Extract from Aronia melanocarpa, Lonicera caerulea, and Vaccinium myrtillus Improves near Visual Acuity in People with Presbyopia
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of AKB Extract
2.3. Quantification of Compounds by HPLC-PDA
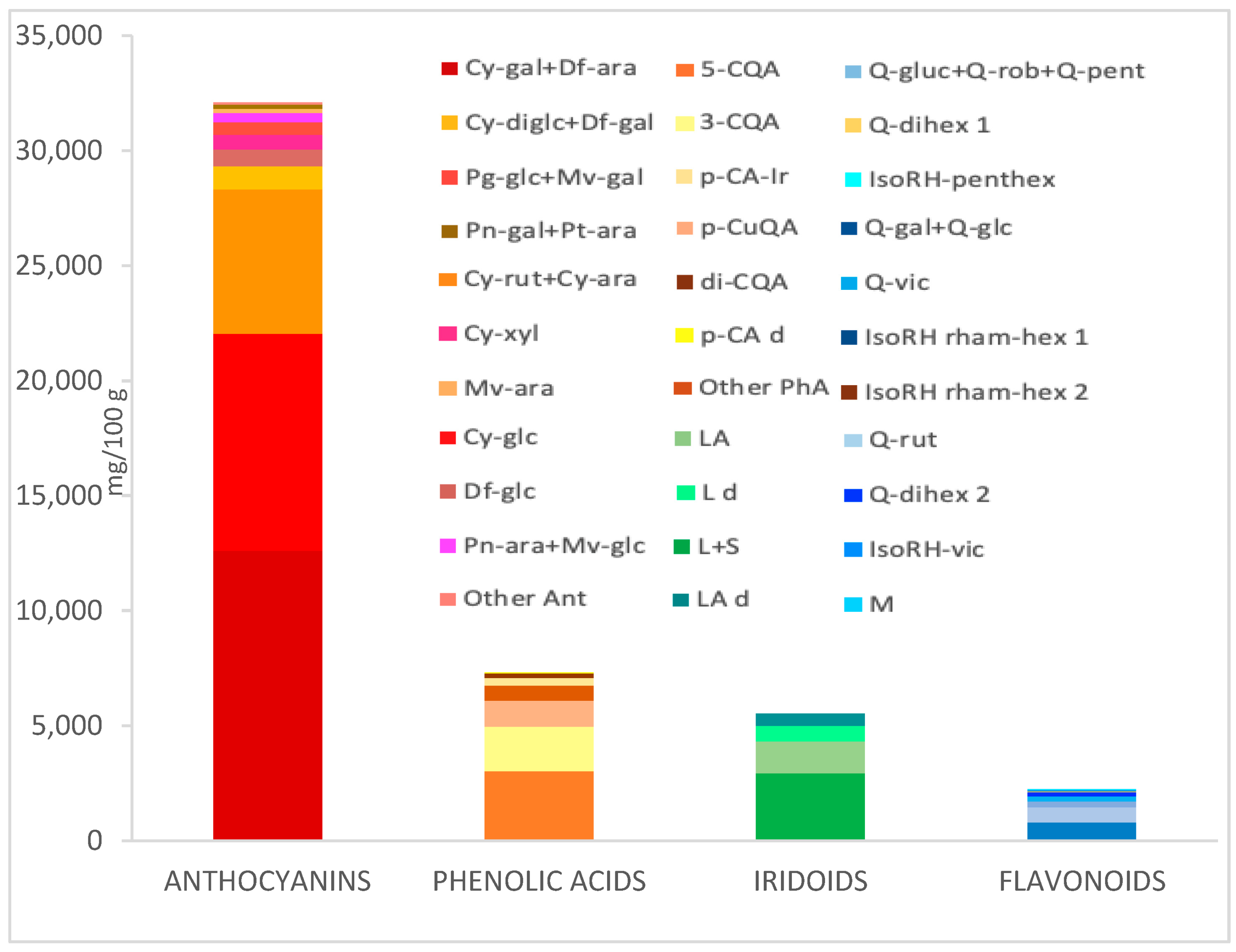
2.4. Composition of the Preparation (Composition of Extracts) AKB Prepared for the Study
2.5. Study Group, Conditions for Inclusion in the Study
2.6. The Study Being Conducted
3. Results
3.1. The Effect of Using the Test Product (AKB) in the First and Second Stages of the Study
3.2. Comparison of the Study Group with the Control Group in Terms of the Occurrence of Improvement in Near Visual Acuity in the First and Second Stages of the Study
Percentage Distributions of Supplementation Effects on Near Visual Acuity in the Test and Control Groups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Korenfeld, M.S.; Robertson, S.M.; Stein, J.M.; Evans, D.G.; Rauchman, S.H.; Sall, K.N.; Venkataraman, S.; Chen, B.-L.; Wuttke, M.; Burns, W. Topical lipoic acid choline ester eye drop for improvement of near visual acuity in subjects with presbyopia: A safety and preliminary efficacy trial. Eye 2021, 35, 3292–3301. [Google Scholar] [CrossRef]
- Mercer, R.N.; Milliken, C.M.; Waring, G.O., IV; Rocha, K.M. Future trends in presbyopia correction. J. Refract. Surg. 2021, 37, S28–S34. [Google Scholar] [CrossRef]
- Grzybowski, A.; Markeviciute, A.; Zemaitiene, R. A Review of Pharmacological Presbyopia Treatment. Asia Pac. J. Ophthalmol. 2020, 9, 226–233. [Google Scholar] [CrossRef]
- Truscott, R.J. Presbyopia. Emerging from a blur towards an understanding of the molecular basis for this most common eye condition. Exp. Eye Res. 2009, 88, 241–247. [Google Scholar] [CrossRef]
- Wolffsohn, J.S.; Arita, R.; Chalmers, R.; Djalilian, A.; Dogru, M.; Dumbleton, K.; Gupta, P.K.; Karpecki, P.; Lazreg, S.; Pult, H. TFOS DEWS II diagnostic methodology report. Ocul. Surf. 2017, 15, 539–574. [Google Scholar] [CrossRef] [PubMed]
- Benozzi, J.; Benozzi, G.; Orman, B. Presbyopia: A new potential pharmacological treatment. Med. Hypothesis Discov. Innov. Ophthalmol. 2012, 1, 3. [Google Scholar] [PubMed]
- Katz, J.A.; Karpecki, P.M.; Dorca, A.; Chiva-Razavi, S.; Floyd, H.; Barnes, E.; Wuttke, M.; Donnenfeld, E. Presbyopia—A Review of Current Treatment Options and Emerging Therapies. Clin. Ophthalmol. 2021, 15, 2167–2178. [Google Scholar] [CrossRef] [PubMed]
- Grzybowski, A.; Ruamviboonsuk, V. Pharmacological treatment in presbyopia. J. Clin. Med. 2022, 11, 1385. [Google Scholar] [CrossRef]
- Johnson, E.J.; Vishwanathan, R.; Rasmussen, H.M.; Lang, J.C. Bioavailability of AREDS1 micronutrients from softgel capsules and tablets: A pilot study. Mol. Vis. 2014, 20, 1228. [Google Scholar]
- Chu, W.K.; Cheung, S.C.M.; Lau, R.A.W.; Benzie, I.F.F. Bilberry (Vaccinium myrtillus L.). In Herbal Medicine: Biomolecular and Clinical Aspects; Benzie, I.F.F., Wachtel-Galor, S., Eds.; CRC Press, Taylor & Francis: Boca Raton, FL, USA, 2011. [Google Scholar]
- Nomi, Y.; Iwasaki-Kurashige, K.; Matsumoto, H. Therapeutic effects of anthocyanins for vision and eye health. Molecules 2019, 24, 3311. [Google Scholar] [CrossRef]
- Viljoen, A.; Mncwangi, N.; Vermaak, I. Anti-inflammatory iridoids of botanical origin. Curr. Med. Chem. 2012, 19, 2104–2127. [Google Scholar] [CrossRef]
- Danielewski, M.; Matuszewska, A.; Nowak, B.; Kucharska, A.Z.; Sozański, T. The effects of natural iridoids and anthocyanins on selected parameters of liver and cardiovascular system functions. Oxidative Med. Cell. Longev. 2020, 2020, 2735790. [Google Scholar] [CrossRef]
- Danielewski, M.; Matuszewska, A.; Szeląg, A.; Sozański, T. The impact of anthocyanins and iridoids on transcription factors crucial for lipid and cholesterol homeostasis. Int. J. Mol. Sci. 2021, 22, 6074. [Google Scholar] [CrossRef]
- Grulová, D.; De Feo, V. Euphrasia rostkoviana Hayne-active components and biological activity for the treatment of eye disorders. Nauk Vìsn. Užgorod. Unìv. Ser. Hìm. 2017, 1, 1–9. [Google Scholar]
- Moisanen, S. The Effect of Anthocyanins on Accommodation in Visual Display Terminal Work. 2023. Available online: www.theseus.fi/bitstream/handle/10024/792458/Moisanen_Suvi.pdf?sequence=4&isAllowed=y (accessed on 1 January 2024).
- Szumny, D.; Sozański, T.; Kucharska, A.Z.; Dziewiszek, W.; Piórecki, N.; Magdalan, J.; Chlebda-Sieragowska, E.; Kupczynski, R.; Szeląg, A.; Szumny, A. Application of Cornelian Cherry Iridoid-Polyphenolic Fraction and Loganic Acid to Reduce Intraocular Pressure. Evid. Based Complement. Altern. Med. 2015, 2015, 939402. [Google Scholar] [CrossRef]
- Ohgami, K.; Ilieva, I.; Shiratori, K.; Koyama, Y.; Jin, X.H.; Yoshida, K.; Kase, S.; Kitaichi, N.; Suzuki, Y.; Tanaka, T.; et al. Anti-inflammatory effects of aronia extract on rat endotoxin-induced uveitis. Invest. Ophthalmol. Vis. Sci. 2005, 46, 275–281. [Google Scholar] [CrossRef]
- Lee, J.; Lee, H.K.; Kim, C.Y.; Hong, Y.J.; Choe, C.M.; You, T.W.; Seong, G.J. Purified high-dose anthocyanoside oligomer administration improves nocturnal vision and clinical symptoms in myopia subjects. Br. J. Nutr. 2005, 93, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Chien, K.J.; Horng, C.T.; Huang, Y.S.; Hsieh, Y.H.; Wang, C.J.; Yang, J.S.; Lu, C.C.; Chen, F.A. Effects of Lycium barbarum (goji berry) on dry eye disease in rats. Mol. Med. Rep. 2018, 17, 809–818. [Google Scholar] [CrossRef]
- Sheppard, J.; Shen Lee, B.; Periman, L.M. Dry eye disease: Identification and therapeutic strategies for primary care clinicians and clinical specialists. Ann. Med. 2023, 55, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Urata, K.; Maeda, S.; Ohno, Y.; Satoh, K.; Yamada, Y.; Suzuki, Y.; Koga, Y.; Sugamoto, K.; Kawaguchi, M. Blueberry leaf extract prevents lacrimal hyposecretion in Sjögren’s syndrome-like model of non-obese diabetic mice. Vivo 2023, 37, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Przybylska, D.; Kucharska, A.Z.; Cybulska, I.; Sozański, T.; Piórecki, N.; Fecka, I. Cornus mas L. stones: A valuable by-product as an ellagitannin source with high antioxidant potential. Molecules 2020, 25, 4646. [Google Scholar] [CrossRef] [PubMed]
- Kucharska, A.Z.; Sokół-Łętowska, A.; Oszmiański, J.; Piórecki, N.; Fecka, I. Iridoids, phenolic compounds and antioxidant activity of edible honeysuckle berries (Lonicera caerulea var. kamtschatica Sevast.). Molecules 2017, 22, 405. [Google Scholar] [CrossRef] [PubMed]
- Mech-Nowak, A.; Kruczek, M.; Kaszycki, P.; Bieniasz, M.; Kostecka-Gugala, A. Polyphenols, carboxylic hydroxyacids and carotenoids in berries of blue honeysuckle (Lonicera coerulea var. kamtschatica). Przem. Chem. 2014, 93, 948–953. [Google Scholar]
- Ren, Y.; Frank, T.; Meyer, G.; Lei, J.; Grebenc, J.R.; Slaughter, R.; Gao, Y.G.; Kinghorn, A.D. Potential Benefits of Black Chokeberry (Aronia melanocarpa) Fruits and Their Constituents in Improving Human Health. Molecules 2022, 27, 7823. [Google Scholar] [CrossRef] [PubMed]
- Shvets, V.; Maslak, H.; Davydov, V.; Berest, H.; Nosulenko, I. The effect of Aronia melanocarpa extract on the phospholipid composition of the rat myocardium during stress. Ceska Slov. Farm 2022, 71, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.S.; Lee, E.B.; Lee, S.J.; Lee, S.P.; Boby, N.; Suk, K.; Birhanu, B.T.; Park, S.C. Aronia melanocarpa Extract Fermented by Lactobacillus plantarum EJ2014 Modulates Immune Response in Mice. Antioxidants 2021, 10, 1276. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, X.; Zheng, Y.; Liu, W.; Ding, C. Aronia melanocarpa polysaccharide ameliorates inflammation and aging in mice by modulating the AMPK/SIRT1/NF-κB signaling pathway and gut microbiota. Sci. Rep. 2021, 11, 20558. [Google Scholar] [CrossRef]
- Her, Y.; Lee, T.K.; Kim, J.D.; Kim, B.; Sim, H.; Lee, J.C.; Ahn, J.H.; Park, J.H.; Lee, J.W.; Hong, J.; et al. Topical Application of Aronia melanocarpa Extract Rich in Chlorogenic Acid and Rutin Reduces UVB-Induced Skin Damage via Attenuating Collagen Disruption in Mice. Molecules 2020, 25, 4577. [Google Scholar] [CrossRef]
- Jurikova, T.; Mlcek, J.; Skrovankova, S.; Sumczynski, D.; Sochor, J.; Hlavacova, I.; Snopek, L.; Orsavova, J. Fruits of Black Chokeberry Aronia melanocarpa in the Prevention of Chronic Diseases. Molecules 2017, 22, 944. [Google Scholar] [CrossRef]
- Denev, P.N.; Kratchanov, C.G.; Ciz, M.; Lojek, A.; Kratchanova, M.G. Bioavailability and antioxidant activity of black chokeberry (Aronia melanocarpa) polyphenols: In vitro and in vivo evidences and possible mechanisms of action: A review. Compr. Rev. Food Sci. Food Saf. 2012, 11, 471–489. [Google Scholar] [CrossRef]
- Xing, Y.; Liang, S.; Zhao, Y.; Yang, S.; Ni, H.; Li, H. Protection of Aronia melanocarpa Fruit Extract from Sodium-Iodate-Induced Damages in Rat Retina. Nutrients 2021, 13, 4411. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.H.; Ohgami, K.; Shiratori, K.; Suzuki, Y.; Koyama, Y.; Yoshida, K.; Ilieva, I.; Tanaka, T.; Onoe, K.; Ohno, S. Effects of blue honeysuckle (Lonicera caerulea L.) extract on lipopolysaccharide-induced inflammation in vitro and in vivo. Exp. Eye Res. 2006, 82, 860–867. [Google Scholar] [CrossRef]
- Choo, P.P.; Woi, P.J.; Bastion, M.C.; Omar, R.; Mustapha, M.; Md Din, N. Review of Evidence for the Usage of Antioxidants for Eye Aging. BioMed Res. Int. 2022, 2022, 5810373. [Google Scholar] [CrossRef]
- Canter, P.H.; Ernst, E. Anthocyanosides of Vaccinium myrtillus (bilberry) for night vision—A systematic review of placebo-controlled trials. Surv. Ophthalmol. 2004, 49, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/214028s000lbl.pdf (accessed on 1 January 2024).
- Haghpanah, N.; Alany, R. Pharmacological treatment of presbyopia: A systematic review. Eur. J. Transl. Myol. 2022, 32, 10781. [Google Scholar] [CrossRef]
- Tsuneyoshi, Y.; Higuchi, A.; Negishi, K.; Tsubota, K. Suppression of presbyopia progression with pirenoxine eye drops: Experiments on rats and non-blinded, randomized clinical trial of efficacy. Sci. Rep. 2017, 7, 6819. [Google Scholar] [CrossRef] [PubMed]
- Kono, K.; Shimizu, Y.; Takahashi, S.; Matsuoka, S.; Yui, K. Effect of Multiple Dietary Supplement Containing Lutein, Astaxanthin, Cyanidin-3-Glucoside, and DHA on Accommodative Ability. Curr. Med. Chem. 2014, 14, 114–125. [Google Scholar] [CrossRef]
- Horng, C.T.; Ma, J.W.; Shieh, P.C. Improvement of Presbyopia Using a Mixture of Traditional Chinese Herbal Medicines, Including Cassiae Semen, Wolfberry, and Dendrobium huoshanense. Evid. Based Complement. Alternat. Med. 2021, 2021, 9902211. [Google Scholar] [CrossRef]
- Traditional Chinese Medicine for Treating Presbyopia. Available online: https://patents.google.com/patent/CN101450176A/en (accessed on 1 January 2024).
- Swaminathan, M.; Chee, C.F.; Chin, S.P.; Buckle, M.J.; Rahman, N.A.; Doughty, S.W.; Chung, L.Y. Flavonoids with M1 muscarinic acetylcholine receptor binding activity. Molecules 2014, 19, 8933–8948. [Google Scholar] [CrossRef]
- Chung, L.Y.; Yap, K.F.; Goh, S.H.; Mustafa, M.R.; Imiyabir, Z. Muscarinic receptor binding activity of polyoxygenated flavones from Melicope subunifoliolata. Phytochemistry 2008, 69, 1548–1554. [Google Scholar] [CrossRef]
- Gholamnezhad, Z.; Ghorani, V.; Saadat, S.; Shakeri, F.; Boskabady, M.H. The effects of medicinal plants on muscarinic receptors in various types of smooth muscle. Phytother. Res. 2018, 32, 2340–2363. [Google Scholar] [CrossRef]
- Khan, H.; Marya; Amin, S.; Kamal, M.A.; Patel, S. Flavonoids as acetylcholinesterase inhibitors: Current therapeutic standing and future prospects. Biomed. Pharmacother. 2018, 101, 860–870. [Google Scholar] [CrossRef]
- Sanjay; Shin, J.H.; Park, M.; Lee, H.J. Cyanidin-3-O-Glucoside Regulates the M1/M2 Polarization of Microglia via PPARγ and Aβ42 Phagocytosis Through TREM2 in an Alzheimer’s Disease Model. Mol. Neurobiol. 2022, 59, 5135–5148. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.S.; Kim, H.B.; Lee, S.; Kim, M.J.; Lee, S.O.; Han, S.M.; Maeng, S.; Park, J.H. Loganin enhances long-term potentiation and recovers scopolamine-induced learning and memory impairments. Physiol. Behav. 2017, 171, 243–248. [Google Scholar] [CrossRef]
- Deng, J.; Qi, X.L.; Guan, Z.Z.; Yan, X.M.; Huang, Y.; Wang, Y.L. Pretreatment of SH-SY5Y cells with dicaffeoylquinic acids attenuates the reduced expression of nicotinic receptors, elevated level of oxidative stress and enhanced apoptosis caused by β-amyloid peptide. J. Pharm. Pharmacol. 2013, 65, 1736–1744. [Google Scholar] [CrossRef]
- Brinza, I.; Raey, M.A.E.; El-Kashak, W.; Eldahshan, O.A.; Hritcu, L. Sweroside Ameliorated Memory Deficits in Scopolamine-Induced Zebrafish (Danio rerio) Model: Involvement of Cholinergic System and Brain Oxidative Stress. Molecules 2022, 27, 5901. [Google Scholar] [CrossRef] [PubMed]
- Dartt, D.A. Neural regulation of lacrimal gland secretory processes: Relevance in dry eye diseases. Prog. Retin. Eye Res. 2009, 28, 155–177. [Google Scholar] [CrossRef]
- Zhu, J.; Inomata, T.; Shih, K.C.; Okumura, Y.; Fujio, K.; Huang, T.; Nagino, K.; Akasaki, Y.; Fujimoto, K.; Yanagawa, A.; et al. Application of Animal Models in Interpreting Dry Eye Disease. Front. Med. 2022, 9, 830592. [Google Scholar] [CrossRef] [PubMed]
- Inaba, T.; Ohnishi-Kameyama, M.; Liu, Y.; Tanaka, Y.; Kobori, M.; Tamaki, S.; Ito, T.; Higa, K.; Shimazaki, J.; Tsubota, K. Quercetin improves lacrimal gland function through its anti-oxidant actions: Evidence from animal studies, and a pilot study in healthy human volunteers. Front. Nutr. 2022, 9, 974530. [Google Scholar] [CrossRef]
- Ng, D.; Altamirano-Vallejo, J.C.; Gonzalez-De la Rosa, A.; Navarro-Partida, J.; Valdez-Garcia, J.E.; Acosta-Gonzalez, R.; Martinez Camarillo, J.C.; Bustamante-Arias, A.; Armendariz-Borunda, J.; Santos, A. An Oral Polyphenol Formulation to Modulate the Ocular Surface Inflammatory Process and to Improve the Symptomatology Associated with Dry Eye Disease. Nutrients 2022, 14, 3236. [Google Scholar] [CrossRef]
- Chen, Y.; Mehta, G.; Vasiliou, V. Antioxidant defenses in the ocular surface. Ocul. Surf. 2009, 7, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, Y.-J.; Chen, Y.-N.; Tsao, Y.-T.; Cheng, C.-M.; Wu, W.-C.; Chen, H.-C. The Pathomechanism, Antioxidant Biomarkers, and Treatment of Oxidative Stress-Related Eye Diseases. Int. J. Mol. Sci. 2022, 23, 1255. [Google Scholar] [CrossRef] [PubMed]
- The Age-Related Eye Disease Study Research Group. The Age-Related Eye Disease Study (AREDS): Design implications. AREDS Report No. 1. Control Clin. Trials 1999, 20, 573–600. [Google Scholar] [CrossRef] [PubMed]
- Muz, O.E.; Orhan, C.; Erten, F.; Tuzcu, M.; Ozercan, I.H.; Singh, P.; Morde, A.; Padigaru, M.; Rai, D.; Sahin, K. A novel integrated active herbal formulation ameliorates dry eye syndrome by inhibiting inflammation and oxidative stress and enhancing glycosylated phosphoproteins in rats. Pharmaceuticals 2020, 13, 295. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, R.D.; Vashist, P.; Gupta, S.K.; Young, I.S.; Maraini, G.; Camparini, M.; Jayanthi, R.; John, N.; Fitzpatrick, K.E.; Chakravarthy, U. Inverse association of vitamin C with cataract in older people in India. Ophthalmology 2011, 118, 1958–1965.e1952. [Google Scholar] [CrossRef]
- Oppenheimer, A. Turmeric (curcumin) in biliary diseases. Lancet 1937, 229, 619–621. [Google Scholar] [CrossRef]
- Nakazawa, Y.; Aoki, M.; Doki, Y.; Morishita, N.; Endo, S.; Nagai, N.; Funakoshi-Tago, M.; Tamura, H. Oral consumption of α-glucosyl-hesperidin could prevent lens hardening, which causes presbyopia. Biochem. Biophys. Rep. 2021, 25, 100885. [Google Scholar] [CrossRef]
- Kan, J.; Wang, M.; Liu, Y.; Liu, H.; Chen, L.; Zhang, X.; Huang, C.; Liu, B.Y.; Gu, Z.; Du, J. A novel botanical formula improves eye fatigue and dry eye: A randomized, double-blind, placebo-controlled study. Am. J. Clin. Nutr. 2020, 112, 334–342. [Google Scholar] [CrossRef]
- Yu, W.Y.; Chan, L.Y.L.; Chung, A.; Lee, P.H.; Woo, G.C. Bilberry-containing supplements on severe dry eye disease in young and middle-aged adults: A 3-month pilot analysis. Front. Nutr. 2023, 10, 1061818. [Google Scholar] [CrossRef]
- Choi, S.Y.; Eom, Y.; Kim, J.Y.; Jang, D.H.; Song, J.S.; Kim, H.M. Effect of natural extract eye drops in dry eye disease rats. Int. J. Ophthalmol. 2020, 13, 1023–1030. [Google Scholar] [CrossRef]
- Zhang, C.; Li, K.; Yang, Z.; Wang, Y.; Si, H. The effect of the aqueous extract of Bidens pilosa L. on androgen deficiency dry eye in rats. Cell. Physiol. Biochem. 2016, 39, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Lem, D.W.; Gierhart, D.L.; Davey, P.G. Can Nutrition Play a Role in Ameliorating Digital Eye Strain? Nutrients 2022, 14, 4005. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, Y.; Kawashima, M.; Inoue, S.; Inagaki, E.; Suzuki, A.; Ooe, E.; Kobayashi, S.; Tsubota, K. Bilberry extract supplementation for preventing eye fatigue in video display terminal workers. J. Nutr. Health Aging 2015, 19, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Uchino, Y.; Uchino, M.; Dogru, M.; Fukagawa, K.; Tsubota, K. Improvement of accommodation with anti-oxidant supplementation in visual display terminal users. J. Nutr. Health Aging 2012, 16, 478–481. [Google Scholar] [CrossRef]


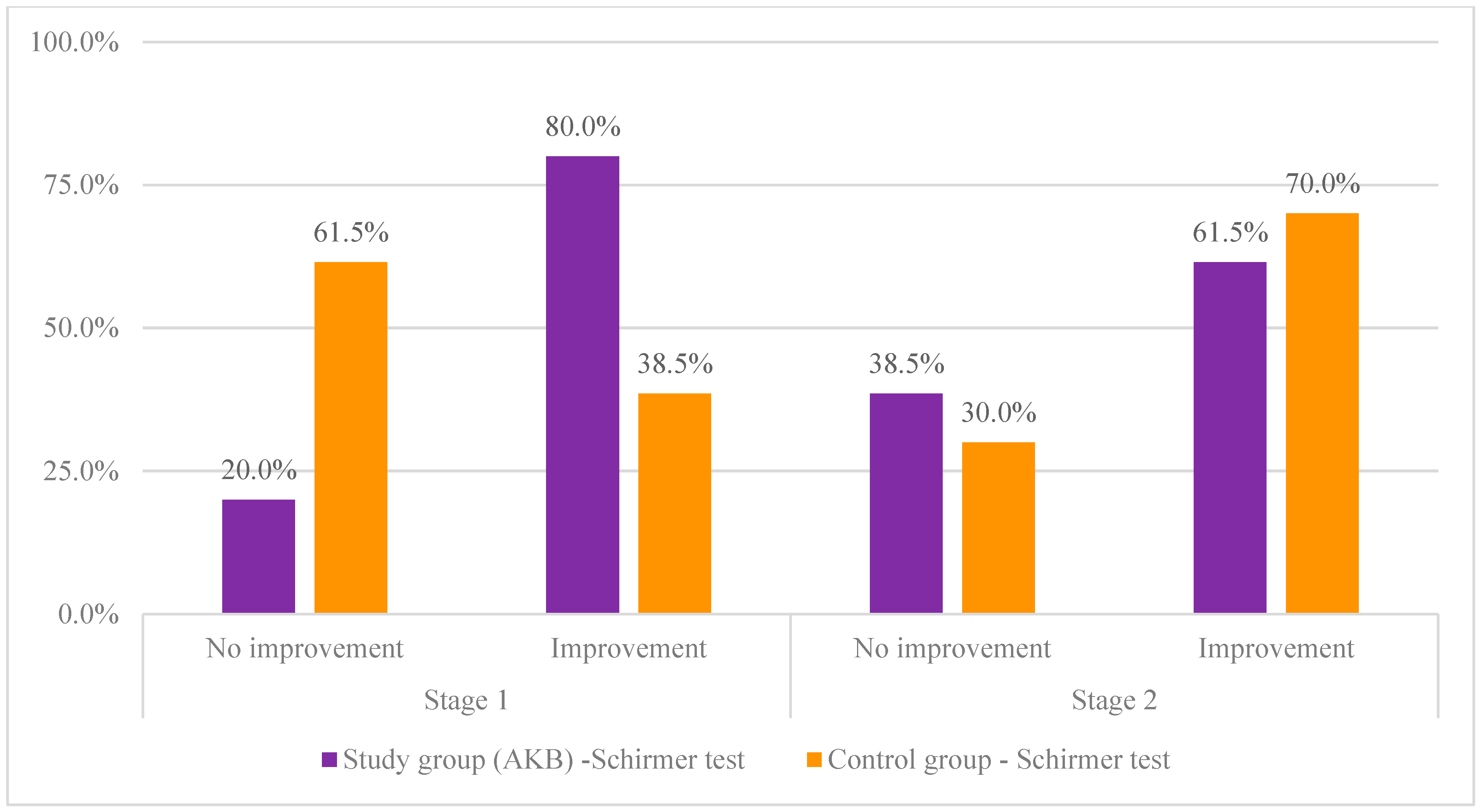
| N | % | χ2 (1) | p | ||
|---|---|---|---|---|---|
| Near visual acuity—Stage 1 | Improve | 5 | 50.0% | <0.01 | 1.000 |
| No improvement | 5 | 50.0% | |||
| Near visual acuity—Stage 2 | Improve | 12 | 92.3% | 9.31 | 0.002 |
| No improvement | 1 | 7.7% | |||
| Schirmer test—Stage 1 | Improve | 8 | 80.0% | 3.60 | 0.058 ^ |
| No improvement | 2 | 20.0% | |||
| Schirmer test—Stage 2 | Improve | 8 | 61.5% | 0.69 | 0.405 |
| No improvement | 5 | 38.5% |
| Near Visual Acuity | Study Group | Control Group | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | χ2 | p | df | ϕ | ||
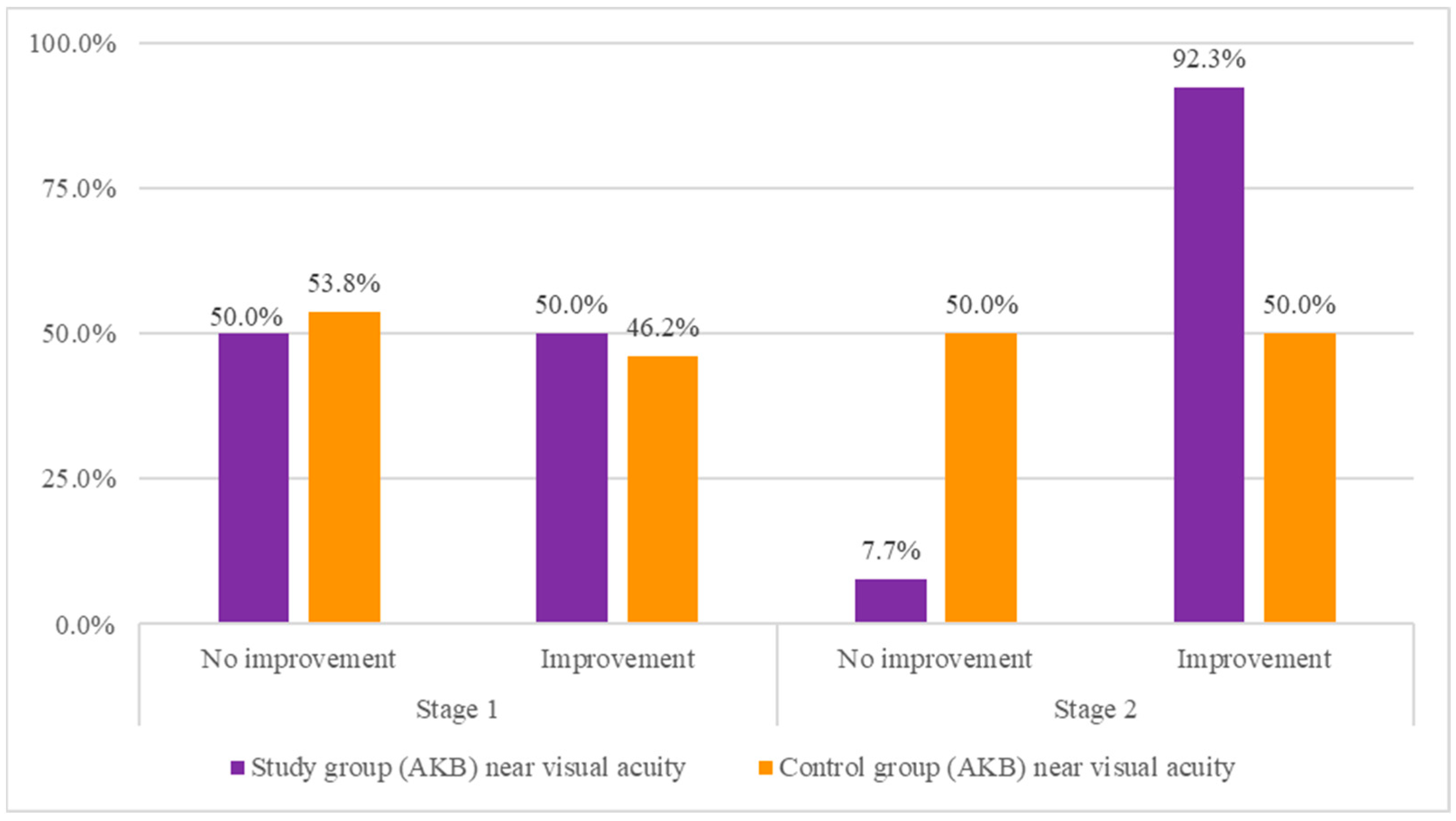
| Stage 1 | No improvement | 5 | 50.0% | 7 | 53.8% | 0.03 | 0.855 | 1 | 0.04 |
| Improve | 5 | 50.0% | 6 | 46.2% | |||||
| Stage 2 | No improvement | 1 | 7.7% | 5 | 50.0% | 5.25 | 0.022 | 1 | 0.48 |
| Improve | 12 | 92.3% | 5 | 50.0% | |||||
| Schirmer Test | Study Group | Control Group | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | χ2 | p | df | ϕ | ||
| Stage 1 | No improvement | 2 | 20.0% | 8 | 61.5% | 3.97 | 0.046 | 1 | 0.42 |
| Improve | 8 | 80.0% | 5 | 38.5% | |||||
| Stage 2 | No improvement | 5 | 38.5% | 3 | 30.0% | 0.18 | 0.673 | 1 | 0.09 |
| Improve | 8 | 61.5% | 7 | 70.0% | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szumny, D.; Kucharska, A.Z.; Czajor, K.; Bernacka, K.; Ziółkowska, S.; Krzyżanowska-Berkowska, P.; Magdalan, J.; Misiuk-Hojło, M.; Sozański, T.; Szeląg, A. Extract from Aronia melanocarpa, Lonicera caerulea, and Vaccinium myrtillus Improves near Visual Acuity in People with Presbyopia. Nutrients 2024, 16, 926. https://doi.org/10.3390/nu16070926
Szumny D, Kucharska AZ, Czajor K, Bernacka K, Ziółkowska S, Krzyżanowska-Berkowska P, Magdalan J, Misiuk-Hojło M, Sozański T, Szeląg A. Extract from Aronia melanocarpa, Lonicera caerulea, and Vaccinium myrtillus Improves near Visual Acuity in People with Presbyopia. Nutrients. 2024; 16(7):926. https://doi.org/10.3390/nu16070926
Chicago/Turabian StyleSzumny, Dorota, Alicja Zofia Kucharska, Karolina Czajor, Karolina Bernacka, Sabina Ziółkowska, Patrycja Krzyżanowska-Berkowska, Jan Magdalan, Marta Misiuk-Hojło, Tomasz Sozański, and Adam Szeląg. 2024. "Extract from Aronia melanocarpa, Lonicera caerulea, and Vaccinium myrtillus Improves near Visual Acuity in People with Presbyopia" Nutrients 16, no. 7: 926. https://doi.org/10.3390/nu16070926
APA StyleSzumny, D., Kucharska, A. Z., Czajor, K., Bernacka, K., Ziółkowska, S., Krzyżanowska-Berkowska, P., Magdalan, J., Misiuk-Hojło, M., Sozański, T., & Szeląg, A. (2024). Extract from Aronia melanocarpa, Lonicera caerulea, and Vaccinium myrtillus Improves near Visual Acuity in People with Presbyopia. Nutrients, 16(7), 926. https://doi.org/10.3390/nu16070926







