Dietary Amino Acid Composition and Glycemic Biomarkers in Japanese Adolescents
Abstract
1. Introduction
2. Materials and Methods
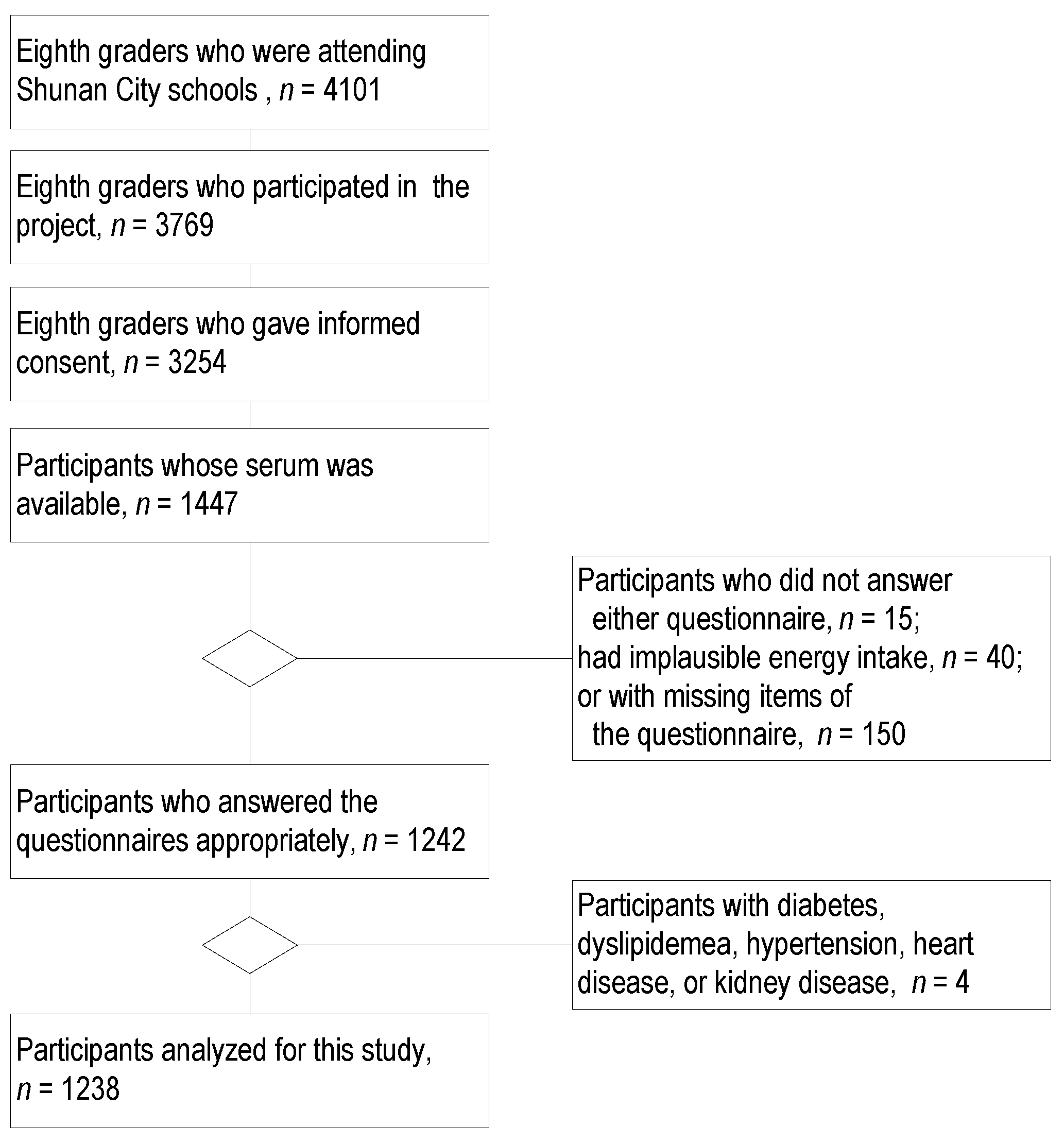
2.1. Participants
2.2. Amino Acids
2.3. Glucose Metabolism
2.4. Covariates
2.5. Statistical Analysis
3. Results
3.1. Participants
3.2. Amino Acid Intake
3.3. Regression Analysis
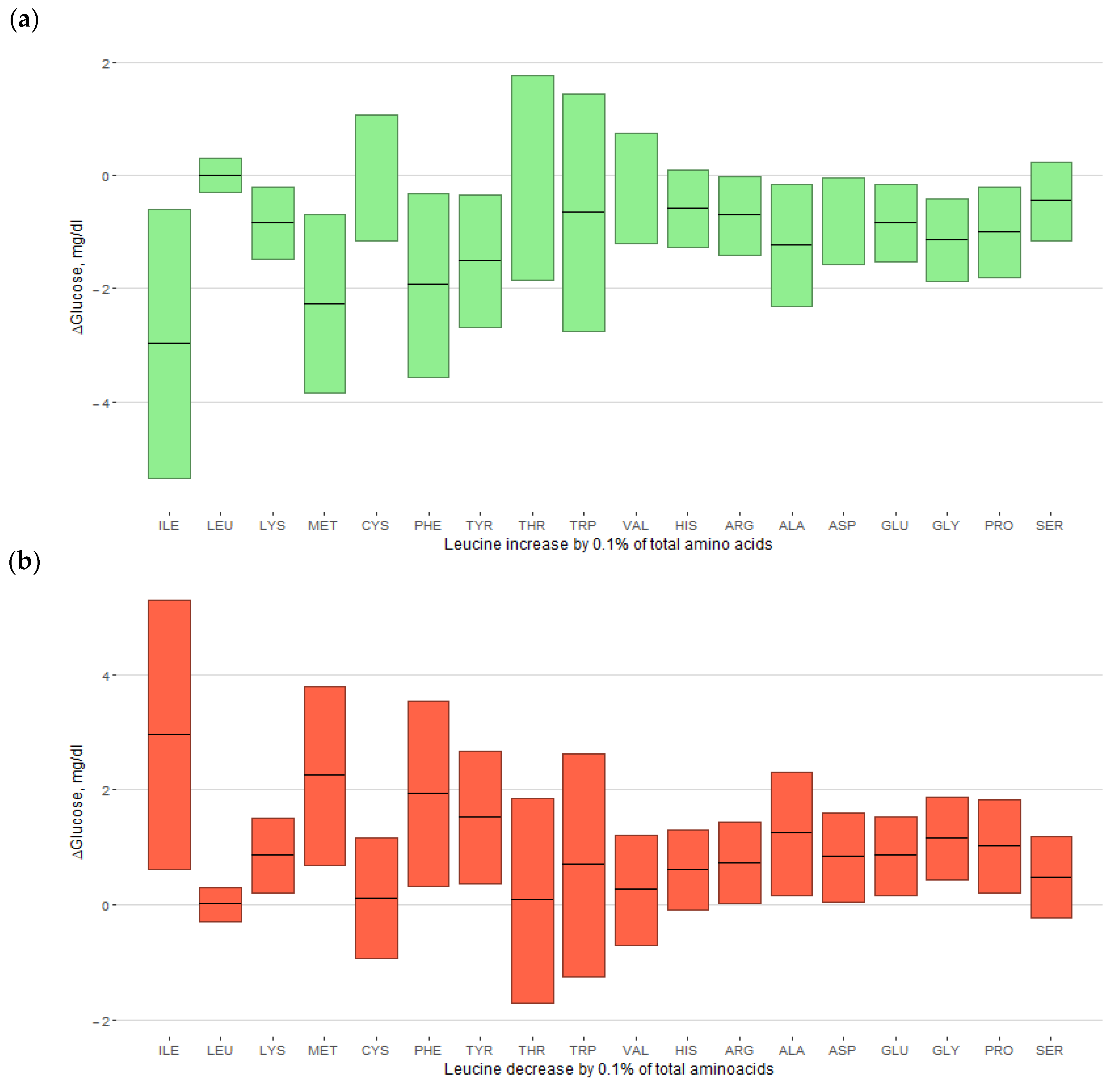
3.4. Replacement of Amino Acids
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Valaiyapathi, B.; Gower, B.; Ashraf, A.P. Pathophysiology of type 2 diabetes in children and adolescents. Curr. Diabetes Rev. 2020, 16, 220–229. [Google Scholar] [CrossRef]
- Daneshzad, E.; Rostami, S.; Aghamahdi, F.; Mahdavi-Gorabi, A.; Qorbani, M. Association of cardiometabolic risk factors with insulin resistance in overweight and obese children. BMC Endocr. Disord. 2022, 22, 320. [Google Scholar] [CrossRef]
- Neuenschwander, M.; Ballon, A.; Weber, K.S.; Norat, T.; Aune, D.; Schwingshackl, L.; Schlesinger, S. Role of diet in type 2 diabetes incidence: Umbrella review of meta-analyses of prospective observational studies. BMJ 2019, 366, l2368. [Google Scholar] [CrossRef]
- Zhao, L.G.; Zhang, Q.L.; Liu, X.L.; Wu, H.; Zheng, J.L.; Xiang, Y.B. Dietary protein intake and risk of type 2 diabetes: A dose-response meta-analysis of prospective studies. Eur. J. Nutr. 2019, 58, 1351–1367. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Li, Y.; Wang, C.; Mao, Z.; Zhou, W.; Zhang, L.; Yang, X.; Cui, S.; Li, L. Dietary protein consumption and the risk of type 2 diabetes: A dose-response meta-analysis of prospective studies. Nutrients 2019, 11, 2783. [Google Scholar] [CrossRef] [PubMed]
- Schulze, M.B.; Haardt, J.; Amini, A.M.; Kalotai, N.; Lehmann, A.; Schmidt, A.; Buyken, A.E.; Egert, S.; Ellinger, S.; Kroke, A.; et al. Protein intake and type 2 diabetes mellitus: An umbrella review of systematic reviews for the evidence-based guideline for protein intake of the German Nutrition Society. Eur. J. Nutr. 2024, 63, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Hoffmann, G.; Lampousi, A.M.; Knüppel, S.; Iqbal, K.; Schwedhelm, C.; Bechthold, A.; Schlesinger, S.; Boeing, H. Food groups and risk of type 2 diabetes mellitus: A systematic review and meta-analysis of prospective studies. Eur. J. Epidemiol. 2017, 32, 363–375. [Google Scholar] [CrossRef]
- Chen, Z.; Franco, O.H.; Lamballais, S.; Ikram, M.A.; Schoufour, J.D.; Muka, T.; Voortman, T. Associations of specific dietary protein with longitudinal insulin resistance, prediabetes and type 2 diabetes: The Rotterdam Study. Clin. Nutr. 2020, 39, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, H.E.K.; Koskinen, T.T.; Voutilainen, S.; Mursu, J.; Tuomainen, T.-P.; Kokko, P.; Virtanen, J.K. Intake of different dietary proteins and risk of type 2 diabetes in men: The Kuopio Ischaemic Heart Disease Risk Factor Study. Br. J. Nutr. 2017, 117, 882–893. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, Y.; Qi, Q.; Hruby, A.; Manson, J.E.; Willett, W.C.; Wolpin, B.M.; Hu, F.B.; Qi, L. Cumulative consumption of branched-chain amino acids and incidence of type 2 diabetes. Int. J. Epidemiol. 2016, 45, 1482–1492. [Google Scholar] [CrossRef]
- Isanejad, M.; LaCroix, A.Z.; Thomson, C.A.; Tinker, L.; Larson, J.C.; Qi, Q.; Qi, L.; Cooper-DeHoff, R.M.; Phillips, L.S.; Prentice, R.L.; et al. Branched-chain amino acid, meat intake and risk of type 2 diabetes in the Women’s Health Initiative. Br. J. Nutr. 2017, 117, 1523–1530. [Google Scholar] [CrossRef]
- Wang, W.; Jiang, H.; Zhang, Z.; Duan, W.; Han, T.; Sun, C. Interaction between dietary branched-chain amino acids and genetic risk score on the risk of type 2 diabetes in Chinese. Genes Nutr. 2021, 16, 4. [Google Scholar] [CrossRef]
- Gadgil, M.D.; Ingram, K.H.; Appiah, D.; Rudd, J.; Whitaker, K.M.; Bennett, W.L.; Shikany, J.M.; Jacobs, D.R., Jr.; Lewis, C.E.; Gunderson, E.P. Prepregnancy protein source and BCAA intake are associated with gestational diabetes mellitus in the CARDIA Study. Int. J. Environ. Res. Public Health 2022, 19, 14142. [Google Scholar] [CrossRef]
- Nagata, C.; Nakamura, K.; Wada, K.; Tsuji, M.; Tamai, Y.; Kawachi, T. Branched-chain amino acid intake and the risk of diabetes in a Japanese community: The Takayama Study. Am. J. Epidemiol. 2013, 178, 1226–1232. [Google Scholar] [CrossRef]
- Dong, Z.; Richie, J.P., Jr.; Gao, X.; Al-Shaar, L.; Nichenametla, S.N.; Shen, B.; Orentreich, D. Cumulative consumption of sulfur amino acids and risk of diabetes: A prospective cohort study. J. Nutr. 2022, 152, 2419–2428. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Li, C.L.; Qi, J.Y.; Huang, L.N.; Shi, D.; Du, S.S.; Liu, L.Y.; Feng, R.N.; Sun, C.H. Relationships of dietary histidine and obesity in northern Chinese adults, an Internet-based cross-sectional study. Nutrients 2016, 8, 420. [Google Scholar] [CrossRef] [PubMed]
- Ezeonwumelu, I.J.; Mode, A.M.; Magaji, U.F.; Nzoniwu, N.A.; Tangaza, M.H.; Tanimu, F.I.; Dandare, S.U. Coadministration of L-alanine and L-glutamine ameliorate blood glucose levels, biochemical indices and histological features in alloxan-induced diabetic rats. J. Food Biochem. 2022, 46, e14420. [Google Scholar] [CrossRef] [PubMed]
- Correa Leite, M.L. Applying compositional data methodology to nutritional epidemiology. Stat. Methods Med. Res. 2016, 25, 3057–3065. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, V.; Koletzko, B.; Luque, V.; Gispert-Llaurado, M.; Gruszfeld, D.; Socha, P.; Verduci, E.; Zuccotti, G.V.; Etienne, L.; Grote, V. Time of dietary energy and nutrient intake and body mass index in children: Compositional data analysis from the Childhood Obesity Project (CHOP) trial. Nutrients 2022, 14, 4356. [Google Scholar] [CrossRef] [PubMed]
- Okuda, M.; Fujiwara, A.; Sasaki, S. Composition of dietary fatty acids and health risks in Japanese youths. Nutrients 2021, 13, 426. [Google Scholar] [CrossRef] [PubMed]
- Movassagh, E.Z.; Baxter-Jones, A.D.G.; Kontulainen, S.; Whiting, S.J.; Vatanparast, H. Tracking dietary patterns over 20 years from childhood through adolescence into young adulthood: The Saskatchewan Pediatric Bone Mineral Accrual Study. Nutrients 2017, 9, 990. [Google Scholar] [CrossRef] [PubMed]
- Appannah, G.; Murray, K.; Trapp, G.; Dymock, M.; Oddy, W.H.; Ambrosini, G.L. Dietary pattern trajectories across adolescence and early adulthood and their associations with childhood and parental factors. Am. J. Clin. Nutr. 2021, 113, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Okuda, M.; Sasaki, S.; Bando, N.; Hashimoto, M.; Kunitsugu, I.; Sugiyama, S.; Terao, J.; Hobara, T. Carotenoid, tocopherol, and fatty acid biomarkers and dietary intake estimated by using a brief self-administered diet history questionnaire for older Japanese children and adolescents. J. Nutr. Sci. Vitaminol. 2009, 55, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Okuda, M.; Fujiwara, A.; Sasaki, S. Added and free sugars intake and metabolic biomarkers in Japanese adolescents. Nutrients 2020, 12, 2046. [Google Scholar] [CrossRef]
- Sasaki, S.; Yanagibori, R.; Amano, K. Self-administered diet history questionnaire developed for health education: A relative validation of the test-version by comparison with 3-day diet record in women. J. Epidemiol. 1998, 8, 203–215. [Google Scholar] [CrossRef]
- Kobayashi, S.; Murakami, K.; Sasaki, S.; Okubo, H.; Hirota, N.; Notsu, A.; Fukui, M.; Date, C. Comparison of relative validity for food group intake estimated by comprehensive and brief-type self-administered diet history questionnaires against 16-day dietary records in Japanese adults. Public Health Nutr. 2011, 14, 1200–1211. [Google Scholar] [CrossRef] [PubMed]
- Science and Technology Agency. Standard Tables of Food Composition in Japan, 2010; Ministry of Education, Culture, Sports, Science and Technology: Tokyo, Japan, 2010.
- Suga, H.; Murakami, K.; Sasaki, S. Development of an amino acid composition database and estimation of amino acid intake in Japanese adults. Asia Pac. J. Clin. Nutr. 2013, 22, 188–199. [Google Scholar] [CrossRef]
- Suga, H.; Asakura, K.; Sasaki, S.; Nojima, M.; Okubo, H.; Hirota, N.; Notsu, A.; Fukui, M.; Date, C. Validation study of a self-administered diet history questionnaire for estimating amino acid intake among Japanese adults. Asia Pac. J. Clin. Nutr. 2018, 27, 638–645. [Google Scholar] [CrossRef]
- Okuda, M.; Asakura, K.; Sasaki, S. Protein intake estimated from brief-type self-administered diet history questionnaire and urinary urea nitrogen level in adolescents. Nutrients 2019, 11, 319. [Google Scholar] [CrossRef]
- Ito, S.; Sasaki, S. (Eds.) Dietary Reference Intakes for Japanese 2020; Daiichi-Shuppan: Tokyo, Japan, 2020. [Google Scholar]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- The American Diabetes Association. TABLE 1—Systeme International (SI) units for plasma, serum, or blood concentrations. Diabetes 1998, 47, 1377. [Google Scholar] [CrossRef]
- Cole, T.J.; Green, P.J. Smoothing reference centile curves: The LMS method and penalized likelihood. Stat. Med. 1992, 11, 1305–1319. [Google Scholar] [CrossRef] [PubMed]
- Kato, N.; Takimoto, H.; Sudo, N. The cubic functions for spline smoothed L, S and M values for BMI reference data of Japanese children. Clin. Pediatr. Endocrinol. 2011, 20, 47–49. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 1 December 2020).
- van den Boogaart, K.G.; Tolosana-Delgado, R.; Bren, M. Compositions: Compositional Data Analysis. R Package Version 2.0-6. Available online: https://CRAN.R-project.org/package=compositions (accessed on 1 September 2023).
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Aitchison, J. The statistical analysis of compositional data. J. R. Stat. Soc. B 1982, 44, 139–160. [Google Scholar] [CrossRef]
- Müller, I.; Hron, K.; Fišerová, E.; Šmahaj, J.; Cakirpaloglu, P.; Vančáková, J. Interpretation of compositional regression with application to time budget analysis. Aust. J. Stat. 2018, 47, 3–19. [Google Scholar] [CrossRef]
- Egozcue, J.J.; Daunis-I-Estadella, J.; Pawlowsky-Glahn, V.; Hron, K.; Filzmoser, P. Simplicial regression. The normal model. J. Appl. Probab. Stat. 2012, 6, 87–108. [Google Scholar]
- Roquetto, A.R.; Moura, C.S.; de Almeida Santos-Junior, V.; Oliveira, P.O.S.; Machado, K.I.A.; Carvalho, G.; Risso, E.M.; Amaya-Farfan, J. Moderate intake of BCAA-rich protein improves glucose homeostasis in high-fat-fed mice. J. Nutr. Biochem. 2020, 80, 108332. [Google Scholar] [CrossRef]
- Utsugi, T.; Yoshida, A.; Kanda, T.; Kobayashi, I.; Kurabayashi, M.; Tomono, S.; Kawazu, S.; Tajima, Y.; Nagai, R. Oral administration of branched chain amino acids improves virus-induced glucose intolerance in mice. Eur. J. Pharmacol. 2000, 398, 409–414. [Google Scholar] [CrossRef]
- Binder, E.; Bermúdez-Silva, F.J.; André, C.; Elie, M.; Romero-Zerbo, S.Y.; Leste-Lasserre, T.; Belluomo, I.; Duchampt, A.; Clark, S.; Aubert, A.; et al. Leucine supplementation protects from insulin resistance by regulating adiposity levels. PLoS ONE 2013, 8, e74705. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Liu, R.; Ma, Y.; Guo, H.; Hao, L.; Yao, P.; Liu, L.; Sun, X.; He, K.; et al. Chronic leucine supplementation increases body weight and insulin sensitivity in rats on high-fat diet likely by promoting insulin signaling in insulin-target tissues. Mol. Nutr. Food Res. 2013, 57, 1067–1079. [Google Scholar] [CrossRef]
- Doi, M.; Yamaoka, I.; Fukunaga, T.; Nakayama, M. Isoleucine, a potent plasma glucose-lowering amino acid, stimulates glucose uptake in C2C12 myotubes. Biochem. Biophys. Res. Commun. 2003, 312, 1111–1117. [Google Scholar] [CrossRef]
- Soleimani, E.; Rashnoo, F.; Farhangi, M.A.; Hosseini, B.; Jafarzadeh, F.; Shakarami, A.; Sadabadi, Y. Dietary branched-chain amino acids intake, glycemic markers, metabolic profile, and anthropometric features in a community-based sample of overweight and obese adults. BMC Endocr. Disord. 2023, 23, 205. [Google Scholar] [CrossRef]
- Rietman, A.; Schwarz, J.; Tomé, D.; Kok, F.J.; Mensink, M. High dietary protein intake, reducing or eliciting insulin resistance? Eur. J. Clin. Nutr. 2014, 68, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Orentreich, N.; Matias, J.R.; DeFelice, A.; Zimmerman, J.A. Low methionine ingestion by rats extends life span. J. Nutr. 1993, 123, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Malloy, V.L.; Krajcik, R.A.; Bailey, S.J.; Hristopoulos, G.; Plummer, J.D.; Orentreich, N. Methionine restriction decreases visceral fat mass and preserves insulin action in aging male Fischer 344 rats independent of energy restriction. Aging Cell 2006, 5, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Richie, J.P., Jr.; Sinha, R.; Dong, Z.; Nichenametla, S.N.; Ables, G.P.; Ciccarella, A.; Sinha, I.; Calcagnotto, A.M.; Chinchilli, V.M.; Reinhart, L.; et al. Dietary methionine and total sulfur amino acid restriction in healthy adults. J. Nutr. Health Aging 2023, 27, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Nimni, M.E.; Han, B.; Cordoba, F. Are we getting enough sulfur in our diet? Nutr. Metab. 2007, 4, 24. [Google Scholar] [CrossRef] [PubMed]
- Lamberg-Allardt, C.; Bärebring, L.; Arnesen, E.K.; Nwaru, B.I.; Thorisdottir, B.; Ramel, A.; Söderlund, F.; Dierkes, J.; Åkesson, A. Animal versus plant-based protein and risk of cardiovascular disease and type 2 diabetes: A systematic review of randomized controlled trials and prospective cohort studies. Food Nutr. Res. 2023, 67. [Google Scholar] [CrossRef]
- Feng, R.N.; Niu, Y.C.; Sun, X.W.; Li, Q.; Zhao, C.; Wang, C.; Guo, F.C.; Sun, C.H.; Li, Y. Histidine supplementation improves insulin resistance through suppressed inflammation in obese women with the metabolic syndrome: A randomised controlled trial. Diabetologia 2013, 56, 985–994. [Google Scholar] [CrossRef]
- Freeman, A.M.; Acevedo, L.A.; Pennings, N. Insulin Resistance. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK507839/ (accessed on 29 January 2024).
- Seino, Y.; Nanjo, K.; Tajima, N.; Kadowaki, T.; Kashiwagi, A.; Araki, E.; Ito, C.; Inagaki, N.; Iwamoto, Y.; Kasuga, M.; et al. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J. Diabetes Investig. 2010, 1, 212–228. [Google Scholar] [CrossRef] [PubMed]
- Peplies, J.; Jiménez-Pavón, D.; Savva, S.C.; Buck, C.; Günther, K.; Fraterman, A.; Russo, P.; Iacoviello, L.; Veidebaum, T.; Tornaritis, M.; et al. Percentiles of fasting serum insulin, glucose, HbA1c and HOMA-IR in pre-pubertal normal weight European children from the IDEFICS cohort. Int. J. Obes. 2014, 38 (Suppl. S2), S39–S47. [Google Scholar] [CrossRef] [PubMed]
- Frithioff-Bøjsøe, C.; Lund, M.A.V.; Kloppenborg, J.T.; Nielsen, T.T.H.; Fonvig, C.E.; Lausten-Thomsen, U.; Hedley, P.L.; Hansen, T.; Pedersen, O.B.; Christiansen, M.; et al. Glucose metabolism in children and adolescents: Population-based reference values and comparisons to children and adolescents enrolled in obesity treatment. Pediatr. Diabetes 2019, 20, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Polakof, S.; Dardevet, D.; Lyan, B.; Mosoni, L.; Gatineau, E.; Martin, J.F.; Pujos-Guillot, E.; Mazur, A.; Comte, B. Time course of molecular and metabolic events in the development of insulin resistance in fructose-fed rats. J. Proteome Res. 2016, 15, 1862–1874. [Google Scholar] [CrossRef]
| Mean, Geometric Mean *, n | SD, 95% CI *, (%) | |
|---|---|---|
| Sex: Male | 651 | (52.6%) |
| Female | 587 | (47.4%) |
| Age, months | 163.5 | 3.4 |
| BMI, kg/m2 | 19.2 | 2.6 |
| zBMI | −0.25 | 0.90 |
| Exercise ≥3 times/week | 947 | (76.5%) |
| Screen time, h | 4.2 | 1.0 |
| Sleep duration, h | 7.4 | 0.7 |
| Energy, kcal | 2266.4 | 634.9 |
| Protein, %E | 14.2 | 2.3 |
| Total dietary fiber, g/1000 kcal | 5.5 | 1.4 |
| Saturated fatty acids, %E | 10.6 | 2.5 |
| Glycemic load, /1000 kcal | 68.4 | 22.5 |
| Single parent | 71 | (5.7%) |
| Siblings: 1 | 118 | (9.5%) |
| 2 | 588 | (47.5%) |
| ≥3 | 532 | (43.0%) |
| Passive smoking in household | 638 | (51.5%) |
| Plasma glucose, mg/dL | 90.6 | 5.6 |
| Serum insulin, μU/mL | 6.19 * | 6.03, 6.35 * |
| HOMA-IR | 1.38 * | 1.34, 1.42 * |
| HOMA-β, % | 82.8 * | 80.8, 84.9 * |
| Geometric Mean, % | Arithmetic Mean ± SD, % | |
|---|---|---|
| Isoleucine | 4.56 | 4.56 ± 0.10 |
| Leucine | 8.24 | 8.24 ± 0.18 |
| Lysine | 6.85 | 6.85 ± 0.43 |
| Methionine | 2.47 | 2.47 ± 0.09 |
| Cysteine | 1.55 | 1.55 ± 0.10 |
| Phenylalanine | 4.66 | 4.66 ± 0.09 |
| Tyrosine | 3.66 | 3.66 ± 0.07 |
| Threonine | 4.10 | 4.10 ± 0.10 |
| Tryptophan | 1.23 | 1.23 ± 0.03 |
| Valine | 5.39 | 5.39 ± 0.13 |
| Histidine | 3.47 | 3.47 ± 0.23 |
| Arginine | 5.65 | 5.64 ± 0.36 |
| Alanine | 4.99 | 4.99 ± 0.27 |
| Aspartic acid | 9.36 | 9.36 ± 0.36 |
| Glutamic acid | 18.68 | 18.68 ± 0.90 |
| Glycine | 4.21 | 4.21 ± 0.30 |
| Proline | 6.10 | 6.10 ± 0.55 |
| Serine | 4.84 | 4.84 ± 0.13 |
| Glucose, mg/dL | log(Insulin, μU/mL) | log(HOMA-IR) | log(HOMA-β, %) | |||||
|---|---|---|---|---|---|---|---|---|
| β (SE) | p | β (SE) | p | β (SE) | p | β (SE) | p | |
| Isoleucine | −119.1 (64.2) | 0.066 | 0.78 (4.89) | 0.818 | −0.56 (5.18) | 0.969 | 5.56 (4.68) | 0.209 |
| Leucine | 50 (22.6) | 0.025 | 1.01 (1.72) | 0.564 | 1.57 (1.82) | 0.393 | −0.95 (1.64) | 0.537 |
| Lysine | 26.5 (27.3) | 0.326 | −0.47 (2.07) | 0.792 | −0.17 (2.20) | 0.913 | −1.59 (1.99) | 0.399 |
| Methionine | −32.6 (18.9) | 0.073 | 0.11 (1.44) | 0.926 | −0.26 (1.52) | 0.868 | 1.45 (1.37) | 0.268 |
| Cysteine | 42.8 (31.8) | 0.159 | −0.51 (2.42) | 0.837 | −0.04 (2.57) | 1.000 | −2.14 (2.32) | 0.337 |
| Phenylalanine | −17.9 (32.8) | 0.511 | 0.58 (2.49) | 0.862 | 0.38 (2.64) | 0.943 | 1.32 (2.39) | 0.574 |
| Tyrosine | −40.5 (33.1) | 0.188 | 0.51 (2.52) | 0.846 | 0.04 (2.67) | 0.996 | 2.19 (2.41) | 0.338 |
| Threonine | 22.6 (36.5) | 0.468 | −0.72 (2.77) | 0.825 | −0.45 (2.94) | 0.920 | −1.78 (2.66) | 0.488 |
| Tryptophan | −23.3 (27.1) | 0.430 | 1.23 (2.06) | 0.518 | 0.97 (2.18) | 0.618 | 2.21 (1.97) | 0.259 |
| Valine | 17.7 (23.3) | 0.480 | −0.98 (1.77) | 0.549 | −0.78 (1.88) | 0.643 | −1.75 (1.70) | 0.294 |
| Histidine | 0.2 (7.0) | 0.890 | 0.06 (0.53) | 0.833 | 0.06 (0.56) | 0.826 | 0.05 (0.51) | 0.891 |
| Arginine | 19 (19.2) | 0.283 | −0.79 (1.46) | 0.615 | −0.58 (1.55) | 0.746 | −1.55 (1.40) | 0.261 |
| Alanine | −17.6 (17.1) | 0.262 | 0.64 (1.30) | 0.686 | 0.44 (1.38) | 0.824 | 1.35 (1.25) | 0.289 |
| Aspartic acid | −2 (14.0) | 0.837 | −0.59 (1.07) | 0.569 | −0.62 (1.13) | 0.568 | −0.45 (1.02) | 0.674 |
| Glutamic acid | 15.4 (12.5) | 0.249 | 1.25 (0.95) | 0.212 | 1.42 (1.01) | 0.182 | 0.67 (0.91) | 0.474 |
| Glycine | −3.1 (13.7) | 0.924 | −0.06 (1.04) | 0.946 | −0.09 (1.10) | 0.955 | −0.03 (0.99) | 0.982 |
| Proline | −19.2 (19.0) | 0.305 | −0.22 (1.45) | 0.852 | −0.44 (1.53) | 0.749 | 0.57 (1.38) | 0.693 |
| Serine | 81.3 (49.9) | 0.109 | −1.81 (3.80) | 0.586 | −0.89 (4.02) | 0.773 | −5.13 (3.63) | 0.142 |
| Glucose, mg/dL | log(Insulin, μU/mL) | log(HOMA-IR) | log(HOMA-β) | |||||
|---|---|---|---|---|---|---|---|---|
| Effect | 95%CI | Effect | 95%CI | Effect | 95%CI | Effect | 95%CI | |
| Isoleucine | 2.09 | (0.11, 4.07) | −0.05 | (−0.20, 0.10) | −0.02 | (−0.18, 0.14) | −0.13 | (−0.28, 0.01) |
| Leucine | −1.02 | (−1.76, −0.28) | −0.01 | (−0.07, 0.04) | −0.02 | (−0.08, 0.04) | 0.03 | (−0.03, 0.08) |
| Lysine | −0.13 | (−0.8, 0.55) | 0.01 | (−0.04, 0.06) | 0.01 | (−0.05, 0.06) | 0.01 | (−0.04, 0.06) |
| Methionine | 1.34 | (0.18, 2.5) | −0.01 | (−0.09, 0.08) | 0.01 | (−0.08, 0.1) | −0.06 | (−0.15, 0.02) |
| Cysteine | −0.92 | (−2.13, 0.3) | 0.00 | (−0.09, 0.09) | −0.01 | (−0.11, 0.09) | 0.03 | (−0.05, 0.12) |
| Phenylalanine | 1.01 | (−0.26, 2.29) | −0.02 | (−0.11, 0.08) | −0.01 | (−0.11, 0.1) | −0.05 | (−0.15, 0.04) |
| Tyrosine | 0.58 | (−0.25, 1.40) | 0.00 | (−0.06, 0.07) | 0.01 | (−0.06, 0.08) | −0.02 | (−0.08, 0.04) |
| Threonine | −0.95 | (−2.7, 0.79) | 0.02 | (−0.11, 0.15) | 0.01 | (−0.13, 0.15) | 0.06 | (−0.06, 0.19) |
| Tryptophan | −0.30 | (−2.43, 1.82) | −0.02 | (−0.18, 0.14) | −0.02 | (−0.19, 0.15) | −0.01 | (−0.16, 0.15) |
| Valine | −0.76 | (−1.76, 0.24) | 0.03 | (−0.05, 0.11) | 0.02 | (−0.06, 0.10) | 0.06 | (−0.01, 0.13) |
| Histidine | −0.39 | (−0.81, 0.03) | 0.00 | (−0.03, 0.04) | 0.00 | (−0.03, 0.03) | 0.02 | (−0.01, 0.05) |
| Arginine | −0.26 | (−0.73, 0.21) | 0.00 | (−0.03, 0.04) | 0.00 | (−0.04, 0.04) | 0.01 | (−0.02, 0.05) |
| Alanine | 0.28 | (−0.44, 1.00) | −0.02 | (−0.07, 0.04) | −0.02 | (−0.07, 0.04) | −0.03 | (−0.08, 0.02) |
| Aspartic acid | −0.16 | (−0.54, 0.22) | 0.00 | (−0.03, 0.03) | 0.00 | (−0.03, 0.03) | 0.01 | (−0.02, 0.03) |
| Glutamic acid | −0.63 | (−1.63, 0.37) | −0.02 | (−0.1, 0.05) | −0.03 | (−0.11, 0.05) | 0.00 | (−0.07, 0.08) |
| Glycine | 0.90 | (−1.13, 2.93) | 0.11 | (−0.04, 0.27) | 0.12 | (−0.04, 0.29) | 0.09 | (−0.06, 0.24) |
| Proline | 0.18 | (−1.13, 1.49) | 0.07 | (−0.03, 0.17) | 0.07 | (−0.03, 0.18) | 0.06 | (−0.03, 0.16) |
| Serine | −2.54 | (−5.76, 0.67) | 0.06 | (−0.19, 0.30) | 0.03 | (−0.23, 0.29) | 0.16 | (−0.07, 0.39) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okuda, M.; Sasaki, S. Dietary Amino Acid Composition and Glycemic Biomarkers in Japanese Adolescents. Nutrients 2024, 16, 882. https://doi.org/10.3390/nu16060882
Okuda M, Sasaki S. Dietary Amino Acid Composition and Glycemic Biomarkers in Japanese Adolescents. Nutrients. 2024; 16(6):882. https://doi.org/10.3390/nu16060882
Chicago/Turabian StyleOkuda, Masayuki, and Satoshi Sasaki. 2024. "Dietary Amino Acid Composition and Glycemic Biomarkers in Japanese Adolescents" Nutrients 16, no. 6: 882. https://doi.org/10.3390/nu16060882
APA StyleOkuda, M., & Sasaki, S. (2024). Dietary Amino Acid Composition and Glycemic Biomarkers in Japanese Adolescents. Nutrients, 16(6), 882. https://doi.org/10.3390/nu16060882






