Gynostemma pentaphyllum Hydrodistillate and Its Major Component Damulin B Promote Hair Growth-Inducing Properties In Vivo and In Vitro via the Wnt/β-Catenin Pathway in Dermal Papilla Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture, Reagents, and Antibodies
2.2. Formulation of Cell Culture Media Using G. pentaphyllum Leaves Extract
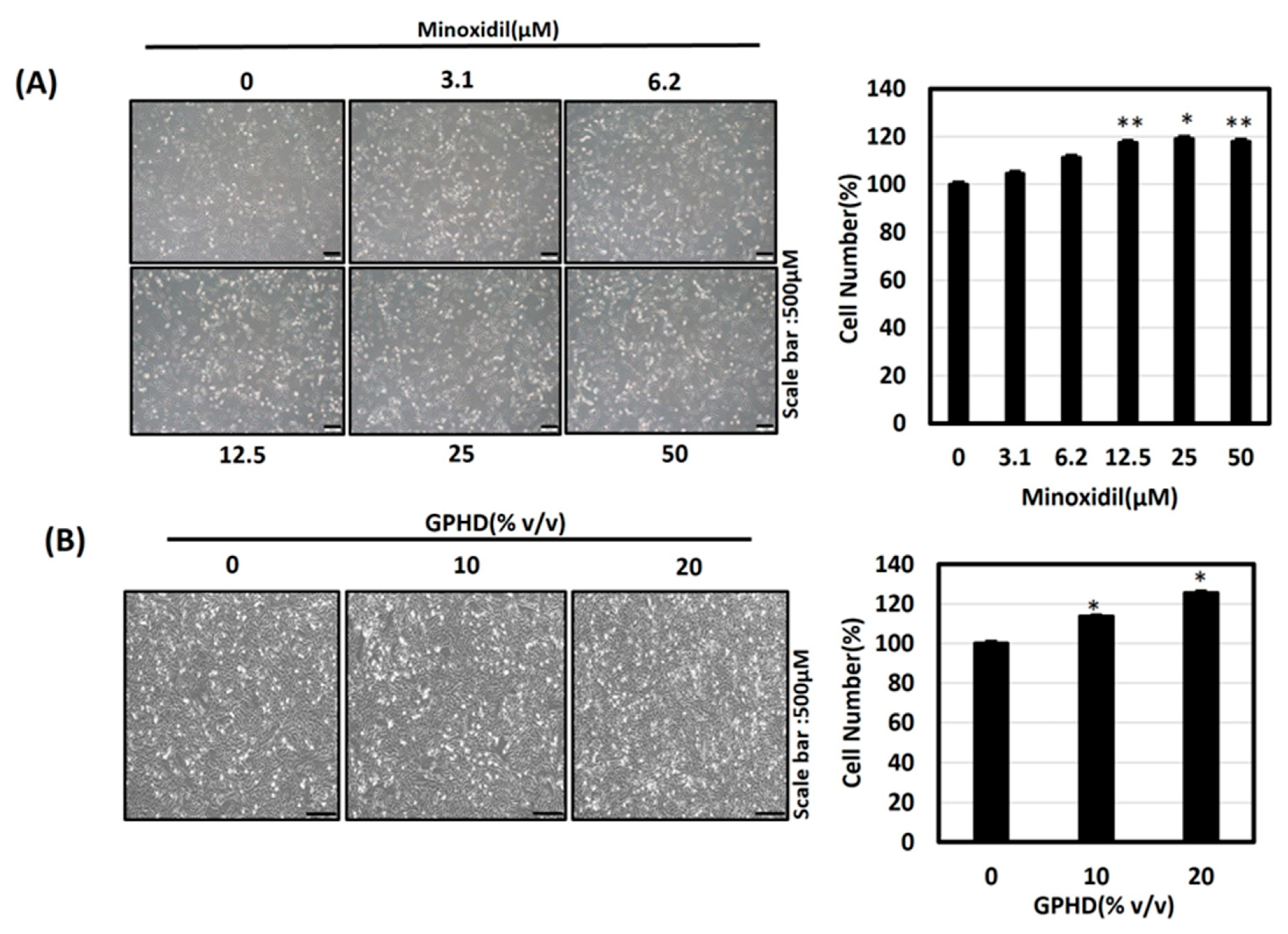
2.3. Trypan Blue Cell Counting Assay
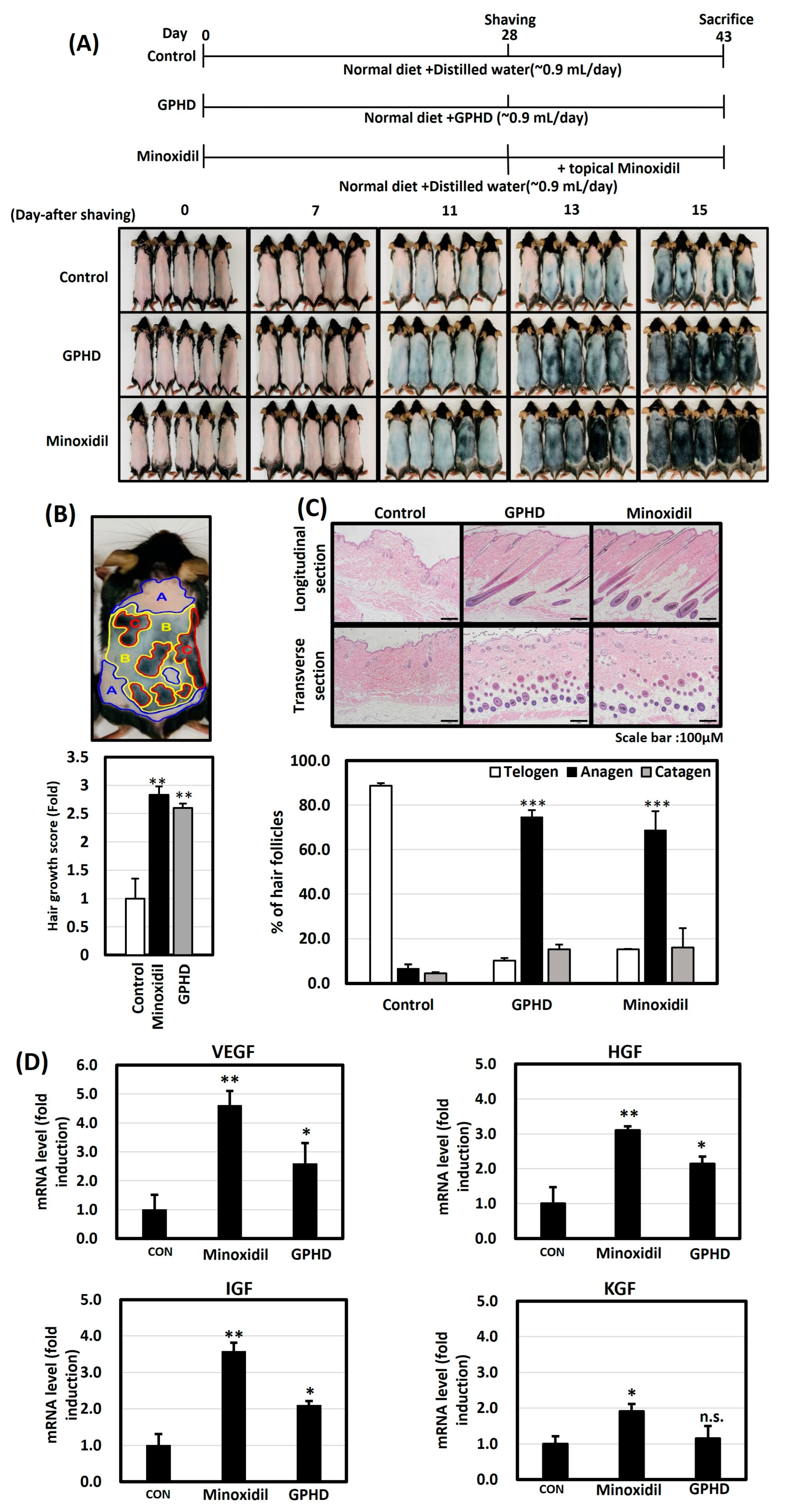
2.4. Animal Experiments
2.5. Hair Growth Score Analysis of Improvement in Hair Growth
2.6. Histopathological Analysis
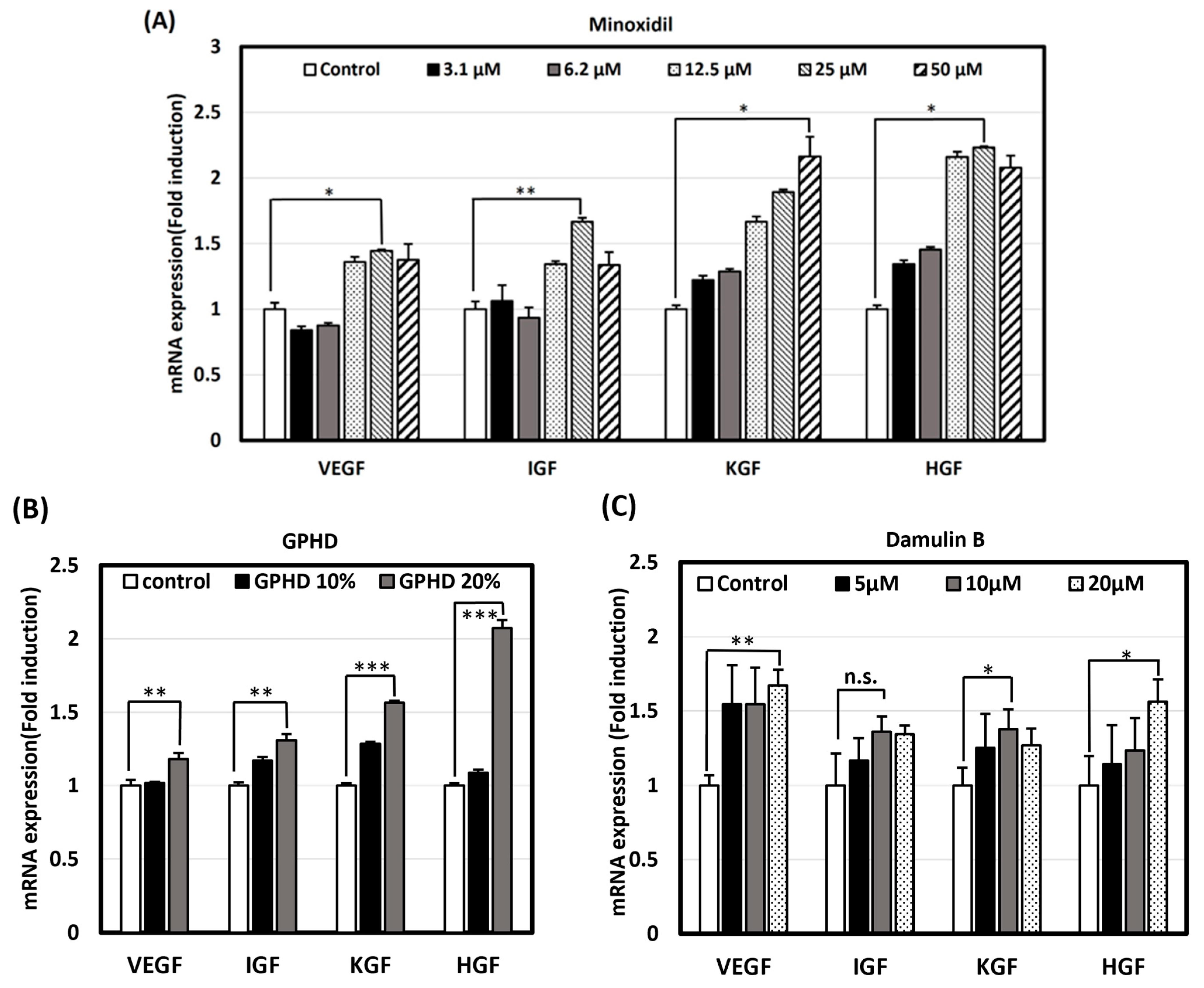
2.7. Real-Time PCR for Mouse Skin Tissue and hDPCs
3. Results
3.1. GPHD Promotes Hair Growth in Mice
3.2. Minoxidil, GPHD, and Damulin B Enhance the Proliferation of Human Dermal Papilla Cells (hDPCs)
3.3. GPHD, Damulin B, and Minoxidil Enhance the Expression of Growth Factors in hDPCs
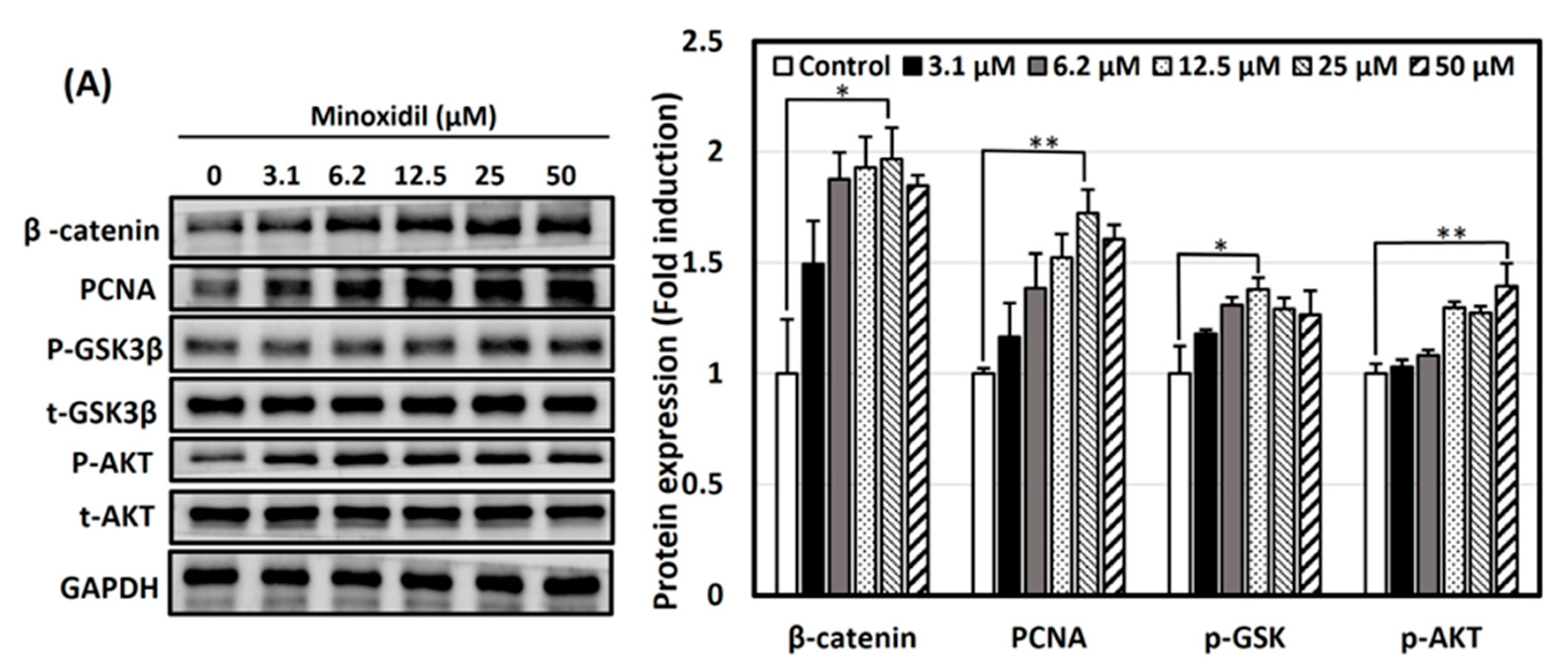
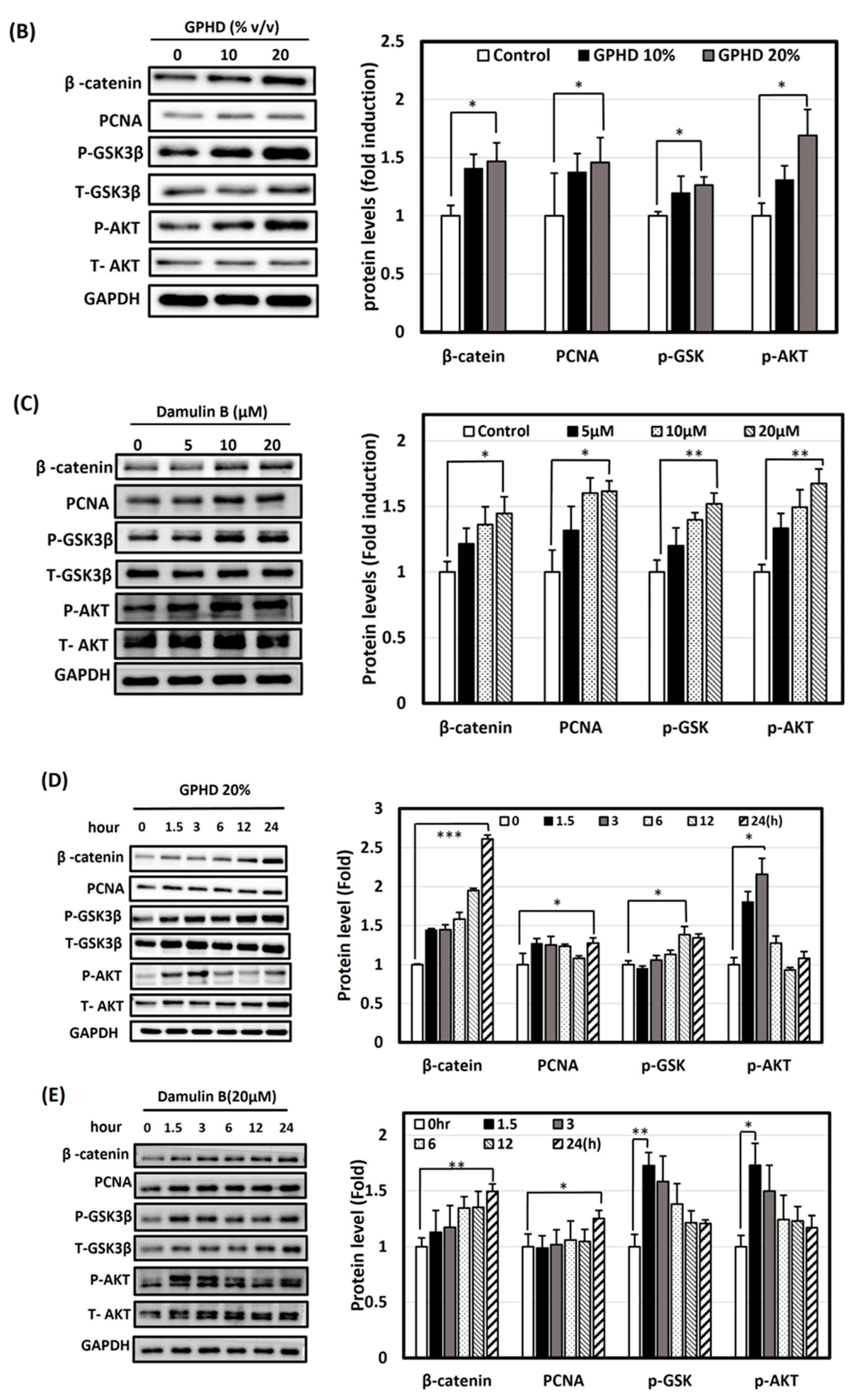
3.4. Activation of Wnt/β-Catenin and Akt Signaling Pathways by Minoxidil, GPHD, and Damulin B
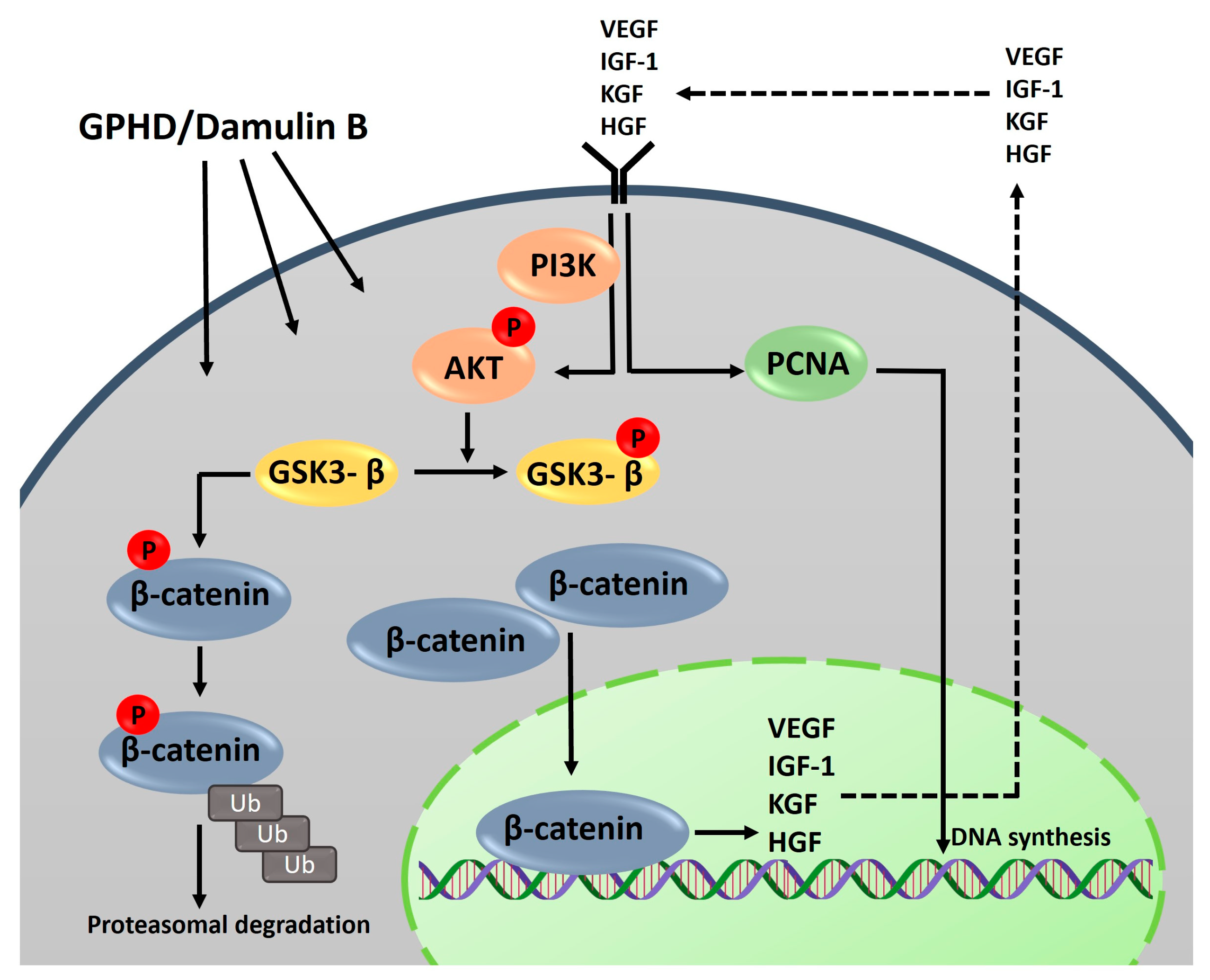
3.5. The Wnt/β-Catenin Pathway Is Activated via AKT in the Presence of GPHD and Damulin B
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Norwood, O.T. Male pattern baldness: Classification and incidence. South. Med. J. 1975, 68, 1359–1365. [Google Scholar] [CrossRef] [PubMed]
- Norwood, O.T. Incidence of female androgenetic alopecia (female pattern alopecia). Dermatol. Surg. 2001, 27, 53–54. [Google Scholar]
- Qi, J.; Garza, L.A. An overview of alopecias. Cold Spring Harb. Perspect. Med. 2014, 4, a013615. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, K.D. Androgens and alopecia. Mol. Cell. Endocrinol. 2002, 198, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.B.; Yuan, X.; Geller, F.; Waterworth, D.; Bataille, V.; Glass, D.; Song, K.; Waeber, G.; Vollenweider, P.; Aben, K.K.; et al. Male-pattern baldness susceptibility locus at 20p11. Nat. Genet. 2008, 40, 1282–1284. [Google Scholar] [CrossRef]
- Trueb, R.M. The impact of oxidative stress on hair. Int. J. Cosmet. Sci. 2015, 37 (Suppl. S2), 25–30. [Google Scholar] [CrossRef] [PubMed]
- Gatherwright, J.; Liu, M.T.; Amirlak, B.; Gliniak, C.; Totonchi, A.; Guyuron, B. The contribution of endogenous and exogenous factors to male alopecia: A study of identical twins. Plast. Reconstr. Surg. 2013, 131, 794e–801e. [Google Scholar] [CrossRef]
- Pratt, C.H.; King, L.E., Jr.; Messenger, A.G.; Christiano, A.M.; Sundberg, J.P. Alopecia areata. Nat. Rev. Dis. Primers 2017, 3, 17011. [Google Scholar] [CrossRef] [PubMed]
- Horev, L. Environmental and cosmetic factors in hair loss and destruction. Curr. Probl. Dermatol. 2007, 35, 103–117. [Google Scholar] [PubMed]
- Nestor, M.S.; Ablon, G.; Gade, A.; Han, H.; Fischer, D.L. Treatment options for androgenetic alopecia: Efficacy, side effects, compliance, financial considerations, and ethics. J. Cosmet. Dermatol. 2021, 20, 3759–3781. [Google Scholar] [CrossRef] [PubMed]
- Freitas, E.; Guttman-Yassky, E.; Torres, T. Baricitinib for the Treatment of Alopecia Areata. Drugs 2023, 83, 761–770. [Google Scholar] [CrossRef]
- Gupta, A.K.; Ravi, S.P.; Vincent, K.; Abramovits, W. LITFULO(TM) (Ritlecitinib) Capsules: A Janus Kinase 3 Inhibitor for the Treatment of Severe Alopecia Areata. Skinmed 2023, 21, 434–438. [Google Scholar]
- Palakkal, S.; Cortial, A.; Frusic-Zlotkin, M.; Soroka, Y.; Tzur, T.; Nassar, T.; Benita, S. Effect of cyclosporine A—Tempol topical gel for the treatment of alopecia and anti-inflammatory disorders. Int. J. Pharm. 2023, 642, 123121. [Google Scholar] [CrossRef] [PubMed]
- Nowaczyk, J.; Makowska, K.; Rakowska, A.; Sikora, M.; Rudnicka, L. Cyclosporine with and without Systemic Corticosteroids in Treatment of Alopecia Areata: A Systematic Review. Dermatol. Ther. 2020, 10, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Avci, P.; Gupta, G.K.; Clark, J.; Wikonkal, N.; Hamblin, M.R. Low-level laser (light) therapy (LLLT) for treatment of hair loss. Lasers Surg. Med. 2014, 46, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Cantisani, C.; Melis, L.; Iorio, A.; Scali, E.; Calvieri, S. Minoxidil use in dermatology, side effects and recent patents. Recent. Pat. Inflamm. Allergy Drug Discov. 2012, 6, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Proctor, P.H. Endothelium-derived relaxing factor and minoxidil: Active mechanisms in hair growth. Arch. Dermatol. 1989, 125, 1146. [Google Scholar] [CrossRef] [PubMed]
- Messenger, A.G.; Rundegren, J. Minoxidil: Mechanisms of action on hair growth. Br. J. Dermatol. 2004, 150, 186–194. [Google Scholar] [CrossRef]
- McClellan, K.J.; Markham, A. Finasteride: A review of its use in male pattern hair loss. Drugs 1999, 57, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.R.; Bae, S.; Kim, J.Y.; Lee, J.; Cho, D.H.; Kim, H.S.; An, I.S.; An, S. Monoterpenoid Loliolide Regulates Hair Follicle Inductivity of Human Dermal Papilla Cells by Activating the Akt/beta-Catenin Signaling Pathway. J. Microbiol. Biotechnol. 2019, 29, 1830–1840. [Google Scholar] [CrossRef]
- Xing, F.; Yi, W.J.; Miao, F.; Su, M.Y.; Lei, T.C. Baicalin increases hair follicle development by increasing canonical Wnt/beta-catenin signaling and activating dermal papillar cells in mice. Int. J. Mol. Med. 2018, 41, 2079–2085. [Google Scholar] [PubMed]
- Park, G.H.; Park, K.Y.; Cho, H.I.; Lee, S.M.; Han, J.S.; Won, C.H.; Chang, S.E.; Lee, M.W.; Choi, J.H.; Moon, K.C.; et al. Red ginseng extract promotes the hair growth in cultured human hair follicles. J. Med. Food 2015, 18, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Li, N.; Ren, R.; Wang, Y.; Su, X.; Lu, F.; Zong, R.; Yang, L.; Ma, X. Progress in the Medicinal Value, Bioactive Compounds, and Pharmacological Activities of Gynostemma pentaphyllum. Molecules 2021, 26, 6249. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.Y. Hair-Growth Potential of Ginseng and Its Major Metabolites: A Review on Its Molecular Mechanisms. Int. J. Mol. Sci. 2018, 19, 2703. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Eneroth, P.; Bruhn, J.G. Gynostemma pentaphyllum: Identification of major sapogenins and differentiation from Panax species. Eur. J. Pharm. Sci. 1999, 8, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.H.; Ha, T.K.Q.; Yang, J.L.; Pham, H.T.T.; Oh, W.K. Triterpenoids from the genus Gynostemma: Chemistry and pharmacological activities. J. Ethnopharmacol. 2021, 268, 113574. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Lim, S.M.; Jung, J.I.; Kim, S.M.; Lee, J.K.; Kim, Y.H.; Cha, K.M.; Oh, T.K.; Moon, J.M.; Kim, T.Y.; et al. Gynostemma Pentaphyllum Extract Ameliorates High-Fat Diet-Induced Obesity in C57BL/6N Mice by Upregulating SIRT1. Nutrients 2019, 11, 2475. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.X.; Wang, Z.Z.; Du, Z.Z. Sensory-guided isolation and identification of new sweet-tasting dammarane-type saponins from Jiaogulan (Gynostemma pentaphyllum) herbal tea. Food Chem. 2022, 388, 132981. [Google Scholar] [CrossRef] [PubMed]
- Cheret, J.; Bertolini, M.; Ponce, L.; Lehmann, J.; Tsai, T.; Alam, M.; Hatt, H.; Paus, R. Olfactory receptor OR2AT4 regulates human hair growth. Nat. Commun. 2018, 9, 3624. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, T.; Yoshizato, K. Role of hair papilla cells on induction and regeneration processes of hair follicles. Wound Repair. Regen. 1998, 6, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Morgan, B.A. The dermal papilla: An instructive niche for epithelial stem and progenitor cells in development and regeneration of the hair follicle. Cold Spring Harb. Perspect. Med. 2014, 4, a015180. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.H.; Park, S.Y.; Kim, M.K.; Kim, J.C.; Sung, Y.K. Establishment and characterization of an immortalized human dermal papilla cell line. BMB Rep. 2011, 44, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Augustyniak, A.; McMahon, H. Effect of Marine-Derived Saccharides on Human Skin Fibroblasts and Dermal Papilla Cells. Mar. Drugs 2023, 21, 330. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Kim, M.; Hoang, D.H.; Kovale, L.M.; Lee, J.; Kim, Y.; Lee, C.; Hong, J.; Park, S.; Choe, W. A Hydrodistillate of Gynostemma pentaphyllum and Damulin B Prevent Cisplatin-Induced Nephrotoxicity In Vitro and In Vivo via Regulation of AMPKalpha1 Transcription. Nutrients 2022, 14, 4997. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.Y.; Kim, B.H.; Kim, D.W.; Lee, W.Y.; Kim, C.E.; Kim, H.Y.; Pyo, J.; Park, E.S.; Kang, K.S. Hair Growth Effect of DN106212 in C57BL/6 Mouse and Its Network Pharmacological Mechanism of Action. Curr. Issues Mol. Biol. 2023, 45, 5071–5083. [Google Scholar] [CrossRef] [PubMed]
- Muller-Rover, S.; Handjiski, B.; van der Veen, C.; Eichmuller, S.; Foitzik, K.; McKay, I.A.; Stenn, K.S.; Paus, R. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J. Investig. Dermatol. 2001, 117, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Suchonwanit, P.; Thammarucha, S.; Leerunyakul, K. Minoxidil and its use in hair disorders: A review. Drug Des. Devel Ther. 2019, 13, 2777–2786. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.Y. Targeting Wnt/beta-Catenin Pathway for Developing Therapies for Hair Loss. Int. J. Mol. Sci. 2020, 21, 4915. [Google Scholar] [CrossRef]
- Veltri, A.; Lang, C.; Lien, W.H. Concise Review: Wnt Signaling Pathways in Skin Development and Epidermal Stem Cells. Stem Cells 2018, 36, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Kwack, M.H.; Kang, B.M.; Kim, M.K.; Kim, J.C.; Sung, Y.K. Minoxidil activates beta-catenin pathway in human dermal papilla cells: A possible explanation for its anagen prolongation effect. J. Dermatol. Sci. 2011, 62, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Enshell-Seijffers, D.; Lindon, C.; Kashiwagi, M.; Morgan, B.A. beta-catenin activity in the dermal papilla regulates morphogenesis and regeneration of hair. Dev. Cell 2010, 18, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Yamane, M.; Seo, J.; Zhou, Y.; Asaba, T.; Tu, S.; Nanmo, A.; Kageyama, T.; Fukuda, J. Effects of the PI3K/Akt signaling pathway on the hair inductivity of human dermal papilla cells in hair beads. J. Biosci. Bioeng. 2022, 134, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fan, Z.; Wang, X.; Mo, M.; Zeng, S.B.; Xu, R.H.; Wang, X.; Wu, Y. PI3K/Akt signaling pathway is essential for de novo hair follicle regeneration. Stem Cell Res. Ther. 2020, 11, 144. [Google Scholar] [CrossRef] [PubMed]
- Abid, M.R.; Guo, S.; Minami, T.; Spokes, K.C.; Ueki, K.; Skurk, C.; Walsh, K.; Aird, W.C. Vascular endothelial growth factor activates PI3K/Akt/forkhead signaling in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Paus, R. Principles of hair cycle control. J. Dermatol. 1998, 25, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Ge, W.; Tan, S.J.; Wang, S.H.; Li, L.; Sun, X.F.; Shen, W.; Wang, X. Single-cell Transcriptome Profiling reveals Dermal and Epithelial cell fate decisions during Embryonic Hair Follicle Development. Theranostics 2020, 10, 7581–7598. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zhu, L.; He, J. Morphogenesis, Growth Cycle and Molecular Regulation of Hair Follicles. Front. Cell. Dev. Biol. 2022, 10, 899095. [Google Scholar] [CrossRef] [PubMed]
- Chi, W.; Wu, E.; Morgan, B.A. Dermal papilla cell number specifies hair size, shape and cycling and its reduction causes follicular decline. Development 2013, 140, 1676–1683. [Google Scholar] [CrossRef] [PubMed]
- Taghiabadi, E.; Nilforoushzadeh, M.A.; Aghdami, N. Maintaining Hair Inductivity in Human Dermal Papilla Cells: A Review of Effective Methods. Skin. Pharmacol. Physiol. 2020, 33, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.Y.; Sennett, R.; Rezza, A.; Clavel, C.; Grisanti, L.; Zemla, R.; Najam, S.; Rendl, M. Wnt/beta-catenin signaling in dermal condensates is required for hair follicle formation. Dev. Biol. 2014, 385, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Huelsken, J.; Vogel, R.; Erdmann, B.; Cotsarelis, G.; Birchmeier, W. beta-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell 2001, 105, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Tanner, M.A.; Bu, X.; Steimle, J.A.; Myers, P.R. The direct release of nitric oxide by gypenosides derived from the herb Gynostemma pentaphyllum. Nitric Oxide 1999, 3, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Liu, Y.; Song, Z.; Hao, F.; Yang, X. Identification of Wnt/beta-catenin signaling pathway in dermal papilla cells of human scalp hair follicles: TCF4 regulates the proliferation and secretory activity of dermal papilla cell. J. Dermatol. 2014, 41, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Ryu, Y.C.; Lee, D.H.; Shim, J.; Park, J.; Kim, Y.R.; Choi, S.; Bak, S.S.; Sung, Y.K.; Lee, S.H.; Choi, K.Y. KY19382, a novel activator of Wnt/beta-catenin signalling, promotes hair regrowth and hair follicle neogenesis. Br. J. Pharmacol. 2021, 178, 2533–2546. [Google Scholar] [CrossRef] [PubMed]
- Manning, B.D.; Cantley, L.C.; Cantley, L.C. AKT/PKB signaling: Navigating downstream. Cell 2007, 129, 1261–1274. [Google Scholar] [CrossRef]
- Kwack, M.H.; Shin, S.H.; Kim, S.R.; Im, S.U.; Han, I.S.; Kim, M.K.; Kim, J.C.; Sung, Y.K. l-Ascorbic acid 2-phosphate promotes elongation of hair shafts via the secretion of insulin-like growth factor-1 from dermal papilla cells through phosphatidylinositol 3-kinase. Br. J. Dermatol. 2009, 160, 1157–1162. [Google Scholar] [CrossRef] [PubMed]
- Lachgar, S.; Moukadiri, H.; Jonca, F.; Charveron, M.; Bouhaddioui, N.; Gall, Y.; Bonafe, J.L.; Plouet, J. Vascular endothelial growth factor is an autocrine growth factor for hair dermal papilla cells. J. Investig. Dermatol. 1996, 106, 17–23. [Google Scholar] [CrossRef]
- Shimaoka, S.; Imai, R.; Ogawa, H. Dermal papilla cells express hepatocyte growth factor. J. Dermatol. Sci. 1994, 7, S79–S83. [Google Scholar] [CrossRef] [PubMed]
- Booth, C.; Potten, C.S. Keratinocyte growth factor increases hair follicle survival following cytotoxic insult. J. Investig. Dermatol. 2000, 114, 667–673. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hunt, N.; McHale, S. The psychological impact of alopecia. BMJ 2005, 331, 951–953. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xie, X.; Zhang, G.; Zhang, Y. Comorbidities in Androgenetic Alopecia: A Comprehensive Review. Dermatol. Ther. 2022, 12, 2233–2247. [Google Scholar] [CrossRef] [PubMed]
- Urysiak-Czubatka, I.; Kmiec, M.L.; Broniarczyk-Dyla, G. Assessment of the usefulness of dihydrotestosterone in the diagnostics of patients with androgenetic alopecia. Postep. Dermatol. Alergol. 2014, 31, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Ustuner, E.T. Cause of androgenic alopecia: Crux of the matter. Plast. Reconstr. Surg. Glob. Open 2013, 1, e64. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovale, L.; Lee, S.; Song, M.; Lee, J.; Son, H.J.; Sung, Y.K.; Kwack, M.H.; Choe, W.; Kang, I.; Kim, S.S.; et al. Gynostemma pentaphyllum Hydrodistillate and Its Major Component Damulin B Promote Hair Growth-Inducing Properties In Vivo and In Vitro via the Wnt/β-Catenin Pathway in Dermal Papilla Cells. Nutrients 2024, 16, 985. https://doi.org/10.3390/nu16070985
Kovale L, Lee S, Song M, Lee J, Son HJ, Sung YK, Kwack MH, Choe W, Kang I, Kim SS, et al. Gynostemma pentaphyllum Hydrodistillate and Its Major Component Damulin B Promote Hair Growth-Inducing Properties In Vivo and In Vitro via the Wnt/β-Catenin Pathway in Dermal Papilla Cells. Nutrients. 2024; 16(7):985. https://doi.org/10.3390/nu16070985
Chicago/Turabian StyleKovale, Lochana, Seoyeon Lee, Minhyeok Song, Jihyun Lee, Hyeong Jig Son, Young Kwan Sung, Mi Hee Kwack, Wonchae Choe, Insug Kang, Sung Soo Kim, and et al. 2024. "Gynostemma pentaphyllum Hydrodistillate and Its Major Component Damulin B Promote Hair Growth-Inducing Properties In Vivo and In Vitro via the Wnt/β-Catenin Pathway in Dermal Papilla Cells" Nutrients 16, no. 7: 985. https://doi.org/10.3390/nu16070985
APA StyleKovale, L., Lee, S., Song, M., Lee, J., Son, H. J., Sung, Y. K., Kwack, M. H., Choe, W., Kang, I., Kim, S. S., & Ha, J. (2024). Gynostemma pentaphyllum Hydrodistillate and Its Major Component Damulin B Promote Hair Growth-Inducing Properties In Vivo and In Vitro via the Wnt/β-Catenin Pathway in Dermal Papilla Cells. Nutrients, 16(7), 985. https://doi.org/10.3390/nu16070985






