Soy Protein Concentrate Diets Inversely Affect LPS-Binding Protein Expression in Colon and Liver, Reduce Liver Inflammation, and Increase Fecal LPS Excretion in Obese Zucker Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Experimental Design
2.3. Quantitation of mRNA Expression by Quantitative PCR (qPCR)
2.4. Lipopolysaccharide (LPS) Quantification Using Limulus Amebocyte Lysate (LAL) Test
2.5. Statistical Analysis
3. Results
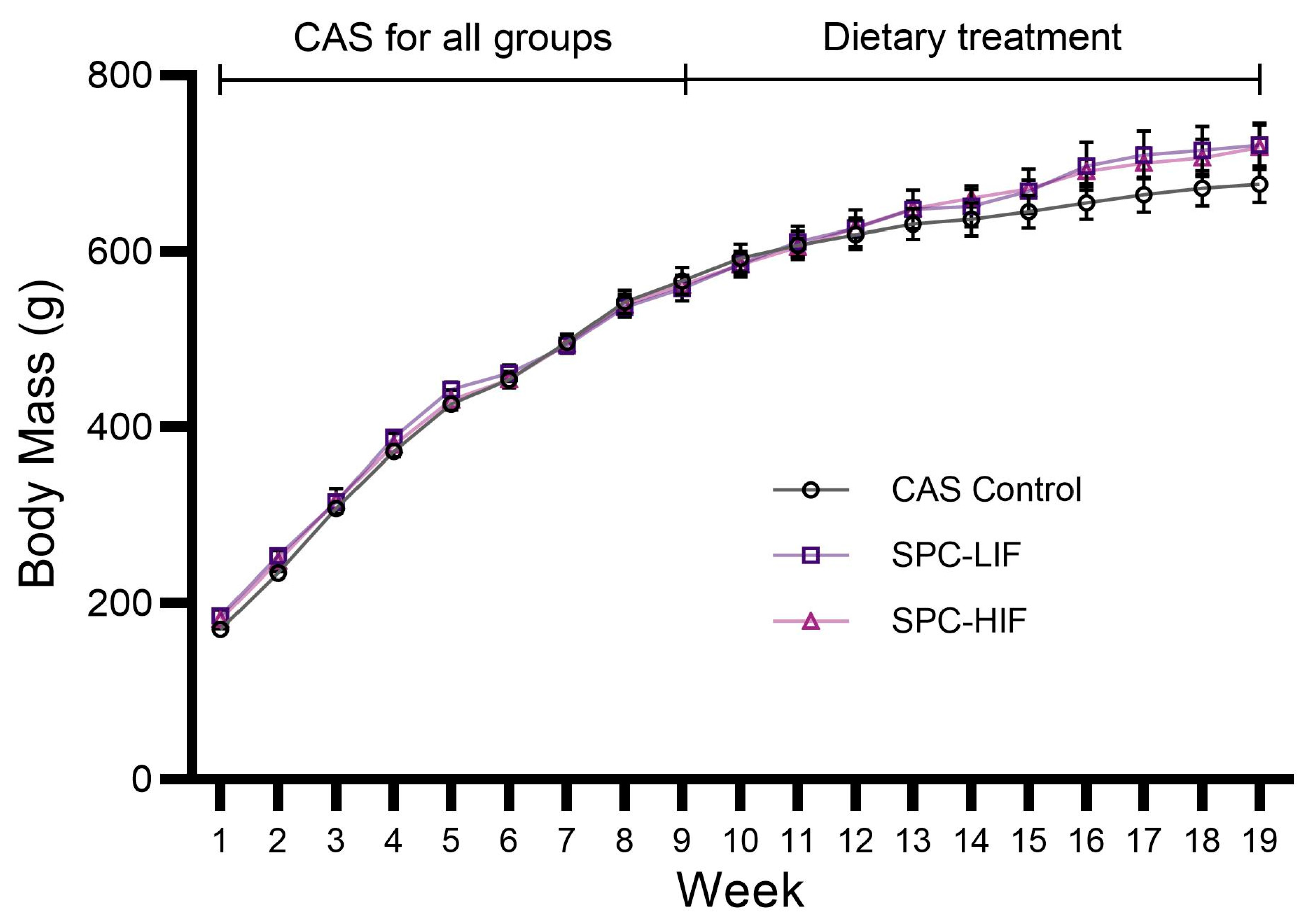
3.1. Body Mass of CAS, SPC-LIF, and SPC-HIF Diet-Fed Rats
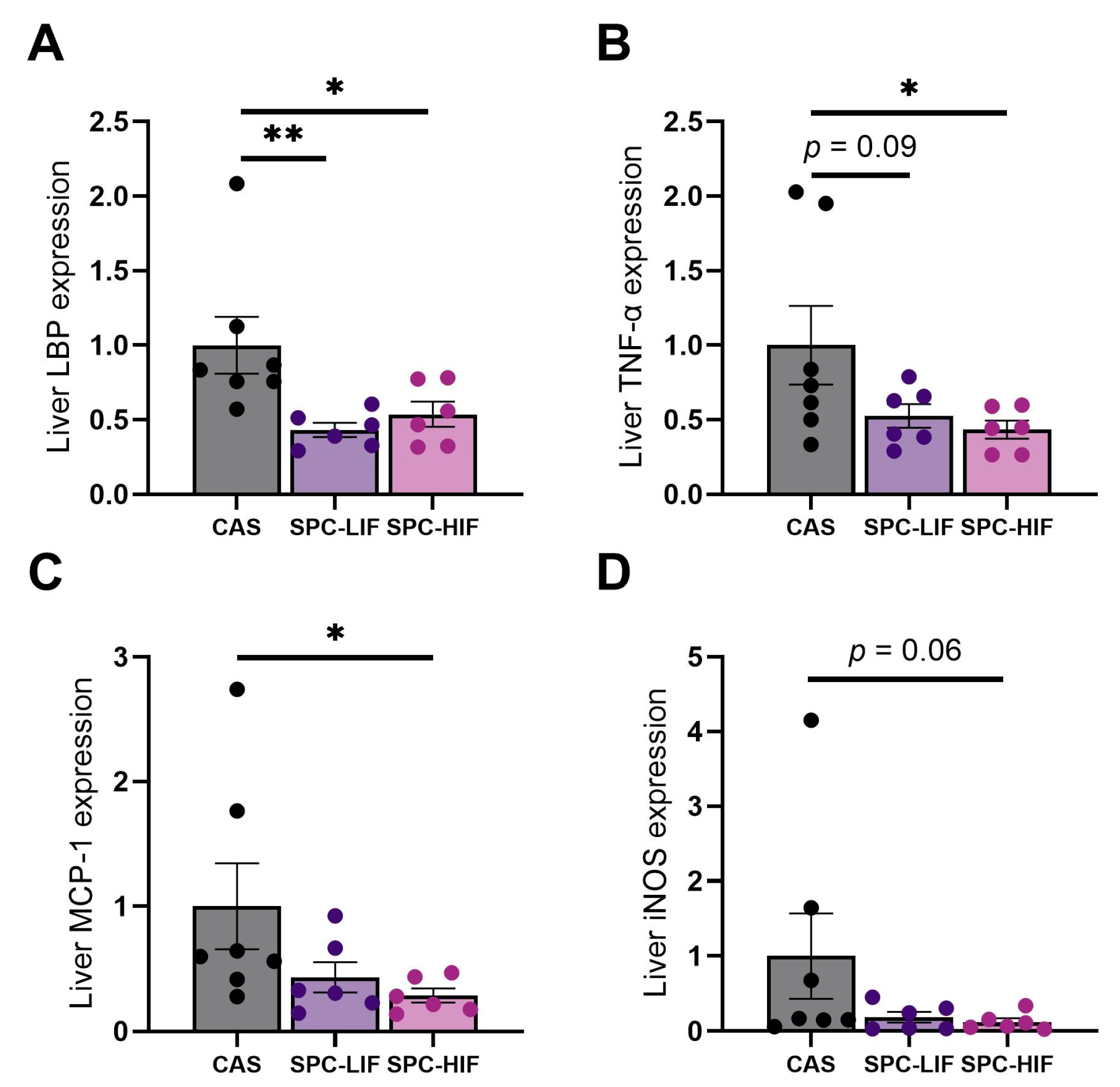
3.2. The Effect of SPC-LIF and SPC-HIF Diets on Liver Gene Expression
3.3. The Effect of SPC-LIF and SPC-HIF Diets on Colon Gene Expression
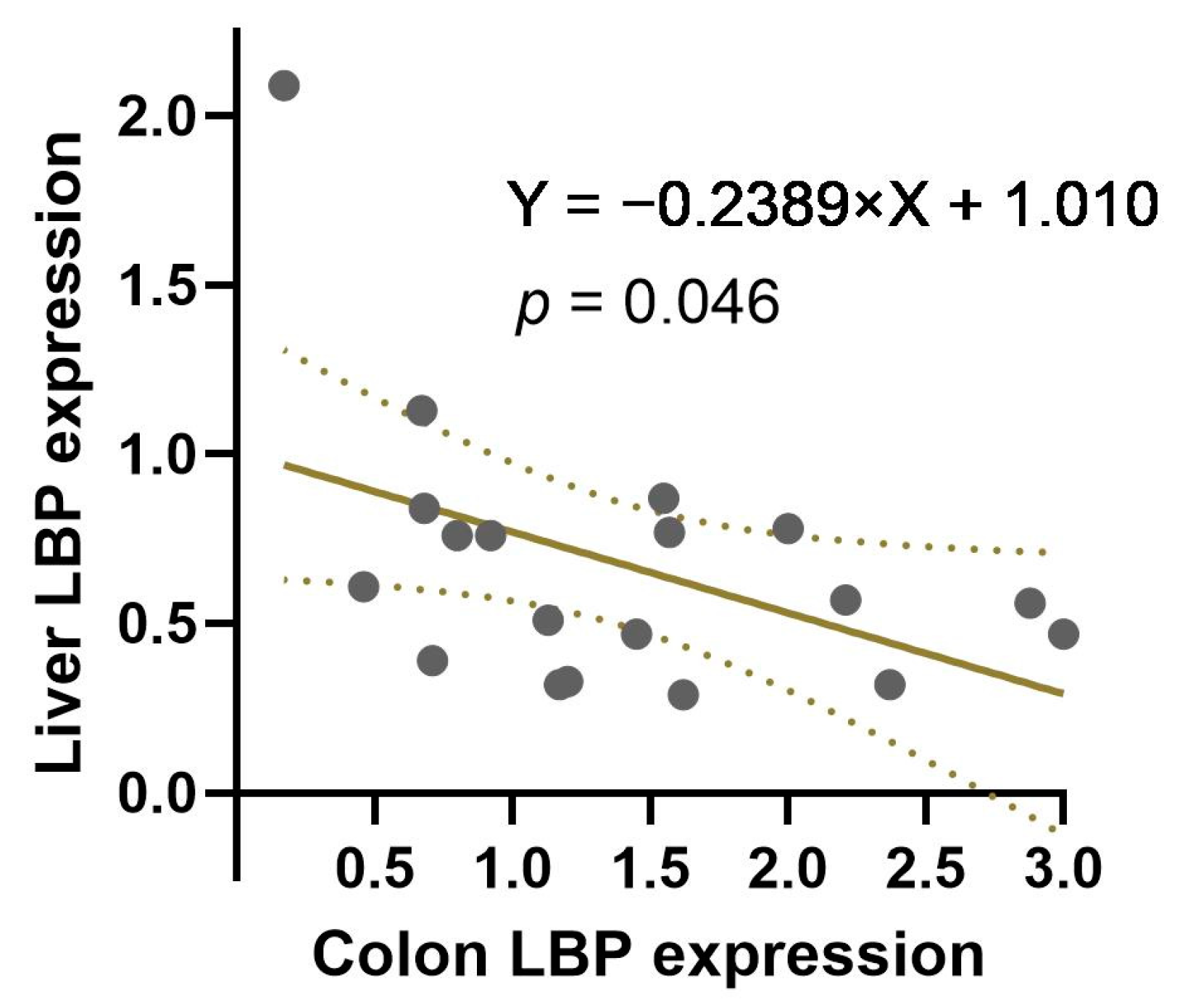
3.4. The Correlation between Colon and Liver LBP Expression
3.5. The Effect of SPC-LIF and SPC-HIF Diets on LPS Concentration in Colon Content and Fecal Samples
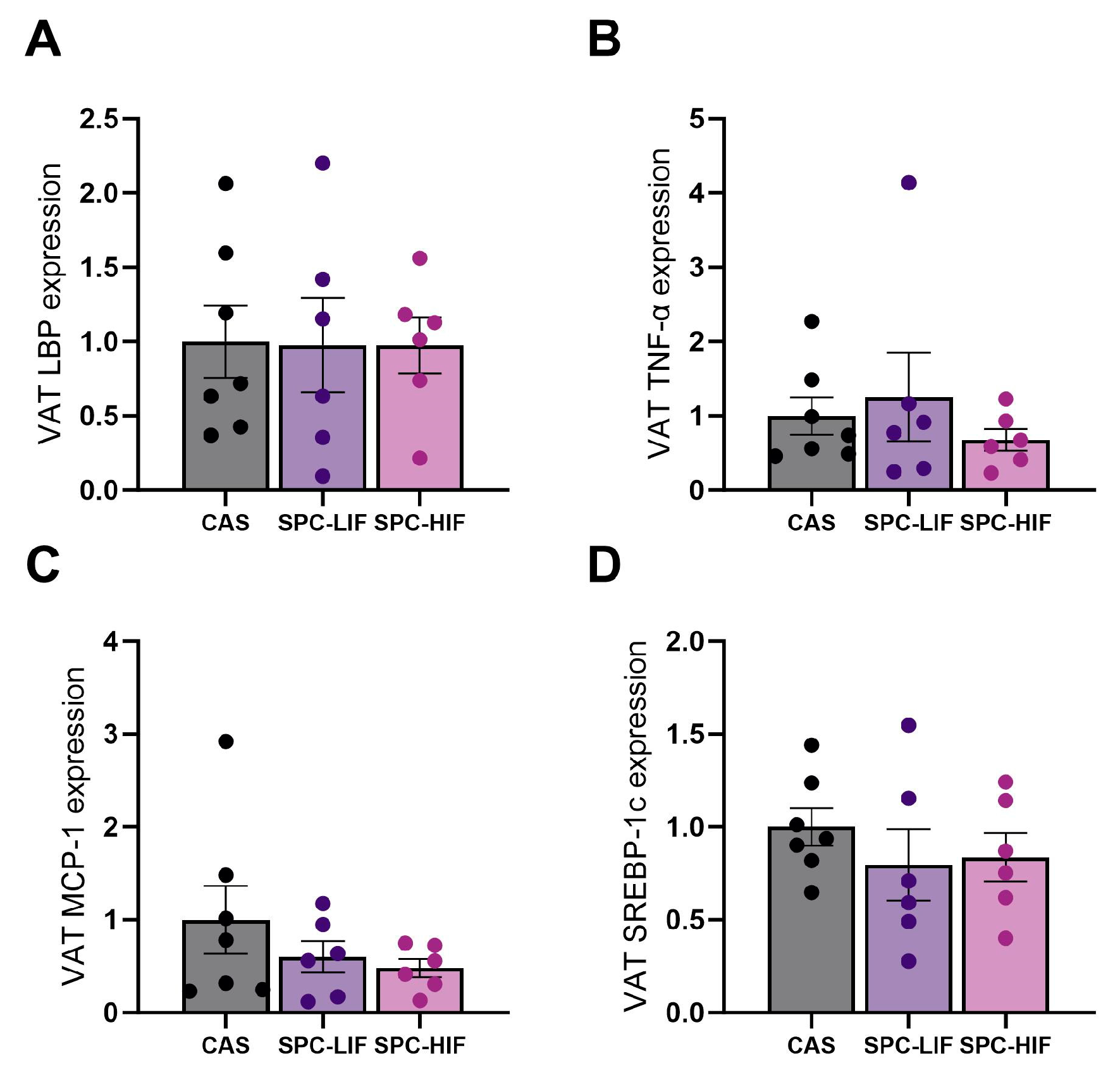
3.6. The Effect of SPC-LIF and SPC-HIF Diets on VAT Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Obesity Federation, World Obesity Atlas 2023. Available online: https://data.worldobesity.org/publications/?cat=19 (accessed on 22 February 2024).
- Nour, T.Y.; Altintaş, K.H. Effect of the COVID-19 pandemic on obesity and it is risk factors: A systematic review. BMC Public Health 2023, 23, 1018. [Google Scholar] [CrossRef]
- Cawley, J.; Biener, A.; Meyerhoefer, C.; Ding, Y.; Zvenyach, T.; Smolarz, B.G.; Ramasamy, A. Direct medical costs of obesity in the United States and the most populous states. J. Manag. Care Spec. Pharm. 2021, 27, 354–366. [Google Scholar] [CrossRef]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol.-Cell Physiol. 2020, 320, C375–C391. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Durack, J.; Lynch, S.V. The gut microbiome: Relationships with disease and opportunities for therapy. J. Exp. Med. 2019, 216, 20–40. [Google Scholar] [CrossRef]
- Afzaal, M.; Saeed, F.; Shah, Y.A.; Hussain, M.; Rabail, R.; Socol, C.T.; Hassoun, A.; Pateiro, M.; Lorenzo, J.M.; Rusu, A.V.; et al. Human gut microbiota in health and disease: Unveiling the relationship. Front. Microbiol. 2022, 13, 999001. [Google Scholar] [CrossRef]
- Ghosh, S.S.; Wang, J.; Yannie, P.J.; Ghosh, S. Intestinal Barrier Dysfunction, LPS Translocation, and Disease Development. J. Endocr. Soc. 2020, 4, bvz039. [Google Scholar] [CrossRef]
- An, L.; Wirth, U.; Koch, D.; Schirren, M.; Drefs, M.; Koliogiannis, D.; Nieß, H.; Andrassy, J.; Guba, M.; Bazhin, A.V.; et al. The Role of Gut-Derived Lipopolysaccharides and the Intestinal Barrier in Fatty Liver Diseases. J. Gastrointest. Surg. 2022, 26, 671–683. [Google Scholar] [CrossRef]
- Violi, F.; Cammisotto, V.; Bartimoccia, S.; Pignatelli, P.; Carnevale, R.; Nocella, C. Gut-derived low-grade endotoxaemia, atherothrombosis and cardiovascular disease. Nat. Rev. Cardiol. 2023, 20, 24–37. [Google Scholar] [CrossRef]
- Neves, A.L.; Coelho, J.; Couto, L.; Leite-Moreira, A.; Roncon-Albuquerque, R. Metabolic endotoxemia: A molecular link between obesity and cardiovascular risk. J. Mol. Endocrinol. 2013, 51, R51–R64. [Google Scholar] [CrossRef]
- Clemente-Postigo, M.; Oliva-Olivera, W.; Coin-Aragüez, L.; Ramos-Molina, B.; Giraldez-Perez, R.M.; Lhamyani, S.; Alcaide-Torres, J.; Perez-Martinez, P.; Bekay, R.E.; Cardona, F.; et al. Metabolic endotoxemia promotes adipose dysfunction and inflammation in human obesity. Am. J. Physiol.-Endocrinol. Metab. 2019, 316, E319–E332. [Google Scholar] [CrossRef] [PubMed]
- Erlanson-Albertsson, C.; Stenkula, K.G. The Importance of Food for Endotoxemia and an Inflammatory Response. Int. J. Mol. Sci. 2021, 22, 9562. [Google Scholar] [CrossRef] [PubMed]
- Messina, M. Soy and Health Update: Evaluation of the Clinical and Epidemiologic Literature. Nutrients 2016, 8, 754. [Google Scholar] [CrossRef] [PubMed]
- Badger, T.M.; Ronis, M.J.; Hakkak, R.; Rowlands, J.C.; Korourian, S. The health consequences of early soy consumption. J. Nutr. 2002, 132, 559s–565s. [Google Scholar] [CrossRef] [PubMed]
- Hakkak, R.; Korourian, S.; Shelnutt, S.R.; Lensing, S.; Ronis, M.J.; Badger, T.M. Diets containing whey proteins or soy protein isolate protect against 7,12-dimethylbenz(a)anthracene-induced mammary tumors in female rats. Cancer Epidemiol. Biomark. Prev. 2000, 9, 113–117. [Google Scholar]
- Belobrajdic, D.P.; James-Martin, G.; Jones, D.; Tran, C.D. Soy and Gastrointestinal Health: A Review. Nutrients 2023, 15, 1959. [Google Scholar] [CrossRef] [PubMed]
- Soy Protein Ingredients Commodity Fact Sheet. Available online: https://2012-2017.usaid.gov/what-we-do/agriculture-and-food-security/food-assistance/resources/soy-protein-ingredients-commodity (accessed on 19 March 2024).
- Sacks, F.M.; Lichtenstein, A.; Horn, L.V.; Harris, W.; Kris-Etherton, P.; Winston, M. Soy Protein, Isoflavones, and Cardiovascular Health. Circulation 2006, 113, 1034–1044. [Google Scholar] [CrossRef]
- Kalaiselvan, V.; Kalaivani, M.; Vijayakumar, A.; Sureshkumar, K.; Venkateskumar, K. Current knowledge and future direction of research on soy isoflavones as a therapeutic agents. Pharmacogn. Rev. 2010, 4, 111–117. [Google Scholar] [CrossRef]
- Miadoková, E. Isoflavonoids—An overview of their biological activities and potential health benefits. Interdiscip. Toxicol. 2009, 2, 211–218. [Google Scholar] [CrossRef]
- Li, W.; Hakkak, R. Feeding soy protein concentrates with low or high isoflavone decreases liver inflammation by reducing lipopolysaccharide translocation. Front. Nutr. 2023, 10, 1278158. [Google Scholar] [CrossRef]
- Hakkak, R.; Spray, B.; Børsheim, E.; Korourian, S. Diet Containing Soy Protein Concentrate with Low and High Isoflavones for 9 Weeks Protects against Non-alcoholic Fatty Liver Steatosis Using Obese Zucker Rats. Front. Nutr. 2022, 9, 913571. [Google Scholar] [CrossRef] [PubMed]
- Robeson, M.S.; Manna, K.; Randolph, C.; Byrum, S.; Hakkak, R. Short-Term Metformin Treatment Enriches Bacteroides dorei in an Obese Liver Steatosis Zucker Rat Model. Front. Microbiol. 2022, 13, 834776. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, Y.; Yu, B.; Lu, R. An optimized TRIzol-based method for isolating RNA from adipose tissue. Biotechniques 2023, 74, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Epeldegui, M.; Magpantay, L.; Guo, Y.; Halec, G.; Cumberland, W.G.; Yen, P.K.; Macatangay, B.; Margolick, J.B.; Rositch, A.F.; Wolinsky, S.; et al. A prospective study of serum microbial translocation biomarkers and risk of AIDS-related non-Hodgkin lymphoma. AIDS 2018, 32, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Boutagy, N.E.; McMillan, R.P.; Frisard, M.I.; Hulver, M.W. Metabolic endotoxemia with obesity: Is it real and is it relevant? Biochimie 2016, 124, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Sankararaman, S.; Noriega, K.; Velayuthan, S.; Sferra, T.; Martindale, R. Gut Microbiome and Its Impact on Obesity and Obesity-Related Disorders. Curr. Gastroenterol. Rep. 2023, 25, 31–44. [Google Scholar] [CrossRef] [PubMed]
- DiMattia, Z.; Damani, J.J.; Van Syoc, E.; Rogers, C.J. Effect of Probiotic Supplementation on Intestinal Permeability in Overweight and Obesity: A Systematic Review of Randomized Controlled Trials and Animal Studies. Adv. Nutr. 2024, 15, 100162. [Google Scholar] [CrossRef] [PubMed]
- Portincasa, P.; Bonfrate, L.; Khalil, M.; Angelis, M.; Calabrese, F.M.; D’Amato, M.; Wang, D.Q.; Di Ciaula, A. Intestinal Barrier and Permeability in Health, Obesity and NAFLD. Biomedicines 2021, 10, 83. [Google Scholar] [CrossRef]
- Wen, Y.; Lambrecht, J.; Ju, C.; Tacke, F. Hepatic macrophages in liver homeostasis and diseases-diversity, plasticity and therapeutic opportunities. Cell. Mol. Immunol. 2021, 18, 45–56. [Google Scholar] [CrossRef]
- Gäbele, E.; Dostert, K.; Hofmann, C.; Wiest, R.; Schölmerich, J.; Hellerbrand, C.; Obermeier, F. DSS induced colitis increases portal LPS levels and enhances hepatic inflammation and fibrogenesis in experimental NASH. J. Hepatol. 2011, 55, 1391–1399. [Google Scholar] [CrossRef]
- Ceccarelli, S.; Panera, N.; Mina, M.; Gnani, D.; De Stefanis, C.; Crudele, A.; Rychlicki, C.; Petrini, S.; Bruscalupi, G.; Agostinelli, L.; et al. LPS-induced TNF-α factor mediates pro-inflammatory and pro-fibrogenic pattern in non-alcoholic fatty liver disease. Oncotarget 2015, 6, 41434–41452. [Google Scholar] [CrossRef]
- Kakino, S.; Ohki, T.; Nakayama, H.; Yuan, X.; Otabe, S.; Hashinaga, T.; Wada, N.; Kurita, Y.; Tanaka, K.; Hara, K.; et al. Pivotal Role of TNF-α in the Development and Progression of Nonalcoholic Fatty Liver Disease in a Murine Model. Horm. Metab. Res. 2018, 50, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Wandrer, F.; Liebig, S.; Marhenke, S.; Vogel, A.; John, K.; Manns, M.P.; Teufel, A.; Itzel, T.; Longerich, T.; Maier, O.; et al. TNF-Receptor-1 inhibition reduces liver steatosis, hepatocellular injury and fibrosis in NAFLD mice. Cell Death Dis. 2020, 11, 212. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Krishnan, H.B.; Pham, Q.; Yu, L.L.; Wang, T.T.Y. Soy and Gut Microbiota: Interaction and Implication for Human Health. J. Agric. Food Chem. 2016, 64, 8695–8709. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Lin, X.; Zhao, F.; Shi, X.; Li, H.; Li, Y.; Zhu, W.; Xu, X.; Li, C.; Zhou, G. Meat, dairy and plant proteins alter bacterial composition of rat gut bacteria. Sci. Rep. 2015, 5, 15220. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.-E.; Kim, K.-A.; Han, M.J.; Kim, D.-H. Doenjang, a Fermented Korean Soybean Paste, Inhibits Lipopolysaccharide Production of Gut Microbiota in Mice. J. Med. Food 2014, 17, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Herath, M.; Hosie, S.; Bornstein, J.C.; Franks, A.E.; Hill-Yardin, E.L. The Role of the Gastrointestinal Mucus System in Intestinal Homeostasis: Implications for Neurological Disorders. Front. Cell. Infect. Microbiol. 2020, 10, 520859. [Google Scholar] [CrossRef] [PubMed]
- Vreugdenhil, A.C.E.; Snoek, A.M.P.; Greve, J.W.M.; Buurman, W.A. Lipopolysaccharide-Binding Protein Is Vectorially Secreted and Transported by Cultured Intestinal Epithelial Cells and Is Present in the Intestinal Mucus of Mice1. J. Immunol. 2000, 165, 4561–4566. [Google Scholar] [CrossRef]
- Richter, J.M.; Schanbacher, B.L.; Huang, H.; Xue, J.; Bauer, J.A.; Giannone, P.J. LPS-binding protein enables intestinal epithelial restitution despite LPS exposure. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 639–644. [Google Scholar] [CrossRef]
- Health Information–Soy. Available online: https://www.nccih.nih.gov/health/soy (accessed on 19 March 2024).


| Gene Name | Species | Assay ID (ThermoFisher) |
|---|---|---|
| Tnfa | Rat | Rn01525859_g1 |
| Ccl2/Mcp1 | Rat | Rn00580555_m1 |
| iNos/Nos2 | Rat | Rn00561646_m1 |
| Lbp | Rat | Rn00567985_m1 |
| Srebp1c | Rat | Rn01495769_m1 |
| Ocln | Rat | Rn00580064_m1 |
| Cldn3 | Rat | Rn00581751_s1 |
| Zo-1/Tjp1 | Rat | Rn02116071_s1 |
| 18s rRna | Rat | Rn03928990_g1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Hakkak, R. Soy Protein Concentrate Diets Inversely Affect LPS-Binding Protein Expression in Colon and Liver, Reduce Liver Inflammation, and Increase Fecal LPS Excretion in Obese Zucker Rats. Nutrients 2024, 16, 982. https://doi.org/10.3390/nu16070982
Li W, Hakkak R. Soy Protein Concentrate Diets Inversely Affect LPS-Binding Protein Expression in Colon and Liver, Reduce Liver Inflammation, and Increase Fecal LPS Excretion in Obese Zucker Rats. Nutrients. 2024; 16(7):982. https://doi.org/10.3390/nu16070982
Chicago/Turabian StyleLi, Wei, and Reza Hakkak. 2024. "Soy Protein Concentrate Diets Inversely Affect LPS-Binding Protein Expression in Colon and Liver, Reduce Liver Inflammation, and Increase Fecal LPS Excretion in Obese Zucker Rats" Nutrients 16, no. 7: 982. https://doi.org/10.3390/nu16070982
APA StyleLi, W., & Hakkak, R. (2024). Soy Protein Concentrate Diets Inversely Affect LPS-Binding Protein Expression in Colon and Liver, Reduce Liver Inflammation, and Increase Fecal LPS Excretion in Obese Zucker Rats. Nutrients, 16(7), 982. https://doi.org/10.3390/nu16070982






