h recall (GloboDiet) data from the EFCOVAL project. Eur. J. Nutr. 2018, 57, 1045–1057. [Google Scholar] [CrossRef]
- D’Angelo, C.; Gloinson, E.R.; Draper, A.; Guthrie, S. Food Consumption in the UK. Trends, Attitudes and Drivers; RAND Corporation: Cambridge, UK, 2020. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Munn, Z.; Moola, S.; Lisy, K.; Riitano, D.; Tufanaru, C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int. J. Evid. Based Heal. 2015, 13, 147–153. [Google Scholar] [CrossRef]
- Munn, Z.; Barker, T.H.; Moola, S.; Tufanaru, C.; Stern, C.; McArthur, A.; Stephenson, M.; Aromataris, E. Methodological quality of case series studies: An introduction to the JBI critical appraisal tool. JBI Evid. Synth. 2020, 18, 2127–2133. [Google Scholar] [CrossRef]
- Roßbach, S.; Diederichs, T.; Bolzenius, K.; Herder, C.; Buyken, A.E.; Alexy, U. Age and time trends in eating frequency and duration of nightly fasting of German children and adolescents. Eur. J. Nutr. 2017, 56, 2507–2517. [Google Scholar] [CrossRef]
- Roßbach, S.; Diederichs, T.; Nöthlings, U.; Buyken, A.E.; Alexy, U. Relevance of chronotype for eating patterns in adolescents. Chronobiol. Int. 2018, 35, 336–347. [Google Scholar] [CrossRef]
- Deeks, J.J.; Higgins, J.P.T.; Altman, D.G. Chapter 10: Analysing data and undertaking meta-analyses. In Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2023; Available online: https://training.cochrane.org/handbook/current/chapter-10 (accessed on 12 October 2023).
- Page, M.J.; Higgins, J.P.T.; Sterne, J.A.C. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2023; Available online: https://training.cochrane.org/handbook/current/chapter-13 (accessed on 12 October 2023).
- Berendsen, M.; Boss, M.; Smits, M.; Pot, G.K. Chrono-Nutrition and Diet Quality in Adolescents with Delayed Sleep-Wake Phase Disorder. Nutrients 2020, 12, 539. [Google Scholar] [CrossRef]
- Duraccio, K.M.; Whitacre, C.; Wright, I.D.; Summer, S.S.; Beebe, D.W. The impact of experimentally shortened sleep on timing of eating occasions in adolescents: A brief report. J. Sleep. Res. 2023, 32, e13806. [Google Scholar] [CrossRef]
- Garcez, M.R.; de Castro, M.A.; César, C.L.G.; Goldbaum, M.; Fisberg, R.M. A chrononutrition perspective of diet quality and eating behaviors of Brazilian adolescents in associated with sleep duration. Chronobiol. Int. 2021, 38, 387–399. [Google Scholar] [CrossRef]
- Intemann, T.; Bogl, L.H.; Hunsberger, M.; Lauria, F.; De Henauw, S.; Molnár, D.; Moreno, L.A.; Tornaritis, M.; Veidebaum, T.; Ahrens, W.; et al. A Late Meal Timing Pattern Is Associated with Insulin Resistance in European Children and Adolescents. Pediatr. Diabetes 2024, 2024, 6623357. [Google Scholar] [CrossRef]
- Jain Gupta, N.; Khare, A. Disruption in daily eating-fasting and activity-rest cycles in Indian adolescents attending school. PLoS ONE 2020, 15, e0227002. [Google Scholar] [CrossRef]
- Spaeth, A.M.; Hawley, N.L.; Raynor, H.A.; Jelalian, E.; Greer, A.; Crouter, S.E.; Coffman, D.L.; Carskadon, M.A.; Owens, J.A.; Wing, R.R.; et al. Sleep, energy balance, and meal timing in school-aged children. Sleep. Med. 2019, 60, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Iglowstein, I.; Jenni, O.G.; Molinari, L.; Largo, R.H. Sleep Duration from Infancy to Adolescence: Reference Values and Generational Trends. Pediatrics 2003, 111, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.A.; Zimmerman, F.J.; Bell, J.F. Norms and Trends of Sleep Time Among US Children and Adolescents. JAMA Pediatr. 2013, 167, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Chaput, J.-P.; Janssen, I. Sleep duration estimates of Canadian children and adolescents. J. Sleep. Res. 2016, 25, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Almoosawi, S.; Vingeliene, S.; Karagounis, L.G.; Pot, G.K. Chrono-nutrition: A review of current evidence from observational studies on global trends in time-of-day of energy intake and its association with obesity. Proc. Nutr. Soc. 2016, 75, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Monzani, A.; Ricotti, R.; Caputo, M.; Solito, A.; Archero, F.; Bellone, S.; Prodam, F. A Systematic Review of the Association of Skipping Breakfast with Weight and Cardiometabolic Risk Factors in Children and Adolescents. What Should We Better Investigate in the Future? Nutrients 2019, 11, 387. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.; Northstone, K.; Perry, R.; Johnson, L.; Leary, S. The association between later eating rhythm and adiposity in children and adolescents: A systematic review and meta-analysis. Nutr. Rev. 2022, 80, 1459–1479. [Google Scholar] [CrossRef] [PubMed]
- Bailey, R.L.; Leidy, H.J.; Mattes, R.D.; Heymsfield, S.B.; Boushey, C.J.; Ahluwalia, N.; Cowan, A.E.; Pannucci, T.; Moshfegh, A.J.; Goldman, J.D.; et al. Frequency of Eating in the US Population: A Narrative Review of the 2020 Dietary Guidelines Advisory Committee Report. Curr. Dev. Nutr. 2022, 6, nzac132. [Google Scholar] [CrossRef]
- Wittmann, M.; Dinich, J.; Merrow, M.; Roenneberg, T. Social Jetlag: Misalignment of Biological and Social Time. Chronobiol. Int. 2006, 23, 497–509. [Google Scholar] [CrossRef]
- Almoosawi, S.; Cribb, V.; Emmett, P.; Lennox, A. Temporal trends in energy and macronutrient distribution in meals eaten by children from the Avon Longitudinal Study of Parents and Children. Longitud. Life Course Stud. 2016, 7, 25–40. [Google Scholar] [CrossRef]
- Magklis, E.; Howe, L.D.; Johnson, L. Eating Style and the Frequency, Size and Timing of Eating Occasions: A cross-sectional analysis using 7-day weighed dietary records. Sci. Rep. 2019, 9, 15133. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.J.; Kumar, V.; Panda, S. A camera-phone based study reveals erratic eating pattern and disrupted daily eating-fasting cycle among adults in India. PLoS ONE 2017, 12, e0172852. [Google Scholar] [CrossRef] [PubMed]
- Adan, A.; Archer, S.N.; Hidalgo, M.P.; Di Milia, L.; Natale, V.; Randler, C. Circadian Typology: A Comprehensive Review. Chronobiol. Int. 2012, 29, 1153–1175. [Google Scholar] [CrossRef] [PubMed]
- Nicklas, T.A.; Morales, M.; Linares, A.; Yang, S.J.; Baranowski, T.; De Moor, C.; Berenson, G. Children’s meal patterns have changed over a 21-year period: The Bogalusa Heart Study. J. Am. Diet. Assoc. 2004, 104, 753–761. [Google Scholar] [CrossRef] [PubMed]

| Study | Study Design | Country | Description | Sample Size | Age in Years (Range) | Gender | Race | Ethnicity | Socioeconomic Status | BMI |
|---|---|---|---|---|---|---|---|---|---|---|
| Berendsen et al. (2020) [32] | Case control | Netherlands | Adolescents with delayed sleep-wake phase disorder and similar aged control group. Data from control group presented. | 43 | 13–20 | Female: 56% | Not reported | Not reported | Pre-vocational secondary 14% a Senior general secondary 49% a Pre-university 37% a | 19.7 (18.7, 22.2) Median (IQR) |
| Duraccio et al. (2023) [33] | Randomised crossover trial | USA | Short sleep (6.5-h sleep opportunity) or healthy sleep (9.5-h sleep opportunity) for five days with two-day washout period. Healthy sleep data presented | 93 | 14–17 | Female: 62% | White 63% Hispanic 2% Black 22% Asian 3% Multiracial 10% | <$50,000 19% b $50,000–$100,000 32% b >$100,000 48% b | 0.33 (0.91) BMIz Mean (SD) | |
| Garcez et al. (2021) [34] | Cross- sectional | Brazil | Data from 2015 Health Survey of Sao Paulo with Focus in Nutrition (2015 ISA Nutrition). | 419 | 12–19 | Female: 47% | White 44% Black 11% Brown 35% Other 5% | ≤1: 69% c >1: 31% c | Overweight 72% (67, 77%) % (95% CI) f | |
| Intemann et al. (2024) [35] | Cross- sectional | Pan-European: Belgium, Cyprus, Estonia, Germany, Hungary, Italy, Spain, Sweden | Data from I.Family study (2013/14). | 2195 | 8–15 | Female: 50% | Not reported | Not reported | Low 49% d High 51% d | 0.59 (1.11) BMIz Mean (SD) |
| Jain Gupta and Khare (2020) [36] | Longitudinal | India | Mobile phone cameras used to collect food data for 21 days in two schools. | 13 | 13–15 | Female: 64% | Not reported | Not reported | Not reported | 21.6 (16.6–36.9) Median (range) |
| 9 | 16–18 | Female: 67% | 26.0 (15.0–32.4) Median (range) | |||||||
| Roßbach et al. (2017) [28] | Cohort | Germany | Data from Dortmund Nutritional and Anthropometric Longitudinally Designed (DONALD) study, collected 1985–2014. | 465 | 6–10 | Male: 100% | Not reported | Not reported | High 62% e | 16.0 (15.1, 17.5) |
| 326 | 11–13 | Male: 100% | High 56% e | 18.4 (16.7, 20.4) | ||||||
| 265 | 14–18 | Male: 100% | High 55% e | 21.0 (19.0, 23.2) | ||||||
| 461 | 6–10 | Female: 100% | High 61% e | 16.0 (14.9, 17.6) | ||||||
| 322 | 11–13 | Female: 100% | High 56% e | 18.2 (16.6, 20.6) | ||||||
| 257 | 14–18 | Female: 100% | High 55% e | 21.1 (19.1, 23.1) Median (IQR) | ||||||
| Roßbach et al. (2018) [29] | Cohort | Germany | Data from DONALD study with chronotype data, collected 2014–2016. | 116 (chronotype T1) | 10–18 | Female: 49% | Not reported | Not reported | High 89% e | Overweight 8%, Adiposity 3%, Underweight 14% g |
| 116 (chronotype T2) | 10–18 | Female: 53% | High 83% e | Overweight 18% Adiposity 1% Underweight 13% g | ||||||
| 114 (chronotype T3) | 10–18 | Female: 39% | High 81% e | Overweight 18% Adiposity 6% Underweight 7% g | ||||||
| Spaeth et al. (2019) [37] | RCT | USA | Baseline data from an RCT of an intervention to enhance sleep. | 87 | 8–11 | Female: 67% | Black 46% White 37% Other 16% Not reported 1% | Hispanic 14% Not Hispanic 86% | Not reported | Normal weight 54% Overweight/obesity 45% Not reported 1% h |
| Tucker et al. (2022) [15] | Cross-sectional | USA | Survey of parents and children within paediatric weight management clinics to assess timing of eating and acceptability/barriers to time-limited eating. | 213 parents/ 159 children | 8–17 | Female: 57% | Black 22% White 52% Hispanic 16% Other 10% | Not reported | Overweight 3% Obesity 24% Severe obesity 73% h | |
| Vidmar et al. (2021) [16] | Pilot RCT | USA | Baseline data from pilot RCT assessing feasibility, safety, and efficacy of time-limited eating in children undergoing treatment for obesity. | 50 | 14–18 | Female: 72% | White 10% Black 6% Asian 8% Hispanic 54% American Indian 4% Mixed race 12% | Hispanic 30% Not Hispanic 64% | Public insurance 74% Annual household income < $50,000 70% | 2.30 (0.5) BMIz score Mean (SD) |
| Author, Year | 1. Sample Frame | 2. Participant Sampling | 3. Sample Size | 4. Participant and Setting Description | 5. Coverage | 6. Validity | 7. Reliability | 8a. Eating Window | 8b. Summary Statistics | 9. Response Rate | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Berendsen et al., 2020 [32] | Yes | No | Unclear | Yes | Unclear | No | Yes | Unclear | Yes | Unclear | 4/10 |
| Duraccio et al., 2023 [33] | Yes | No | Unclear | Yes | Unclear | No | Yes | Unclear | Unclear | No | 3/10 a,b |
| Garcez et al., 2021 [34] | Yes | Yes | Yes | Yes | Unclear | No | Yes | Yes | Unclear | Unclear | 6/10 a,b |
| Intemann et al., 2024 [35] | Yes | Yes | Yes | Yes | Unclear | Unclear | Yes | Unclear | Unclear | Unclear | 5/10 a |
| Jain Gupta and Khare, 2020 [36] | Ye | No | Unclear | No | Unclear | Yes | Yes | Yes | Unclear | No | 4/10 |
| Roßbach et al., 2017 [28] | No | No | Unclear | Yes | Unclear | No | Yes | Yes | No | Unclear | 3/10 |
| Roßbach et al., 2018 [29] | No | No | Unclear | Yes | Unclear | No | Yes | Yes | No | Unclear | 3/10 |
| Spaeth et al., 2019 [37] | Unclear | Unclear | Unclear | Yes | Unclear | Yes | Yes | No | No | No | 3/10 |
| Tucker et al., 2022 [15] | Yes | Unclear | Unclear | Yes | Unclear | No | No | No | Unclear | Yes | 3/10 a,b |
| Vidmar et al., 2021 [16] | Yes | Yes | No | Yes | Unclear | Yes | Yes | Unclear | Yes | No | 6/10 a,b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Townley, J.; Northstone, K.; Hinton, E.C.; Hamilton-Shield, J.; Searle, A.; Leary, S. Daily Duration of Eating for Children and Adolescents: A Systematic Review and Meta-Analysis. Nutrients 2024, 16, 993. https://doi.org/10.3390/nu16070993
Townley J, Northstone K, Hinton EC, Hamilton-Shield J, Searle A, Leary S. Daily Duration of Eating for Children and Adolescents: A Systematic Review and Meta-Analysis. Nutrients. 2024; 16(7):993. https://doi.org/10.3390/nu16070993
Chicago/Turabian StyleTownley, Jill, Kate Northstone, Elanor C. Hinton, Julian Hamilton-Shield, Aidan Searle, and Sam Leary. 2024. "Daily Duration of Eating for Children and Adolescents: A Systematic Review and Meta-Analysis" Nutrients 16, no. 7: 993. https://doi.org/10.3390/nu16070993
APA StyleTownley, J., Northstone, K., Hinton, E. C., Hamilton-Shield, J., Searle, A., & Leary, S. (2024). Daily Duration of Eating for Children and Adolescents: A Systematic Review and Meta-Analysis. Nutrients, 16(7), 993. https://doi.org/10.3390/nu16070993






