Abstract
The implications of soy consumption on human health have been a subject of debate, largely due to the mixed evidence regarding its benefits and potential risks. The variability in responses to soy has been partly attributed to differences in the metabolism of soy isoflavones, compounds with structural similarities to estrogen. Approximately one-third of humans possess gut bacteria capable of converting soy isoflavone daidzein into equol, a metabolite produced exclusively by gut microbiota with significant estrogenic potency. In contrast, lab-raised rodents are efficient equol producers, except for those raised germ-free. This discrepancy raises concerns about the applicability of traditional rodent models to humans. Herein, we designed a gnotobiotic mouse model to differentiate between equol producers and non-producers by introducing synthetic bacterial communities with and without the equol-producing capacity into female and male germ-free mice. These gnotobiotic mice display equol-producing phenotypes consistent with the capacity of the gut microbiota received. Our findings confirm the model’s efficacy in mimicking human equol production capacity, offering a promising tool for future studies to explore the relationship between endogenous equol production and health outcomes like cardiometabolic health and fertility. This approach aims to refine dietary guidelines by considering individual microbiome differences.
1. Introduction
The benefits of consuming soy for human health remain largely inconclusive and controversial. For example, soy consumption has been related to poor sperm quality, likely due to the estrogenic activity of soy isoflavones [1,2]. On the contrary, for health challenges related to estrogen deficiency, such as those related to menopause, soy supplementation has been shown to sometimes be beneficial [3,4]. Soy isoflavones are plant-derived phytoestrogens that have structural similarities to mammalian-synthesized estrogen and have binding capacity to estrogen receptors (ER)-alpha and -beta to act as estrogen agonists or antagonists [5]. However, inconsistent outcomes in clinical human feeding trials of soy suggest that the existence of responders and non-responders may be driven by a person’s ability to produce (S)-equol (hereafter denoted as equol) [6,7,8]. Equol is an exclusively microbial-produced metabolite of one of the soy isoflavones, daidzein, with the highest estrogenic potency among all soy isoflavones and their metabolites [9]. Higher equol production has also been reported to increase estradiol excretion and lower blood estradiol levels [10]. Only ~30–50% of humans possess equol-producing bacteria within their gut microbiome and can convert daidzein to equol, likely contributing to the interpersonal variations observed in the clinical outcomes of soy consumption [11]. In contrast, lab-raised rodents are efficient equol-producers except those raised germ-free [12]. Therefore, soy-feeding studies using conventionally raised rodent models may only be relevant to half of the human population at best. Developing a proper negative control (i.e., a model with a gut microbiome that cannot produce equol) is critical to discern the impact of soy consumption based on equol-producing status.
Exogenous supplementation of equol that was synthesized through in vitro bacterial fermentation has been tested to determine its potential benefits in humans, particularly those that do not produce equol themselves. A high level of oral equol supplementation appears to successfully reduce cardiovascular risks and bone resorption [13,14,15]. However, the pharmacokinetics of oral equol supplementation differs significantly from endogenous production within the gastrointestinal tract and has been reported to provide little to no cardiometabolic benefits in non-equol-producing humans [16,17,18]. In fact, a recent study showed an opposing effect that dietary supplementation of equol exacerbated metabolic dysfunction in high-fat diet-induced obese mice by suppressing physical activity and energy expenditure and causing hyperglycemia, hyperinsulinemia, and hypoleptinemia [19]. Therefore, intestinal-produced equol likely exerts a distinct physiological role than the exogenous supplementation of equol.
Germ-free rodents do not produce equol due to the absence of intestinal bacteria required to metabolize daidzein into equol [12]. However, living in a sterile environment is associated with various developmental and physiological abnormalities, making germ-free rodents a poor control when examining physiological and metabolic outcomes of bacterial-produced equol. On the other hand, gnotobiotic mice (i.e., germ-free mice colonized with a known microbial community) provide the potential to serve as an appropriate negative control as non-equol producers. Bowey et al. have demonstrated this concept by colonizing gnotobiotic rats with a human microbiome of a poor equol producer to create a non-equol-producing rodent [20]. While this approach has translational relevance to human health, several downsides exist, including (1) reproducibility is low when colonization of a community relies on a human fecal sample and (2) the ecological dynamics of a complex human microbial community when encountering a novel equol-producing species is likely highly individualized due to the large interpersonal variations of the gut microbiome [21]. An alternative approach to creating a rodent model to test the endogenously synthesized equol status is to create a gnotobiotic rodent model using synthetic bacterial communities that are designed and assembled in vitro with precision before inoculating germ-free mice [22,23,24]. The benefits of using synthetic bacterial communities are greater community stability and reproducibility of colonization. A couple of attempts have been made involving the synthetic community to create equol-producing and non-equol-producing rodent models. One study compares a synthetic community that consists of eight mouse-derived bacterial strains as the non-equol-producing “control” to a conventional microbiome that produces equol to study the benefits of equol production in ApoE-null mice on reproductive health [25]. While this method utilizes a synthetic microbiome, the comparison to a complex microbiome introduces confounding variables that are not accounted for. Further, mouse-derived bacterial strains may have less relevance to human health. Another study bred gnotobiotic rats colonized with a synthetic community built with human-derived bacterial strains called the simplified human microbiota (SIHUMI), which cannot produce equol [26]. Once rats reached adulthood at 12 weeks of age, an equol-producing strain Slackia isoflavoniconvertens DSM 22006 was introduced to create equol-producing rats. Although much better controlled than comparing a synthetic microbiota to a complex microbiota where multiple confounding factors exist, this approach cannot be used to study the developmental or transgenerational impact of equol production as the breeders remain non-equol producers.
Herein, we aimed to develop a gnotobiotic mouse model colonized with synthetic microbiota from human-derived bacterial strains with distinct equol-producing capacity at the time of inoculation (Figure 1). We hypothesize that when two communities differ by only the presence of one bacterial strain that is capable of producing equol, the corresponding equol phenotype will be present when inoculated into germ-free mice. Developing a model system is essential to understanding the impact of microbial metabolites of nutrients on human health and contributing to developing novel strategies to maximize the nutritional efficacy of soy foods.
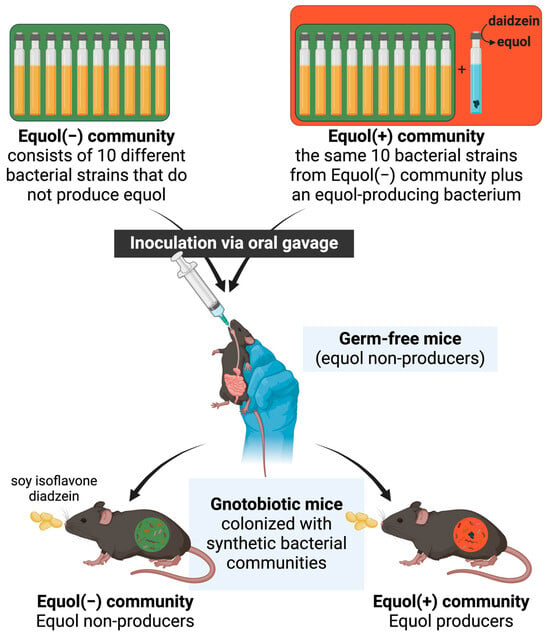
Figure 1.
Schematic of the study.
2. Materials and Methods
2.1. Design of Synthetic Bacterial Communities
To design these synthetic bacterial communities with disparate equol-producing capacity, we first selected a total of 10 strains that (1) are commonly found in the human gut, (2) have not been reported with equol-producing capability, and (3) were used previously in synthetic bacterial communities to colonize rodent gut [27,28,29]. These 10 strains were used as the “core” microbiota without equol-producing capacity (Equol(−)): Bacteroides caccae, Bacteroides thetaiotaomicron, Bacteroides uniformis, Roseburia intestinalis, Faecalibacterium duncaniae, Agathobacter rectalis, Coprococcus comes, Akkermansia muciniphila, Providencia stuartii, and Collinsella aerofaciens. To create the equol-producing (Equol(+)) community, an equol-producing strain of Adlercreutzia equolifaciens was added to the Equol(−) community (Table 1). All strains were acquired from either the German Collection of Microorganisms and Cell Cultures (DSMZ, Braunschweig, Germany) or the American Type Culture Collection (ATCC, Manassas, VA, USA) and revived as recommended by the ATCC or DSMZ. The culture media recipes and incubation times used for this study are designated in Supplemental Table S1. All strains were grown anaerobically in an anaerobic chamber (Vinyl Anaerobic Chamber Type B, Coy Laboratory Products Inc., Grass Lake, MI, USA) at 37 °C. Each strain was cultured individually and screened for equol-producing capacity in two ways: (1) conducting National Center for Biotechnology Information (NCBI) nucleotide Basic Local Alignment Search Tool (BLAST) searches against the three genes known to be involved in equol production: dzr, ddr, and tdr (sequences from the whole genome of the equol-producing strain A. equolifaciens DSMZ 19450, GenBank Accession no.: GCA_000478885.1) [30], and (2) in vitro culture to confirm presence and absence of equol-producing capacity before pooling to form communities. The identity of each strain was confirmed via 16S rRNA gene sequencing using the primer pairs 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-TACGGYTACCTTGTTACGACTT-3′) [31,32,33] with the Invitrogen Platinum II Taq Hot-Start DNA Polymerase kit (Cat. No. 14966001, Invitrogen, Waltham, MA, USA). The 16S rRNA gene amplicons were sequenced with GENEWIZ (Azenta Life Sciences, South Plainfield, NJ, USA). The taxonomy of the cleaned nucleotide sequences was then determined using the NCBI nucleotide BLAST.

Table 1.
Bacterial strains included in the equol-producing (Equol(+)) and non-equol-producing (Equol(−)) communities.
Following the confirmation of the purity, identity, and equol-producing capabilities, each bacterial strain was grown separately and then pooled into the inoculants that can be used to colonize mice. Strains were first grown on agar, picked into their respective broth, and incubated. The inoculants were made by pooling each strain in equal optical density (OD600) at an approximate of 1.0. Two bacterial strains were not able to reach the OD600 of 1.0 (A. equolifaciens [OD600: 0.16], P. stuartii [OD600: 0.15]), so they were added into the inoculants without dilution. Using an equal volume of each strain, inoculants of the Equol(−) and Equol(+) communities were prepared and supplemented with 20% glycerol in sterile glass Balch-type tubes and crimp-sealed and stored at −80 °C. All culture media used in this process was prepared with the oxygen indicator, resazurin (Cat. No. AC189900010, ACROS Organics, Waltham, MA, USA) to ensure anaerobic conditions.
2.2. Gnotobiotic Mouse Study
All animal protocols were approved by the Purdue Animal Care and Use Committee under protocol #1909001951 (14 November 2019). Female and male germ-free C57BL/6 mice were bred and maintained at the Purdue Gnotobiotic Facility and fed Teklad Global 19% protein extruded rodent diet (sterilizable 2019S, Envigo, Indianapolis, IN, USA) before starting this study. Mice of 4–6 weeks of age were removed from the breeding isolators and housed in ventilated cages (Sentry SPP™ Mouse, Allentown, LLC, Allentown, NJ, USA) for the duration of the experimentation. Mice were placed on a semi-purified diet formulated based on AIN-93G formulation but adjusted to contain fermentable fibers and supplemented with 1.5 g/kg daidzein (Cat. No. D-2946, LC Laboratories, Woburn, MA, USA). The composition of the diet is shown in Table 2. This diet was sterilized through double gamma irradiation at 10–20 kGy (Research Diets, New Brunswick, NJ, USA). Food and water were provided ad libitum throughout the study. Mice were allowed to adjust to this diet for two weeks before bacterial inoculation. At 6–8 weeks of age, mice were colonized with one of the synthetic bacterial communities, Equol(−) or Equol(+), through oral gavage (n = 10–12/group). Inoculants were thawed on ice and remained anaerobic during this process. Immediately before colonization, fresh fecal pellets were collected to confirm the sterility of the mice before bacterial colonization. Four weeks after colonization, mice were fasted for 4 h and euthanized using carbon dioxide asphyxiation. Cecal contents and serum were collected and flash-frozen in liquid nitrogen. Serum samples were collected from blood acquired through cardiac puncture after euthanasia and allowed to clot at room temperature for approximately 45 min, then centrifuged at 2000× g for 10 min at 4 °C. All samples were stored at −80 °C until analysis.

Table 2.
Composition of study diet.
The diet for this study was formulated based on the AIN-93G diet and was modified to be supplemented with 1.5 g/kg daidzein (LC Laboratories, D-2946) and made to contain fermentable fibers. The cellulose in the AIN-93 diet was replaced with an equal mixture by weight of the following fermentable fibers: inulin, short-chain fructo-oligosaccharides (scFOS), beta-glucan, pectin, and glucomannan. Additional alterations include using mineral acid casein instead of lactic casein, increasing to a 1.5× vitamin mix, and using corn oil in place of soybean oil. This diet was prepared by Research Diets (Research Diets, New Brunswick, NJ, USA).
2.3. Quantification of Daidzein and Equol
The equol-producing capability of each bacterial strain included in the synthetic bacterial communities was confirmed before colonizing the mice. At the conclusion of the mouse study, the concentrations of the daidzein and equol were also quantified in the serum collected from the mice to determine the equol-producing status of the mice. Each bacterial strain was grown in culture media supplemented with 100 μM daidzein (Cat. No. D-2946, LC Laboratories, Woburn, MA, USA). The concentrations of equol and daidzein in the culture media were quantified using an Ultivo Triple Quadrupole LC-MS/MS (Model G6465A, Agilent Technologies, Santa Clara, CA, USA) after a double ethyl acetate extraction [34,35,36]. An internal standard, 4-hydroxybenzophenone (4-HBP) (Cat. No. H20202, Sigma-Aldrich, St. Louis, MO, USA), was added to each sample before extractions. For each sample, 1 mL of culture media was mixed with 6 mL of HPLC grade ethyl acetate, vortexed for 60 s, and centrifuged at 3220× g for 5 min at room temperature. This extraction was repeated, and the solvent phase was removed to another tube. Samples were dried using nitrogen and re-suspended in HPLC-grade methanol and filtered through a 4 mm PVFD membrane 0.45 μM filter unit (Cat. No. SLHV004SL, Millipore Sigma, St. Louis, MO, USA) before analysis. Serum collected from gnotobiotic mice colonized with the Equol(−) and Equol(+) communities were analyzed for levels of daidzein and equol [37]. From each mouse, 100 μL of serum was mixed with 100 μL of acetate buffer (0.2 mol/L, pH 5.0) containing 100 units of ß-glucuronidase/aryl sulfatase (Cat. No. 1041140002, Sigma-Aldrich, St. Louis, MO, USA) and 50 μM of the internal standard 4-HBP (Cat. No. H20202, Sigma-Aldrich, St. Louis, MO, USA). Samples were incubated for 15 h at 37 °C and then vortexed with 400 μL of HPLC-grade methanol and sonicated for 5 min. The samples were then centrifuged at 5000× g for 5 min at 4 °C. The supernatant was filtered through a 4 mm PVFD membrane 0.45 μM filter (Cat. No. SLHV004SL, Millipore Sigma, St. Louis, MO, USA) to eliminate proteins. Samples were subjected to the same LC-MS/MS procedure as for bacterial culture extractions. Serum from germ-free mice was used as a negative control for equol. Standard curves were established by spiking germ-free mouse serum with a gradient of known concentrations of daidzein and equol ranging from 0.05 μM to 50 μM.
2.4. Microbiota Analysis
The gut microbiota composition of the gnotobiotic mice was determined through 16S rRNA gene sequencing of the cecal content. The inoculants used to colonize the mice were also sequenced. DNA was first isolated from the cecal contents and inoculants using a phenol–chloroform method [38]. Briefly, homogenization of the samples was achieved by adding extraction buffer (200 mM Tris (pH 8.0), 200 mM NaCl, 20 mM EDTA), 20% SDS, and phenol:chloroform:isoamyl alcohol (25:24:1, pH 7.9) (Cat. No. 15593-049, Invitrogen, Waltham, MA, USA) and bead beating with 0.1 mm diameter zirconia–silicate beads (Cat. No. 11079101z, BioSpec Products, Bartlesville, OK, USA) and a single 3.2 mm stainless steel bead (Cat. No. 11079132ss, BioSpec Products, Bartlesville, OK, USA) using a Mini-Beadbeater-96 (Cat. No. 1001, BioSpec Products, Bartlesville, OK, USA). The homogenates were centrifuged at 7200× g for 3 min at 4 °C, and the aqueous layer was removed. DNA was precipitated using isopropanol and sodium acetate (3M, pH 5.2). The DNA pellets were rinsed with 100% ethanol, speed vacuumed using a Vacufuge plus (Cat. No. 022820001, Eppendorf, Hamburg, Germany), and re-suspended in T10E1 buffer (10 mM Tris, 1 mM EDTA, pH8). The QIAquick 96 PCR purification kit (Cat. No. 28183, Qiagen, Hilden, Germany) was used to further purify the DNA. Samples were quantified using a Qubit Flex Fluorometer (Cat. No. Q3326, Invitrogen, Waltham, MA, USA) using the Qubit broad range dsDNA kit (Cat. No. Q32853, Invitrogen, Waltham, MA, USA). The hypervariable 4 (V4) region of the 16S rRNA gene was amplified [39]. Briefly, PCR was carried out using the KAPA HiFi HotStart Ready Mix (Cat. No. 7958935001, Roche, Basel, Switzerland). The amplified products were purified using the QIAquick PCR purification kit and underwent size selection using 1.5% low-melt agarose gels and the Gel DNA Recovery Kit (Cat. No. D4002, Zymo Research, Irvine, CA, USA). A final pool was made after quantification of each sample using the Qubit high-sensitivity dsDNA kit (Cat. No. Q32851, Cat. No. Invitrogen, Waltham, MA, USA). Sequencing of the pool was performed using the Illumina MiSeq platform to generate 2 × 250 bp reads by the Purdue Genomics Core. Reads were analyzed using the Quantitative Insights into Microbial Ecology version 2 (QIIME2, version 2021.11.0) pipeline. Sequences were trimmed and filtered using the DADA2 plugin [40]. Taxonomy was assigned to reads using a custom reference database containing only the 11 bacterial species in the communities.
2.5. Strain-Specific qPCRs
To confirm the presence and quantity of the two strains that were challenging to detect through 16S rRNA gene sequencing, strain-specific quantitative PCRs (qPCRs) were performed using the DNA isolated from the cecal contents of each mouse and the bacterial inoculants. Primer sequences used to target A. equolifaciens (ddr gene, Forward: 5′-CTCGAYCTSGTSTACAACGT-3′, Reverse: 5′-GARTTGCAGCGRATKCCGAA-3′) and F. duncaniae (Forward: 5′-TGCCCCCGGGTGGTTCT-3′, Reverse: 5′-CGTTATTCAAAGCCCCGTTATCAA-3′) have been previously described [28,41]. Primer specificity was confirmed by testing for PCR amplification of primers against all strains used in the synthetic community. qPCR was performed using Powerup™ SYBR™ Green Master Mix (Cat. No. A25743, Applied Biosystems, Waltham, MA, USA) following the manufacturer’s directions on a QuantStudio 7 Flex Real-Time PCR System (Cat. No. 4485701, Applied Biosystems, Waltham, MA, USA). The amplification was performed under the following conditions: an initial UDG activation step at 50 °C for 2 min, followed by Dual-Lock DNA Polymerase activation at 95 °C for 15 min. This was succeeded by 40 cycles of denaturation at 95 °C for 15 s and annealing/extension at 60 °C for 1 min. The amplification was finalized with a melt curve stage, gradually increasing the temperature to 95 °C over the course of 1 min. Amplification data and melt curves were analyzed using the QuantStudio Real-Time PCR software v1.7.1. Standard curves were established to quantify the bacterial load of each strain in the samples. Briefly, each bacterial culture was quantified through serial dilution and agar spread plating after entry into the stationary phase to determine the colony-forming units per ml of culture media (CFU/mL). DNA was isolated from the primary dilution tube and then serially diluted ten-fold to create ten standards.
2.6. Cecal Short Chain Fatty Acids
Short-chain fatty acids (SCFAs) were quantified from the cecal contents of mice colonized with Equol(+) and Equol(−) communities using a published method [42]. Briefly, cecal contents were weighed and transferred to tubes containing 1.2 g of zirconia–silicate beads (Cat. No. 11079101z, BioSpec Products, Bartlesville, OK, USA). Cecal contents were homogenized using a bullet blender and a vortex in 1 mL of 0.5% phosphoric acid per 100 mg of sample. The supernatant was mixed with an equal volume of ethyl acetate containing 0.14 mL heptanoic acid/L as an internal standard. This mixture was vortexed for 5 min before centrifugation at 17,000× g at 4 °C for 10 min. The ethyl acetate phase was recovered and stored at −80 °C. An Agilent 7890A gas chromatograph (GC-FID 7890A, Santa Clara, CA, USA) with a fused silica capillary column (Nukon SUPELCO No: 40369-03A, Bellefonte, PA, USA) was used to quantify the SCFA concentration. Peak areas for all SCFAs were recorded, corrected for extraction efficiency and sample volume variability using the internal standard heptanoic acid, and quantified using a standard curve.
2.7. Statistical Analysis
Data were analyzed and graphed in GraphPad Prism version 9.4.1 (GraphPad Software, San Diego, CA, USA). Outliers for daidzein and equol concentration in the serum were identified and removed using the robust regression and outlier removal (ROUT) method. For daidzein and equol serum quantification and qPCR data, normality was assessed using the Shapiro–Wilk test (alpha = 0.05). Variables that passed the Shapiro–Wilk test were analyzed using an unpaired t-test or a one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparisons test. For variables that did not pass the Shapiro–Wilk test, a Mann–Whitney or a Kruskal–Wallis test followed by Dunn’s multiple comparison test was used. For the microbiota data obtained through 16S rRNA gene Illumina sequencing, statistical differences in beta diversity were tested using permutational multivariate analysis of variance (PERMANOVA) in QIIME2. Permutational multivariate analysis of dispersions (PERMDISP) was also performed in QIIME2 to determine significant differences in group variances. Distance matrices were graphed as principal coordinates analysis (PCoA) plots using the package qiime2R via RStudio version 4.1.2 [43]. Ellipses presented on these graphs were calculated based on a multi-variable t distribution with a radius of 0.95 around the center of the data for each group using the stat_ellipses function in ggplot through R. Data are presented as the mean ± standard error of the mean (SEM). p-values below 0.05 were considered statistically significant. Statistical significance is indicated as follows: * = p < 0.05; ** = p < 0.01; *** = p < 0.001, **** = p < 0.0001.
3. Results
3.1. Body Weight, Mesenteric Fat, and Gonadal Fat Mass Did Not Differ in Mice Based on the Microbiota Received
In this study, we first introduced the diet high in daidzein and formulated with fermentable fibers two weeks before colonization of the synthetic bacterial communities designed (Equol(−) and Equol(+) communities) in these gnotobiotic mice. Four weeks after colonization of the gut microbiota, we assessed the body weight, mesenteric fat mass, and gonadal fat mass at the time of euthanasia. Unsurprisingly, we did not discover any differences in these outcomes based on treatment groups (Table 3).

Table 3.
Body weight and fat mass of male and female gnotobiotic mice colonized with the non-equol-producing (Equol(−)) and equol-producing (Equol(+)) communities.
3.2. The Synthetic Bacterial Communities Produce Equol-Producing Capacity as Designed in Gnotobiotic Mice Fed a High Daidzein Diet
The synthetic communities designed include five phyla commonly found in human intestinal microbiomes, and the predominant phyla Bacteroidota and Bacillota are represented by a higher diversity of strains [44,45,46,47]. Before forming the communities, we tested each bacterial strain individually for its equol-producing capability in vitro and confirmed that equol production was only detected for the equol-producing strain of A. equolifaciens, as expected (Table 4). The equol-producing capability of these two synthetic communities was then tested in vivo by inoculating male and female germ-free mice to create gnotobiotic mice colonized with Equol(−) or Equol(+) communities. It has been suggested that females are more likely to become equol producers, so we included both sexes to observe potential sex differences [48]. Two weeks after colonization, the concentration of soy isoflavone daidzein and the microbial metabolite equol was assessed in the serum of gnotobiotic mice. Daidzein was present in all serum samples and did not differ significantly between groups within each sex (Figure 2). As expected, equol was absent in the serum of all mice colonized with the Equol(−) communities and was detected in the serum of all mice colonized with the Equol(+) communities. Sex differences were not observed as the levels of daidzein and equol did not differ statistically between males and females.

Table 4.
Equol-producing capacity of each bacterial strain cultured in vitro. Each bacterial isolate used in this study was grown in daidzein-supplemented culture broth, and the concentrations of daidzein and equol were analyzed using LC-MS/MS analysis.
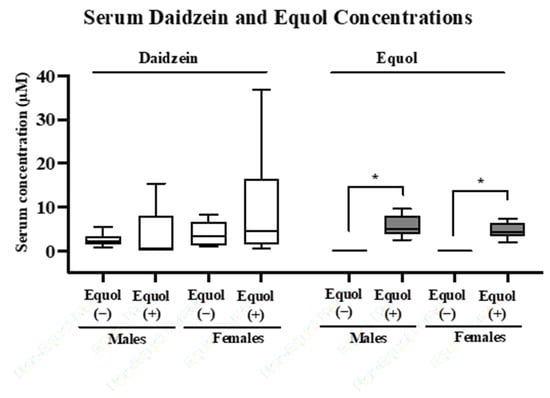
Figure 2.
Concentrations of serum daidzein and equol in male and female gnotobiotic mice colonized with the non-equol-producing (Equol(−)) and equol-producing (Equol(+)) communities. Box plots illustrate the median with a central line inside the box, which encompasses the interquartile range (IQR). Whiskers extend from the box to the lowest and highest data points. Statistical significance is denoted with asterisks. n = 7–11/group.
3.3. Equol-Producing Status Did Not Affect the Concentration of Short-Chain Fatty Acids in the Cecum of Gnotobiotic Mice
Greater short-chain fatty acid productions have been suggested to support the synthesis of equol, likely through increasing hydrogen availability from fermentable substrates to provide electron donors in the bioconversion of daidzein to equol [34]. Therefore, we formulated the mouse diet to contain various fermentable fibers to facilitate the colonization of the synthetic bacterial communities and the synthesis of equol. However, to ensure that the presence of the equol-producing strain of A. equolifaciens will not alter SCFAs, which may pose a confounding factor, we have assessed the level of SCFAs in the cecal content of these gnotobiotic mice.
SCFAs, including acetic acid, propionic acid, butyric acid, isobutyric acid, valeric acid, and isovaleric acid, were analyzed using a GC-MS. There were no significant differences in cecal SCFA between groups colonized with Equol(−) and Equol(+) communities within each sex (Figure 3). A significant difference in cecal propionic acid level was observed in male mice colonized with the Equol(+) community, which had greater (p = 0.02) propionic acid than the female mice colonized with the same microbial community.
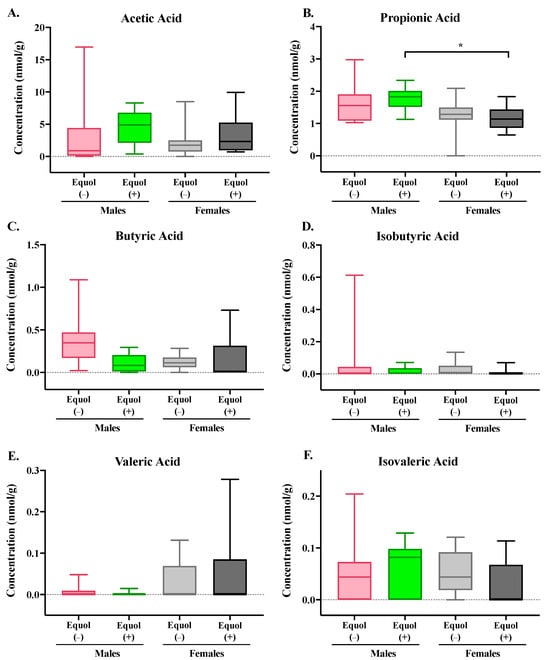
Figure 3.
Concentrations of short-chain fatty acids in the cecal content of male and female gnotobiotic mice colonized with non-equol-producing (Equol(−)) and equol-producing (Equol(+)) communities. Acetic acid (A), propionic acid (B), butyric acid (C), isobutyric acid (D), valeric acid (E), and isovaleric acid (F) were measured. Box plots illustrate the median with a central line inside the box, which encompasses the interquartile range (IQR). Whiskers extend from the box to the lowest and highest data points. Statistical significance is denoted with asterisks. n = 10–11/group.
3.4. Community-Level Assessment of the Gut Microbiota Revealed Differences between the Bacterial Inoculants and Mouse Gut, but Similarity Exists among Treatment Groups in Mice
To assess the colonization similarity of each synthetic bacterial community designed in male and female germ-free mice, the community composition of the gut microbiota was assessed using 16S rRNA gene sequencing of the mouse cecal contents and bacterial inoculants used to colonize these mice. PCoA plots show that the mouse cecal microbiota clustered separately (p < 0.05) from the inoculants, as expected (Figure 4A). The Equol(−) and Equol(+) groups cluster tightly together without statistical differences for the bacterial inoculants. When assessing the mouse cecal microbiota alone, there were significant differences between Equol(+) females and Equol(−) males (Figure 4B; Bray–Curtis PERMANOVA, p = 0.008; Weighted UniFrac PERMANOVA, p = 0.016) and between Equol(+) males and Equol(−) males (Bray–Curtis PERMANOVA, p = 0.002; Weighted UniFrac PERMANOVA, p = 0.002). There were no significant differences in the dispersion of the data of the groups, indicating that differences in the mean distances between groups drove the statistical significance observed. However, a large overlap of the gut microbiota among the treatment groups suggests that the colonization between groups was generally similar, whether accounting for the phylogenetic relationship between the bacterial species or not.
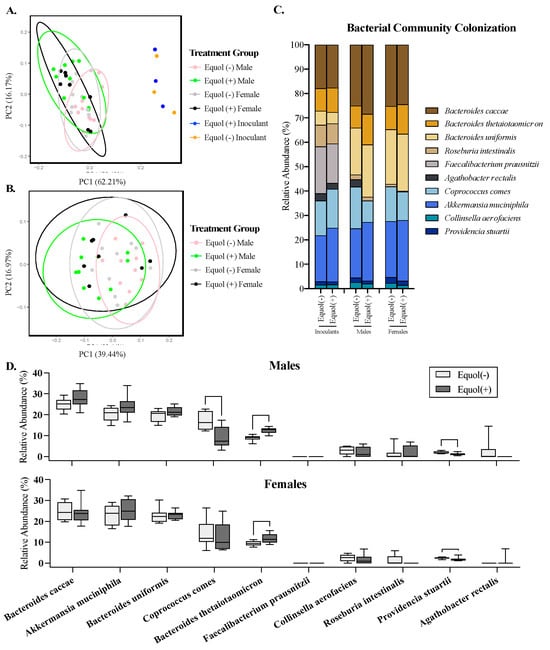
Figure 4.
Microbiota profiling of the cecal microbiota in male and female gnotobiotic mice colonized with the non-equol-producing (Equol(−)) and equol-producing (Equol(+)) communities using 16S rRNA sequencing. Community level differences among the inoculants of synthetic bacterial communities designed and those eventually colonized in the cecal content of mice were analyzed using the Bray–Curtis dissimilarity and displayed using a principal coordinate analysis (PCoA) plot (A). Analysis was also performed in the cecal microbiota of mice without the inoculants (B). Each sphere in the PCoA plots represents a unique microbial community, with communities closer in proximity being more similar to each other. The percent of variation explained by each axis of the PCoA plot is listed in parentheses on each axis. The average relative abundance of each strain within a treatment group is summarized (C), with a differential abundance of each taxon being compared between Equol(−) and Equol(+) in males and females (D). Statistical significance is denoted with asterisks. n = 10–12/group for mouse samples and n = 3/group for inoculants.
3.5. The Relative Abundance of Some Bacterial Taxa in Mice Colonized with Equol(−) and Equol(+) Differ Significantly Statistically despite the Similarity Observed in the Overall Community Structure
The mean relative abundance of the bacterial species in the inoculants and mouse cecal contents was assessed. While the inoculants were prepared by pooling equal OD600, except for the two strains that cannot be grown to OD600 of 1.0 (A. equolifaciens and P. stuartii), the relative abundance of each bacterial strain was not proportionally equal in the inoculants (Figure 4C). This is not surprising, considering the biases present in the nature of the methodology chosen for sequencing, but confirms that all but one taxon, A. equolifaciens, were present at a detectable range in the inoculants prepared. In the mouse cecal content, on the other hand, A. equolifaciens and F. duncaniae were not detectable in the cecal microbiota using the 16S rRNA sequencing method through the Illumina platform (Figure 4C,D). The relative abundance of B. thetaiotaomicron was greater (p < 0.05) in both male and female mice colonized with the Equol(+) community compared to those colonized with the Equol(−) community (Figure 4D). The relative abundance of P. stuartii was lower (p < 0.05) in both male and female mice colonized with the Equol(+) community than the Equol(−) community. And C. comes was present at a lower relative abundance in male mice colonized with Equol(+) community but not in female mice. For the two strains that were not detected in the cecal microbiota of the mice using sequencing, we performed targeted qPCR to assess their presence. As expected, A. equolifaciens was present in the inoculant and mouse cecal microbiota of the Equol(+) community at 105–106 CFU and absent in samples associated with Equol(−) community (Figure 5A). F. duncaniae, on the other hand, was only detected in the inoculants but not the cecal content (Figure 5B).
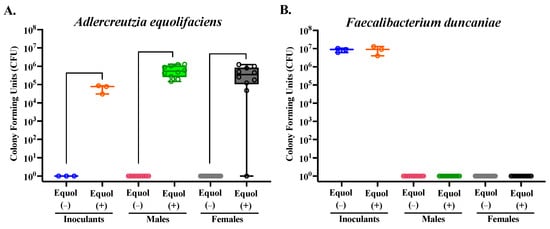
Figure 5.
Quantification of Adlercreutzia equolifaciens (A) and Faecalibacterium duncaniae (B) strains in mouse cecal contents and inoculants using qPCR. Standard curves were generated for each strain to quantify the level of each strain present in the inoculants and mouse cecal samples for each treatment group. The average CFU/mL of each strain within each group was calculated. To ensure visibility on a logarithmic scale, values of 0 were plotted as 1, allowing all data points to be visible. n = 9–12/group for mouse samples and n = 3/group for inoculants.
4. Discussion
As most rodent models harboring natural microbiota are equol producers, outcomes generated from studies related to dietary soy and health are likely biased towards humans who are equol producers. The current study aimed to create a gnotobiotic mouse model without equol-producing capacity by combining non-equol-producing bacteria strains to form a synthetic community. An equol-producing bacterial strain was added to the non-equol-producing community to form a synthetic bacterial community with equol-producing capability. Analysis of blood equol concentration successfully demonstrated our ability to create gnotobiotic mice with divergent equol-producing capabilities that consistently display the expected equol-producing phenotypes. Even though strain-specific differences were detected between Equol(−) and Equol(+) communities in this study, variability of the communities remained low in the microbiomes of the equol producers and non-equol producers in this model compared to if using complex human microbiome samples to colonize the mice [21,27,44]. This mouse model will allow us to mechanistically test and isolate the effect of endogenous bacterial equol production on disease predisposition and prognosis related to soy and/or equol.
The average serum levels of equol observed in the gnotobiotic mouse model in the current study are similar to levels observed in previous soy studies of rodents (1.7–10 µM) [26,49,50,51]. Interestingly, the concentrations of serum equol that we and others have found in rodents are much higher than those reported in humans. Equol-producing humans have been defined to be those with plasma equol concentrations of 83 nmol/L, or 0.083 µM [52]. A human study showed improved cardiovascular health outcomes at average serum equol concentrations of 0.236 µM [18]. These data suggest that, even when colonized with human-derived bacterial strains, rodents are superior equol-producers that produce 7–120 times higher levels of equol than humans. However, the lack of reporting on serum equol levels in numerous soy-related human studies complicates the assessment of whether the high serum concentrations of equol observed in this rodent model are attainable in humans.
A few bacterial genera, particularly those within the Eggerthellaceae family, have been identified to have the equol-producing capacity, including Adlercreutzia, Slackia, Eggerthella, Paraeggerthella, Asaccharobacter, and Enterorhabdus, as summarized in a narrative review study that was published by our research team [53]. Some genera outside of the Eggerthellaceae family have been identified as well, including certain species and strains within genera Lactobacillus, Lactococcus, and Bifidobacterium [14,54,55,56,57,58]. In this study, we chose to use A. equolifaciens as the equol-producing strain. We first utilized the 16S rRNA sequencing technique to assess the microbial communities that were used to inoculate mice and also those eventual colonizers in the cecal content of mice. Although an average sequencing depth of 19,182 reads per sample was achieved, indicating sufficient coverage, equol-producing A. equolifaciens strain were not detected in any of the mice in this study through 16S rRNA sequencing even though equol was detected in the serum. This is somewhat surprising as approximately 4% of the total sequencing reads have been reported to be A. equolifaciens in a recent report assessing equol-producing strains in human microbiota using a similar technique [59]. We speculated that a similar or even higher proportion of the total bacterial reads would be detected in a simplified synthetic bacterial community like those used in the current study. We then successfully confirmed the presence of A. equolifaciens in our study at an approximation of 106 CFU using qPCR. Unfortunately, the one published study utilizing gnotobiotic mice to create divergent equol phenotype did not sequence or quantify equol-producing bacterial strain. These authors did not assess the community structure of the gut microbiota, so we cannot compare colonization with gnotobiotic rodent models [25]. Primer bias may contribute to reads associated with A. equolifaciens being lower than detectable in 16S rRNA-targeted gene sequencing. Future attempts can be made using different sequencing methods, such as short-read or long-read metagenomic sequencing, to detect A. equolifaciens in this synthetic community. Our study emphasizes that the sequencing approach targeting the 16S rRNA gene may not be sensitive enough to detect the presence of bacterial strains with important microbial phenotypes, such as equol production.
One of the strains included in our synthetic bacterial community, F. duncaniae, was recently proposed to be renamed as such [60]. F. duncaniae used to be classified as Faecalibacterium prausnitzii, a butyrate-producing bacterium with a very strict anaerobe [61]. One limitation of this study is that F. duncaniae was not able to colonize despite being present in the inoculant. The presence of oxygen in the stomach and small intestine of germ-free mice due to the complete lack of oxygen may have hindered the colonization of this strain. Others have had some success in improving the colonization of synthetic communities by consecutive inoculation [62,63]. We have attempted similar consecutive inoculation in a separate study by providing a second oral gavage a week after the first inoculation took place. However, this effort did not significantly affect the strain colonization of F. duncaniae. It is worth noting that another butyrate-producing strain is present in this successfully colonized community. Nevertheless, a replacement of F. duncaniae could be considered if the presence of an F. prausnitzii-like strain is desired.
5. Conclusions
The gnotobiotic mouse model system designed in this study can successfully establish microbiomes with divergent equol-producing phenotypes in vivo and be utilized to establish a causal relationship between equol and the consumption of soy isoflavones. Breeding of these gnotobiotic mice with disparate equol phenotypes would make it possible to assess the developmental impact of equol with potential transgenerational influence from parents with the same equol phenotype. This model system provides great potential for future research on the health benefits of equol, as it has far fewer confounding variables than human studies. The benefit of having a true negative control for equol production sets this model apart from conventional rodent models, and minimizing differences between the microbiomes of the mice further reduces confounding variables. The use of this system can provide causative evidence linking the benefits of soy consumption to the microbial production of equol. The applications of this model system are broad, as this model can be used as a foundation to study a range of health outcomes in future studies, such as a reduced risk of breast cancer, improved cardiovascular health, and improved bone health. Further, it also can be used to determine potential health concerns regarding the consumption of soy, such as the reproductive health of men.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/nu16071079/s1, Table S1: Bacterial culture media and incubation times. The following broth and agar were used for the growth of each strain in this study. All strains were grown anaerobically at 37 °C. Agar incubation requires an additional 1–2 days to achieve optimal colony growth.
Author Contributions
Conceptualization, T.-W.L.C. and L.R.; validation, L.M.L., A.M.R.S. and T.-W.L.C.; formal analysis, L.M.L. and A.M.R.S.; investigation, L.M.L. and A.M.R.S.; data curation, L.M.L., A.M.R.S. and S.L.; writing—original draft preparation, L.M.L., A.M.R.S. and T.-W.L.C.; writing—review and editing, T.-W.L.C. and L.R.; visualization, L.M.L., A.M.R.S. and T.-W.L.C.; supervision, T.-W.L.C.; funding acquisition, T.-W.L.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Agricultural Science and Extension for Economic Development (AgSeed) program by the College of Agriculture at Purdue University.
Institutional Review Board Statement
The animal study protocol was approved by the Purdue Animal Care and Use Committee under protocol #1909001951 (14 November 2019).
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
We would like to thank the animal care staff at the Purdue Gnotobiotic Facility for their assistance with our rodent work and acknowledge the Purdue Genomics Core at the Purdue Bindley Bioscience Center.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Salas-Huetos, A.; Bulló, M.; Salas-Salvadó, J. Dietary Patterns, Foods and Nutrients in Male Fertility Parameters and Fecundability: A Systematic Review of Observational Studies. Hum. Reprod. Update 2017, 23, 371–389. [Google Scholar] [CrossRef] [PubMed]
- Cederroth, C.R.; Zimmermann, C.; Beny, J.-L.; Schaad, O.; Combepine, C.; Descombes, P.; Doerge, D.R.; Pralong, F.P.; Vassalli, J.-D.; Nef, S. Potential Detrimental Effects of a Phytoestrogen-Rich Diet on Male Fertility in Mice. Mol. Cell. Endocrinol. 2010, 321, 152–160. [Google Scholar] [CrossRef]
- Nagata, C.; Takatsuka, N.; Kawakami, N.; Shimizu, H. Soy Product Intake and Hot Flashes in Japanese Women: Results from a Community-Based Prospective Study. Am. J. Epidemiol. 2001, 153, 790–793. [Google Scholar] [CrossRef]
- Zhang, Y.-B.; Chen, W.-H.; Guo, J.-J.; Fu, Z.-H.; Yi, C.; Zhang, M.; Na, X.-L. Soy Isoflavone Supplementation Could Reduce Body Weight and Improve Glucose Metabolism in Non-Asian Postmenopausal Women—A Meta-Analysis. Nutr. Burbank Los Angel. Cty. Calif 2013, 29, 8–14. [Google Scholar] [CrossRef]
- Setchell, K.D. Phytoestrogens: The Biochemistry, Physiology, and Implications for Human Health of Soy Isoflavones. Am. J. Clin. Nutr. 1998, 68, 1333S–1346S. [Google Scholar] [CrossRef]
- Yoshikata, R.; Myint, K.Z.; Ohta, H. Relationship between Equol Producer Status and Metabolic Parameters in 743 Japanese Women: Equol Producer Status Is Associated with Antiatherosclerotic Conditions in Women around Menopause and Early Postmenopause. Menopause 2017, 24, 216–224. [Google Scholar] [CrossRef]
- Acharjee, S.; Zhou, J.-R.; Elajami, T.K.; Welty, F.K. Effect of Soy Nuts and Equol Status on Blood Pressure, Lipids and Inflammation in Postmenopausal Women Stratified by Metabolic Syndrome Status. Metabolism 2015, 64, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Anzai, Y.; Tanji, N.; Imaizumi, H.; Fujita, M.; Hayashi, M.; Abe, K.; Ohira, H. Association of Equol with Obesity in Postmenopausal Women. Menopause 2021, 28, 807. [Google Scholar] [CrossRef]
- Lampe, J.W. Is Equol the Key to the Efficacy of Soy Foods? Am. J. Clin. Nutr. 2009, 89, 1664S–1667S. [Google Scholar] [CrossRef]
- Fujitani, T.; Fujii, Y.; Lyu, Z.; Harada Sassa, M.; Harada, K.H. Urinary Equol Levels Are Positively Associated with Urinary Estradiol Excretion in Women. Sci. Rep. 2021, 11, 19532. [Google Scholar] [CrossRef]
- Liu, B.; Qin, L.; Liu, A.; Uchiyama, S.; Ueno, T.; Li, X.; Wang, P. Prevalence of the Equol-Producer Phenotype and Its Relationship with Dietary Isoflavone and Serum Lipids in Healthy Chinese Adults. J. Epidemiol. 2010, 20, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Axelson, M.; Setchell, K.D.R. The Excretion of Lignans in Rats—Evidence for an Intestinal Bacterial Source for This New Group of Compounds. FEBS Lett. 1981, 123, 337–342. [Google Scholar] [CrossRef]
- Usui, T.; Tochiya, M.; Sasaki, Y.; Muranaka, K.; Yamakage, H.; Himeno, A.; Shimatsu, A.; Inaguma, A.; Ueno, T.; Uchiyama, S.; et al. Effects of Natural S -Equol Supplements on Overweight or Obesity and Metabolic Syndrome in the Japanese, Based on Sex and Equol Status. Clin. Endocrinol. 2013, 78, 365–372. [Google Scholar] [CrossRef]
- Yee, S.; Burdock, G.A.; Kurata, Y.; Enomoto, Y.; Narumi, K.; Hamada, S.; Itoh, T.; Shimomura, Y.; Ueno, T. Acute and Subchronic Toxicity and Genotoxicity of SE5-OH, an Equol-Rich Product Produced by Lactococcus Garvieae. Food Chem. Toxicol. 2008, 46, 2713–2720. [Google Scholar] [CrossRef]
- Tousen, Y.; Ezaki, J.; Fujii, Y.; Ueno, T.; Nishimuta, M.; Ishimi, Y. Natural S-Equol Decreases Bone Resorption in Postmenopausal, Non-Equol-Producing Japanese Women: A Pilot Randomized, Placebo-Controlled Trial. Menopause 2011, 18, 563–574. [Google Scholar] [CrossRef]
- Setchell, K.D.R.; Zhao, X.; Shoaf, S.E.; Ragland, K. The Pharmacokinetics of S-(-)Equol Administered as SE5-OH Tablets to Healthy Postmenopausal Women. J. Nutr. 2009, 139, 2037–2043. [Google Scholar] [CrossRef] [PubMed]
- Legette, L.L.; Prasain, J.; King, J.; Arabshahi, A.; Barnes, S.; Weaver, C.M. Pharmacokinetics of Equol, a Soy Isoflavone Metabolite, Changes with the Form of Equol (Dietary versus Intestinal Production) in Ovariectomized Rats. J. Agric. Food Chem. 2014, 62, 1294–1300. [Google Scholar] [CrossRef]
- Hazim, S.; Curtis, P.J.; Schär, M.Y.; Ostertag, L.M.; Kay, C.D.; Minihane, A.-M.; Cassidy, A. Acute Benefits of the Microbial-Derived Isoflavone Metabolite Equol on Arterial Stiffness in Men Prospectively Recruited According to Equol Producer Phenotype: A Double-Blind Randomized Controlled Trial. Am. J. Clin. Nutr. 2016, 103, 694–702. [Google Scholar] [CrossRef]
- Bax, E.N.; Cochran, K.E.; Mao, J.; Wiedmeyer, C.E.; Rosenfeld, C.S. Opposing Effects of S-Equol Supplementation on Metabolic and Behavioral Parameters in Mice Fed a High-Fat Diet. Nutr. Res. 2019, 64, 39–48. [Google Scholar] [CrossRef]
- Bowey, E.; Adlercreutz, H.; Rowland, I. Metabolism of Isoflavones and Lignans by the Gut Microflora: A Study in Germ-Free and Human Flora Associated Rats. Food Chem. Toxicol. 2003, 41, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Franzosa, E.A.; Huang, K.; Meadow, J.F.; Gevers, D.; Lemon, K.P.; Bohannan, B.J.M.; Huttenhower, C. Identifying Personal Microbiomes Using Metagenomic Codes. Proc. Natl. Acad. Sci. USA 2015, 112, E2930–E2938. [Google Scholar] [CrossRef] [PubMed]
- Orcutt, R.P.; Gianni, F.J.; Judge, R.J. Development of an “Altered Schaedler Flora” for NCI Gnotobiotic Rodents. Microecol. Ther. 1987, 17, 59. [Google Scholar]
- Becker, N.; Kunath, J.; Loh, G.; Blaut, M. Human Intestinal Microbiota: Characterization of a Simplified and Stable Gnotobiotic Rat Model. Gut Microbes 2011, 2, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Brugiroux, S.; Beutler, M.; Pfann, C.; Garzetti, D.; Ruscheweyh, H.-J.; Ring, D.; Diehl, M.; Herp, S.; Lötscher, Y.; Hussain, S.; et al. Genome-Guided Design of a Defined Mouse Microbiota That Confers Colonization Resistance against Salmonella Enterica Serovar Typhimurium. Nat. Microbiol. 2017, 2, 16215. [Google Scholar] [CrossRef] [PubMed]
- Dewi, F.N.; Wood, C.E.; Lampe, J.W.; Hullar, M.A.J.; Franke, A.A.; Golden, D.L.; Adams, M.R.; Cline, J.M. Endogenous and Exogenous Equol Are Antiestrogenic in Reproductive Tissues of Apolipoprotein E-Null Mice. J. Nutr. 2012, 142, 1829–1835. [Google Scholar] [CrossRef]
- Matthies, A.; Loh, G.; Blaut, M.; Braune, A. Daidzein and Genistein Are Converted to Equol and 5-Hydroxy-Equol by Human Intestinal Slackia Isoflavoniconvertens in Gnotobiotic Rats1–23. J. Nutr. 2012, 142, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A Human Gut Microbial Gene Catalogue Established by Metagenomic Sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell 2016, 167, 1339–1353.e21. [Google Scholar] [CrossRef]
- Kasahara, K.; Krautkramer, K.A.; Org, E.; Romano, K.A.; Kerby, R.L.; Vivas, E.I.; Mehrabian, M.; Denu, J.M.; Bäckhed, F.; Lusis, A.J.; et al. Interactions between Roseburia Intestinalis and Diet Modulate Atherogenesis in a Murine Model. Nat. Microbiol. 2018, 3, 1461–1471. [Google Scholar] [CrossRef]
- Flórez, A.B.; Vázquez, L.; Rodríguez, J.; Redruello, B.; Mayo, B. Transcriptional Regulation of the Equol Biosynthesis Gene Cluster in Adlercreutzia Equolifaciens DSM19450T. Nutrients 2019, 11, 993. [Google Scholar] [CrossRef]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S Ribosomal DNA Amplification for Phylogenetic Study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Heuer, H.; Krsek, M.; Baker, P.; Smalla, K.; Wellington, E.M. Analysis of Actinomycete Communities by Specific Amplification of Genes Encoding 16S rRNA and Gel-Electrophoretic Separation in Denaturing Gradients. Appl. Environ. Microbiol. 1997, 63, 3233–3241. [Google Scholar] [CrossRef]
- dos Santos, H.R.M.; Argolo, C.S.; Argôlo-Filho, R.C.; Loguercio, L.L. A 16S rDNA PCR-Based Theoretical to Actual Delta Approach on Culturable Mock Communities Revealed Severe Losses of Diversity Information. BMC Microbiol. 2019, 19, 74. [Google Scholar] [CrossRef] [PubMed]
- Decroos, K.; Vanhemmens, S.; Cattoir, S.; Boon, N.; Verstraete, W. Isolation and Characterisation of an Equol-Producing Mixed Microbial Culture from a Human Faecal Sample and Its Activity under Gastrointestinal Conditions. Arch. Microbiol. 2005, 183, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Minamida, K.; Tanaka, M.; Abe, A.; Sone, T.; Tomita, F.; Hara, H.; Asano, K. Production of Equol from Daidzein by Gram-Positive Rod-Shaped Bacterium Isolated from Rat Intestine. J. Biosci. Bioeng. 2006, 102, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Zhao, L.; Zhang, J.; Fang, X.; Zhong, Q.; Liao, Z.; Wang, J.; Guo, Y.; Liang, H.; Wang, L. Colonization Potential to Reconstitute a Microbe Community in Pseudo Germ-Free Mice After Fecal Microbe Transplant From Equol Producer. Front. Microbiol. 2020, 11, 1221. [Google Scholar] [CrossRef] [PubMed]
- Kano, M.; Takayanagi, T.; Harada, K.; Sawada, S.; Ishikawa, F. Bioavailability of Isoflavones after Ingestion of Soy Beverages in Healthy Adults1. J. Nutr. 2006, 136, 2291–2296. [Google Scholar] [CrossRef]
- Kemis, J.H.; Linke, V.; Barrett, K.L.; Boehm, F.J.; Traeger, L.L.; Keller, M.P.; Rabaglia, M.E.; Schueler, K.L.; Stapleton, D.S.; Gatti, D.M.; et al. Genetic Determinants of Gut Microbiota Composition and Bile Acid Profiles in Mice. PLoS Genet. 2019, 15, e1008073. [Google Scholar] [CrossRef] [PubMed]
- Kozich, J.J.; Westcott, S.L.; Baxter, N.T.; Highlander, S.K.; Schloss, P.D. Development of a Dual-Index Sequencing Strategy and Curation Pipeline for Analyzing Amplicon Sequence Data on the MiSeq Illumina Sequencing Platform. Appl. Environ. Microbiol. 2013, 79, 5112–5120. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Vázquez, L.; Guadamuro, L.; Giganto, F.; Mayo, B.; Flórez, A.B. Development and Use of a Real-Time Quantitative PCR Method for Detecting and Quantifying Equol-Producing Bacteria in Human Faecal Samples and Slurry Cultures. Front. Microbiol. 2017, 8, 1155. [Google Scholar] [CrossRef]
- Li, S.; Wang, T.; Fu, W.; Kennett, M.; Cox, A.D.; Lee, D.; Vanamala, J.K.P.; Reddivari, L. Role of Gut Microbiota in the Anti-Colitic Effects of Anthocyanin-Containing Potatoes. Mol. Nutr. Food Res. 2021, 65, 2100152. [Google Scholar] [CrossRef]
- Bisanz, J.E. qiime2R: Importing QIIME2 Artifacts and Associated Data into R Sessions. Version 099. 2018, Volume 13. Available online: https://github.com/jbisanz/qiime2R (accessed on 5 March 2024).
- Huttenhower, C.; Gevers, D.; Knight, R.; Abubucker, S.; Badger, J.H.; Chinwalla, A.T.; Creasy, H.H.; Earl, A.M.; FitzGerald, M.G.; Fulton, R.S.; et al. Structure, Function and Diversity of the Healthy Human Microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef]
- Manor, O.; Dai, C.L.; Kornilov, S.A.; Smith, B.; Price, N.D.; Lovejoy, J.C.; Gibbons, S.M.; Magis, A.T. Health and Disease Markers Correlate with Gut Microbiome Composition across Thousands of People. Nat. Commun. 2020, 11, 5206. [Google Scholar] [CrossRef]
- Vaiserman, A.; Romanenko, M.; Piven, L.; Moseiko, V.; Lushchak, O.; Kryzhanovska, N.; Guryanov, V.; Koliada, A. Differences in the Gut Firmicutes to Bacteroidetes Ratio across Age Groups in Healthy Ukrainian Population. BMC Microbiol. 2020, 20, 221. [Google Scholar] [CrossRef]
- Zafar, H.; Saier, M.H. Gut Bacteroides Species in Health and Disease. Gut Microbes 2021, 13, 1848158. [Google Scholar] [CrossRef]
- Lu, L.J.; Anderson, K.E. Sex and Long-Term Soy Diets Affect the Metabolism and Excretion of Soy Isoflavones in Humans. Am. J. Clin. Nutr. 1998, 68, 1500S–1504S. [Google Scholar] [CrossRef]
- Ohta, A.; Sakai, K.; Takasaki, M.; Uehara, M.; Adlercreutz, H.; Morohashi, T.; Ishimi, Y. A Combination of Dietary Fructooligosaccharides and Isoflavone Conjugates Increases Femoral Bone Mineral Density and Equol Production in Ovariectomized Mice. J. Nutr. 2002, 132, 2048–2054. [Google Scholar] [CrossRef]
- Tousen, Y.; Abe, F.; Ishida, T.; Uehara, M.; Ishimi, Y. Resistant Starch Promotes Equol Production and Inhibits Tibial Bone Loss in Ovariectomized Mice Treated with Daidzein. Metabolism 2011, 60, 1425–1432. [Google Scholar] [CrossRef]
- Tousen, Y.; Matsumoto, Y.; Matsumoto, C.; Nishide, Y.; Nagahata, Y.; Kobayashi, I.; Ishimi, Y. The Combined Effects of Soya Isoflavones and Resistant Starch on Equol Production and Trabecular Bone Loss in Ovariectomised Mice. Br. J. Nutr. 2016, 116, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.R.; Brown, N.M.; Lydeking-Olsen, E. The Clinical Importance of the Metabolite Equol-a Clue to the Effectiveness of Soy and Its Isoflavones. J. Nutr. 2002, 132, 3577–3584. [Google Scholar] [CrossRef] [PubMed]
- Leonard, L.M.; Choi, M.S.; Cross, T.-W.L. Maximizing the Estrogenic Potential of Soy Isoflavones through the Gut Microbiome: Implication for Cardiometabolic Health in Postmenopausal Women. Nutrients 2022, 14, 553. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-L.; Hur, H.-G.; Lee, J.H.; Kim, K.T.; Kim, S.-I. Enantioselective Synthesis of S -Equol from Dihydrodaidzein by a Newly Isolated Anaerobic Human Intestinal Bacterium. Appl. Environ. Microbiol. 2005, 71, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, S.; Ueno, T.; Suzuki, T. Identification of a Newly Isolated Equol-Producing Lactic Acid Bacterium from the Human Feces. J. Intest. Microbiol. 2007, 21, 217–220. [Google Scholar]
- Shimada, Y.; Yasuda, S.; Takahashi, M.; Hayashi, T.; Miyazawa, N.; Sato, I.; Abiru, Y.; Uchiyama, S.; Hishigaki, H. Cloning and Expression of a Novel NADP(H)-Dependent Daidzein Reductase, an Enzyme Involved in the Metabolism of Daidzein, from Equol-Producing Lactococcus Strain 20–92. Appl. Environ. Microbiol. 2010, 76, 5892–5901. [Google Scholar] [CrossRef] [PubMed]
- Elghali, S. Bioconversion of Daidzein to Equol by Bifidobacterium Breve 15700 and Bifidobacterium Longum BB536. J. Funct. Foods 2012, 10, 736–745. [Google Scholar] [CrossRef]
- Heng, Y.; Kim, M.J.; Yang, H.J.; Kang, S.; Park, S. Lactobacillus Intestinalis Efficiently Produces Equol from Daidzein and Chungkookjang, Short-Term Fermented Soybeans. Arch. Microbiol. 2019, 201, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Iino, C.; Shimoyama, T.; Iino, K.; Yokoyama, Y.; Chinda, D.; Sakuraba, H.; Fukuda, S.; Nakaji, S. Daidzein Intake Is Associated with Equol Producing Status through an Increase in the Intestinal Bacteria Responsible for Equol Production. Nutrients 2019, 11, 433. [Google Scholar] [CrossRef]
- Sakamoto, M.; Sakurai, N.; Tanno, H.; Iino, T.; Ohkuma, M.; Endo, A. Genome-Based, Phenotypic and Chemotaxonomic Classification of Faecalibacterium Strains: Proposal of Three Novel Species Faecalibacterium duncaniae sp. nov., Faecalibacterium hattorii sp. nov. and Faecalibacterium gallinarum sp. nov. Int. J. Syst. Evol. Microbiol. 2022, 72. [Google Scholar] [CrossRef]
- Duncan, S.H. Growth Requirements and Fermentation Products of Fusobacterium Prausnitzii, and a Proposal to Reclassify It as Faecalibacterium prausnitzii gen. nov., comb. nov. Int. J. Syst. Evol. Microbiol. 2002, 52, 2141–2146. [Google Scholar] [CrossRef] [PubMed]
- Rezzonico, E.; Mestdagh, R.; Delley, M.; Combremont, S.; Dumas, M.-E.; Holmes, E.; Nicholson, J.; Bibiloni, R. Bacterial Adaptation to the Gut Environment Favors Successful Colonization: Microbial and Metabonomic Characterization of a Simplified Microbiota Mouse Model. Gut Microbes 2011, 2, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Eberl, C.; Ring, D.; Münch, P.C.; Beutler, M.; Basic, M.; Slack, E.C.; Schwarzer, M.; Srutkova, D.; Lange, A.; Frick, J.S.; et al. Reproducible Colonization of Germ-Free Mice with the Oligo-Mouse-Microbiota in Different Animal Facilities. Front. Microbiol. 2020, 10, 2999. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).