Associations between Cholesterol Intake, Food Sources and Cardiovascular Disease in Chinese Residents
Abstract
1. Introduction
2. Materials and Methods
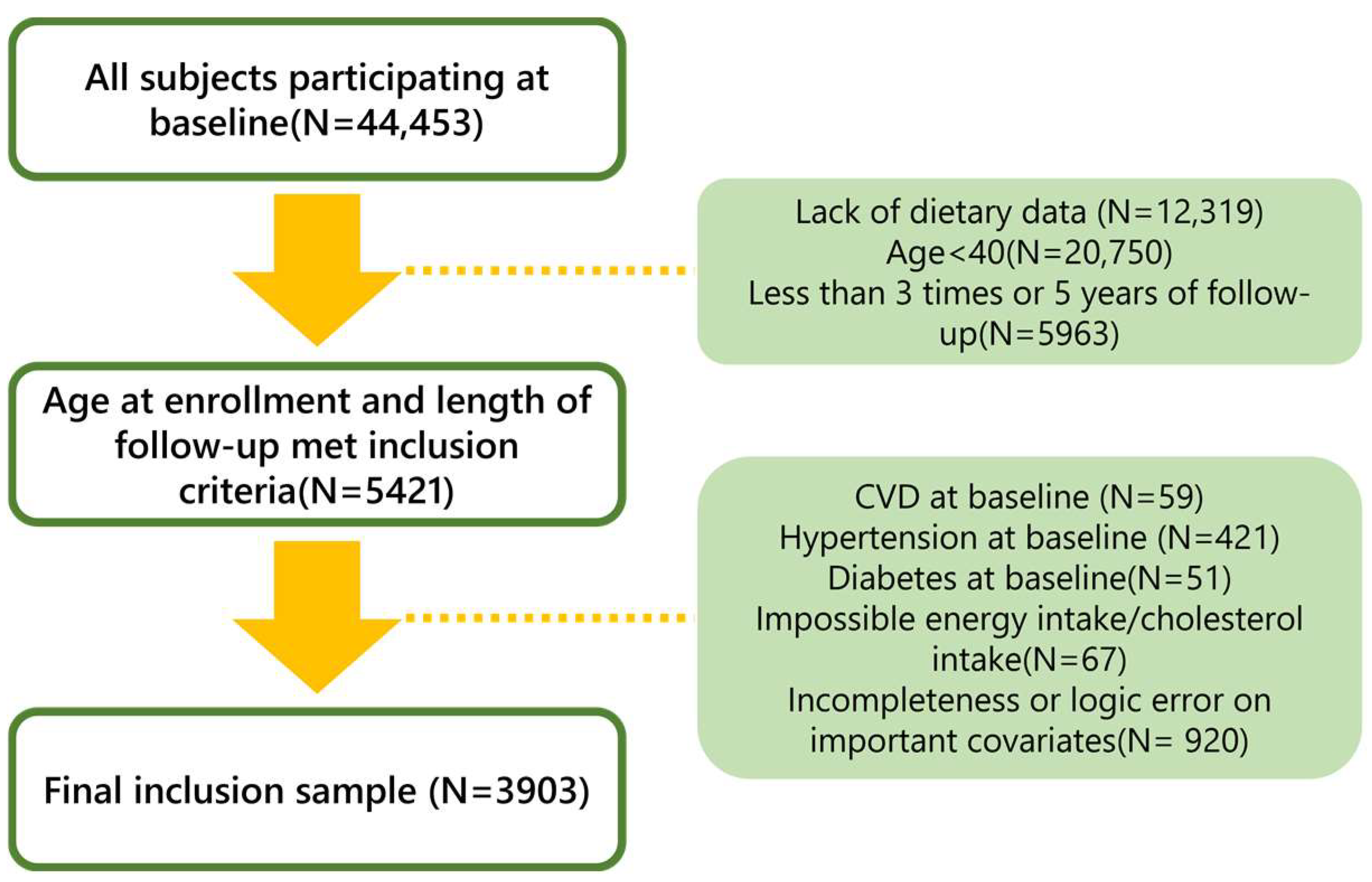
2.1. Study Population
2.2. Study Variables
2.3. Statistic
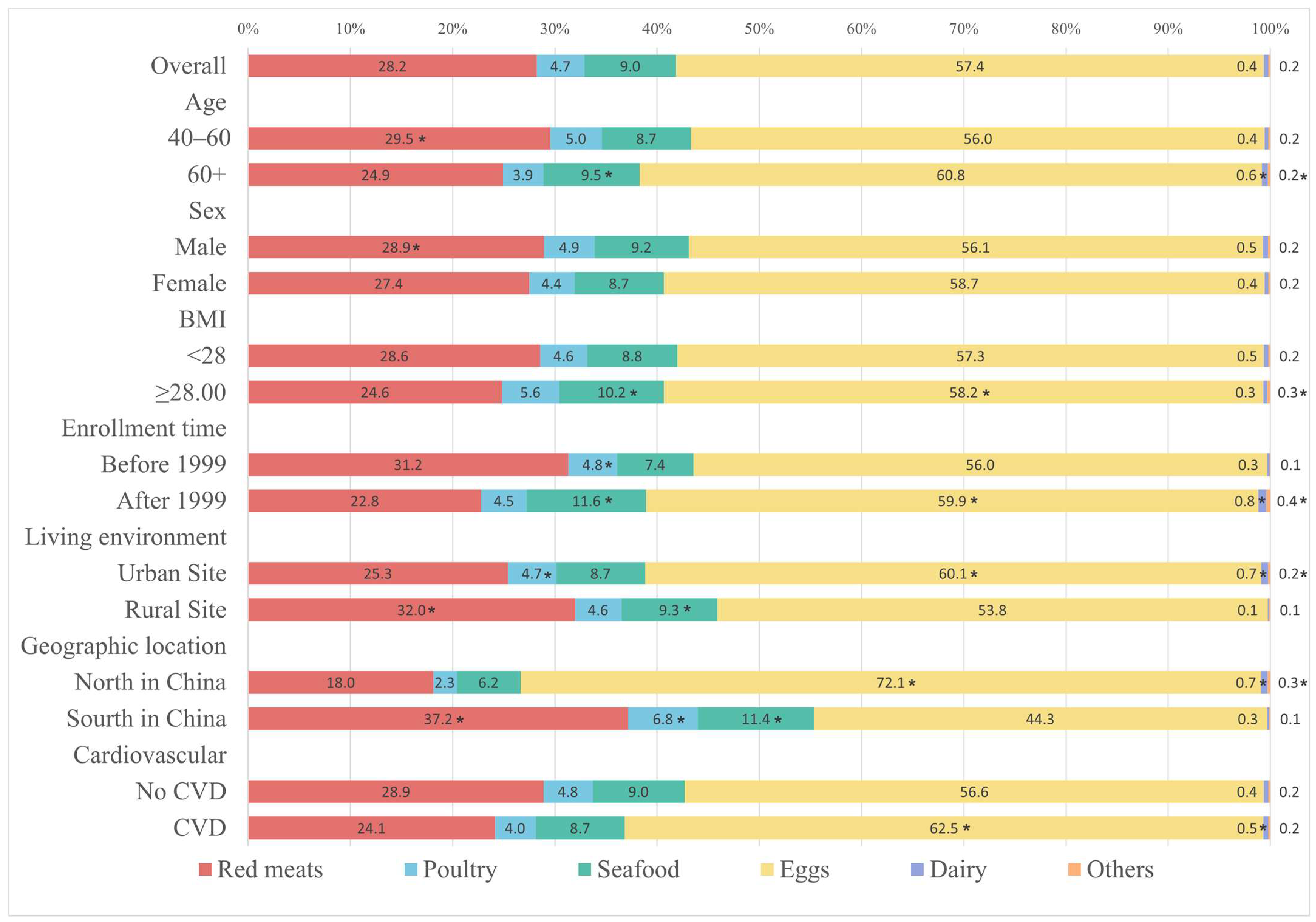
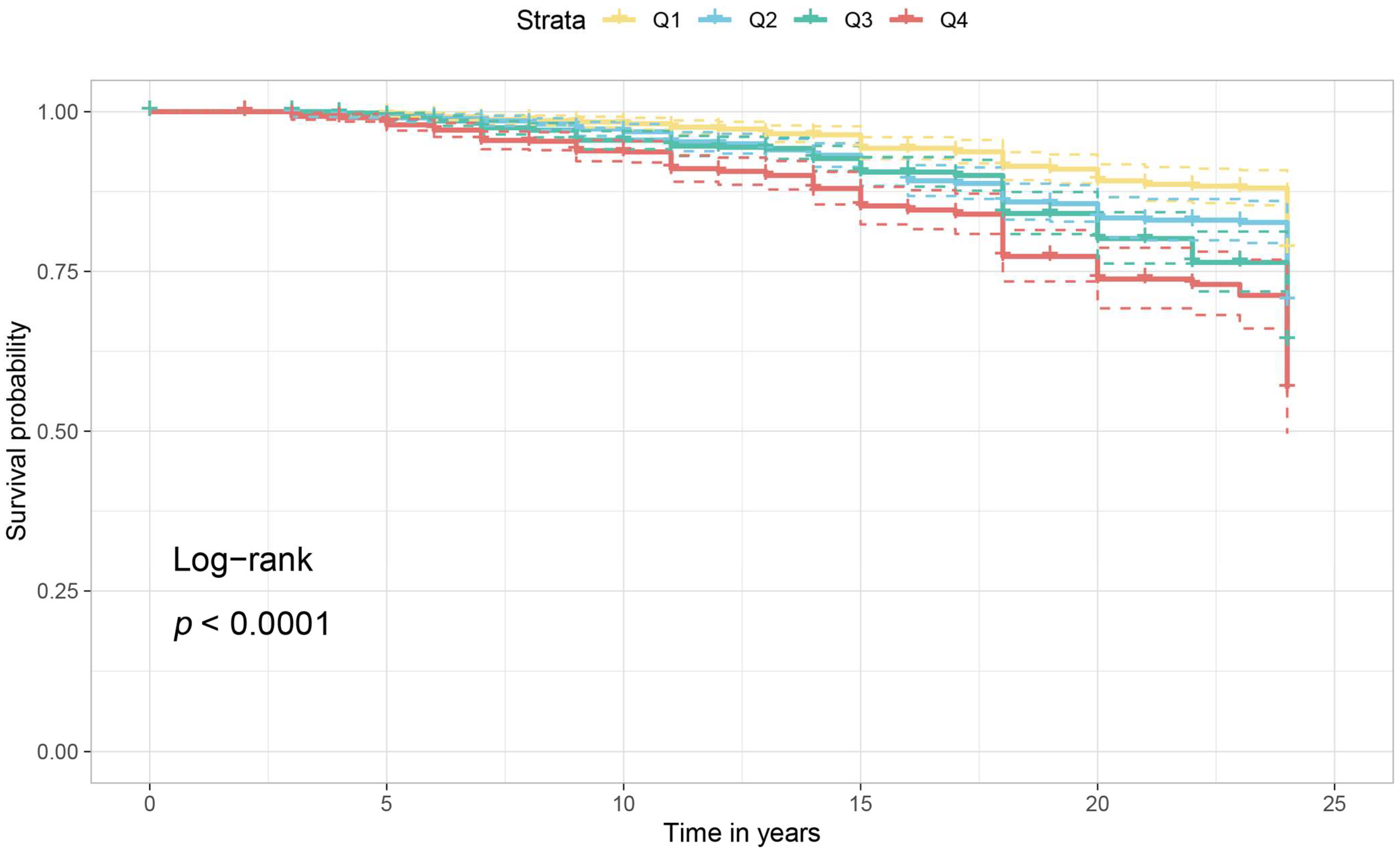
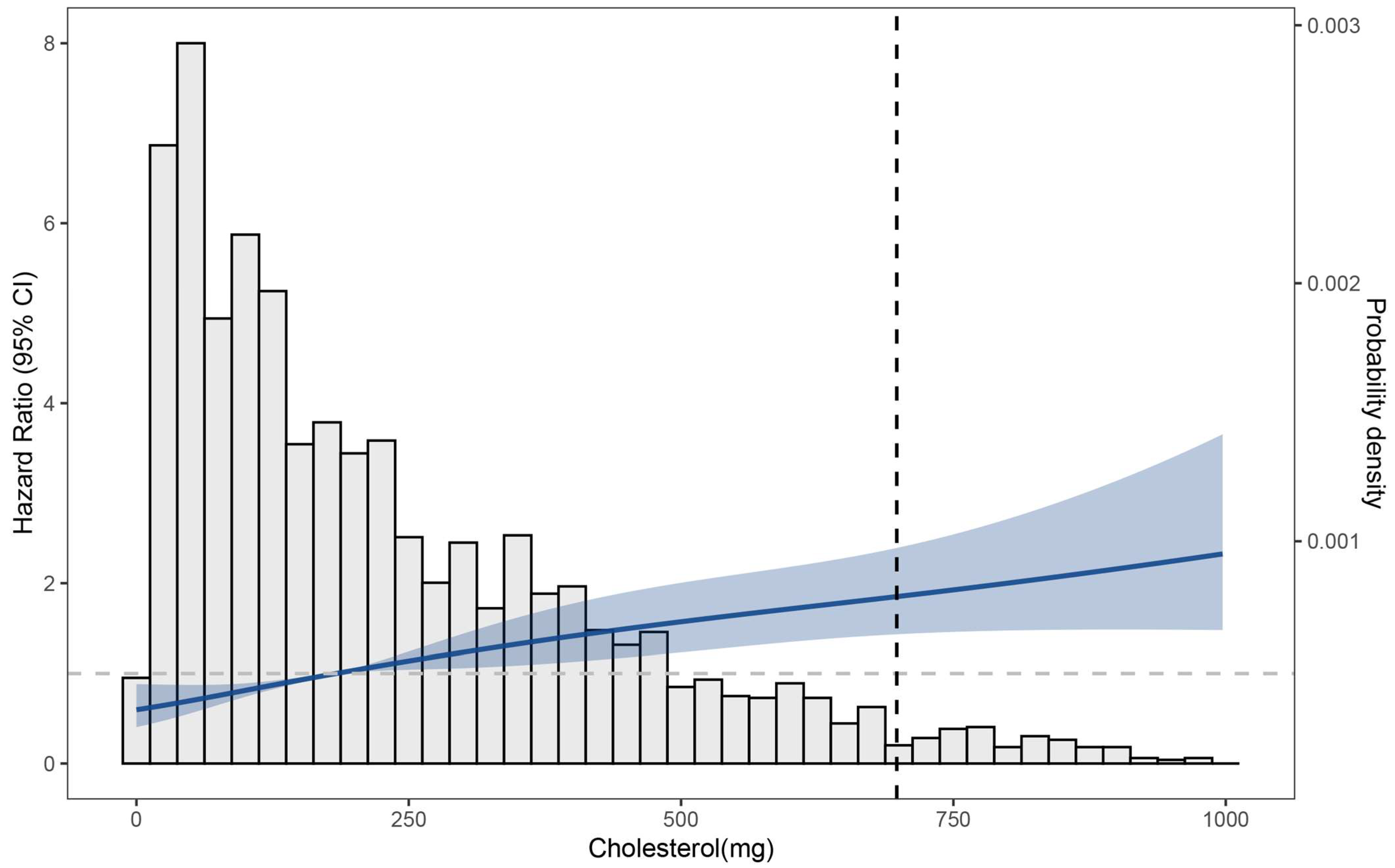
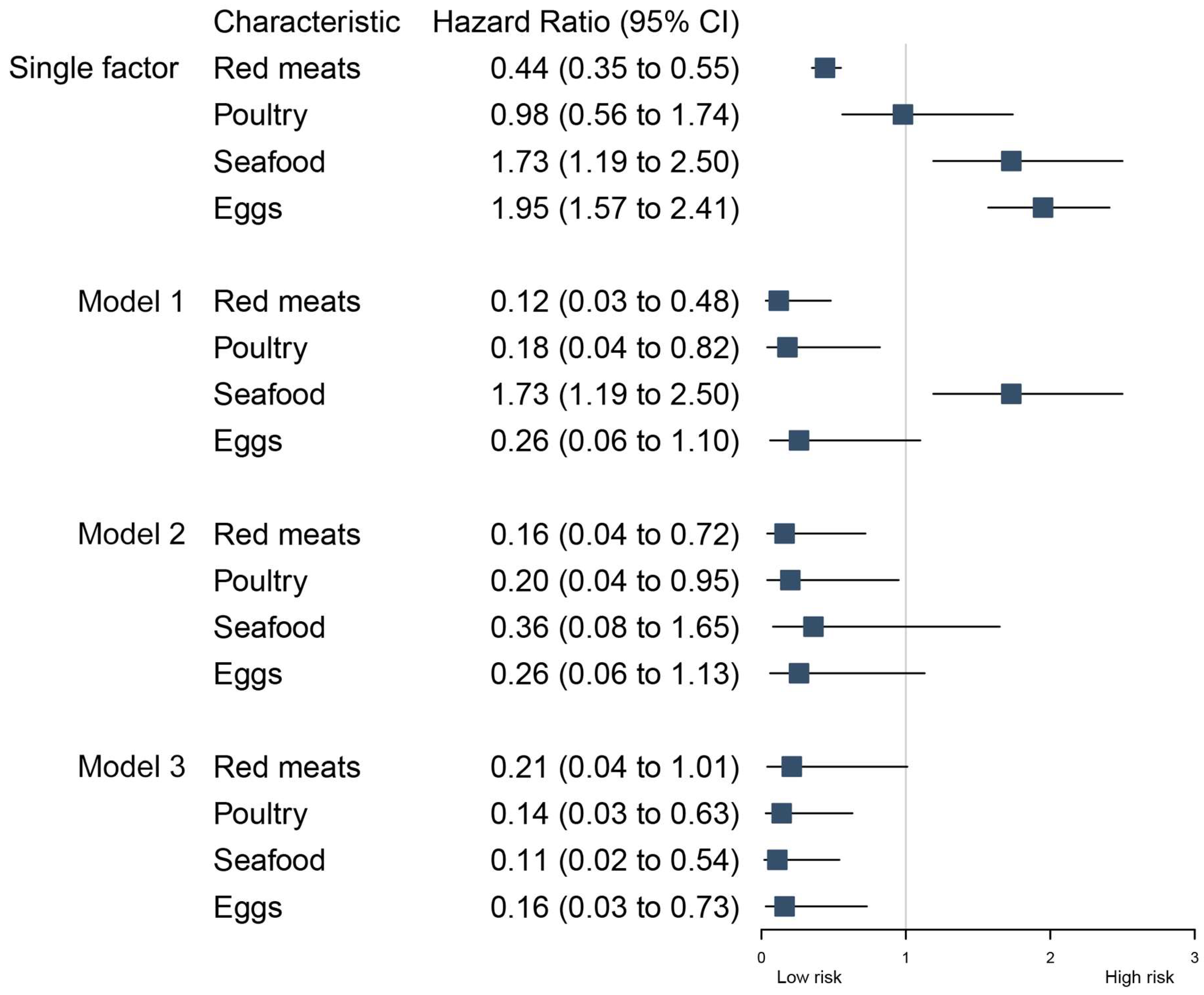
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dietschy, J.M.; Siperstein, M.D. Effect of Cholesterol Feeding and Fasting on Sterol Synthesis in Seventeen Tissues of the Rat. J. Lipid Res. 1967, 8, 97–104. [Google Scholar] [CrossRef]
- Wilson, J.D.; Lindsey, C.A.; Dietschy, J.M. Influence of Dietary Cholesterol on Cholesterol Metabolism. Ann. N. Y. Acad. Sci. 1968, 149, 808–821. [Google Scholar] [CrossRef]
- Espenshade, P.J.; Hughes, A.L. Regulation of Sterol Synthesis in Eukaryotes. Annu. Rev. Genet. 2007, 41, 401–427. [Google Scholar] [CrossRef]
- McNamara, D.J. Dietary Cholesterol and Atherosclerosis. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2000, 1529, 310–320. [Google Scholar] [CrossRef]
- Ministry Tweaks Eating Guidelines. Available online: http://en.nhc.gov.cn/2016-05/16/c_69773.htm (accessed on 16 January 2024).
- 2015–2020 Dietary Guidelines|Health.Gov. Available online: https://health.gov/our-work/nutrition-physical-activity/dietary-guidelines/previous-dietary-guidelines/2015 (accessed on 16 January 2024).
- Carson, J.A.S.; Lichtenstein, A.H.; Anderson, C.A.M.; Appel, L.J.; Kris-Etherton, P.M.; Meyer, K.A.; Petersen, K.; Polonsky, T.; Van Horn, L.; On behalf of the American Heart Association Nutrition Committee of the Council on Lifestyle and Cardiometabolic Health; et al. Dietary Cholesterol and Cardiovascular Risk: A Science Advisory from the American Heart Association. Circulation 2020, 141, e39–e53. [Google Scholar] [CrossRef] [PubMed]
- Zhong, V.W.; Van Horn, L.; Cornelis, M.C.; Wilkins, J.T.; Ning, H.; Carnethon, M.R.; Greenland, P.; Mentz, R.J.; Tucker, K.L.; Zhao, L.; et al. Associations of Dietary Cholesterol or Egg Consumption with Incident Cardiovascular Disease and Mortality. JAMA 2019, 321, 1081. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Gan, L.; Graubard, B.I.; Männistö, S.; Albanes, D.; Huang, J. Associations of Dietary Cholesterol, Serum Cholesterol, and Egg Consumption with Overall and Cause-Specific Mortality: Systematic Review and Updated Meta-Analysis. Circulation 2022, 145, 1506–1520. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; McClure, S.; Appel, L. Dietary Cholesterol Intake and Sources among U.S Adults: Results from National Health and Nutrition Examination Surveys (NHANES), 2001–2014. Nutrients 2018, 10, 771. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-C.; Chen, L.-H.; Mossavar-Rahmani, Y.; Kamensky, V.; Shadyab, A.H.; Haring, B.; Wild, R.A.; Silver, B.; Kuller, L.H.; Sun, Y.; et al. Dietary Cholesterol and Egg Intake in Relation to Incident Cardiovascular Disease and All-Cause and Cause-Specific Mortality in Postmenopausal Women. Am. J. Clin. Nutr. 2021, 113, 948–959. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Virtamo, J.; Wolk, A. Dietary Fats and Dietary Cholesterol and Risk of Stroke in Women. Atherosclerosis 2012, 221, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Sauvaget, C.; Nagano, J.; Hayashi, M.; Yamada, M. Animal Protein, Animal Fat, and Cholesterol Intakes and Risk of Cerebral Infarction Mortality in the Adult Health Study. Stroke 2004, 35, 1531–1537. [Google Scholar] [CrossRef]
- Zhuang, P.; Jiao, J.; Wu, F.; Mao, L.; Zhang, Y. Egg and Egg-Sourced Cholesterol Consumption in Relation to Mortality: Findings from Population-Based Nationwide Cohort. Clin. Nutr. 2020, 39, 3520–3527. [Google Scholar] [CrossRef]
- Zhang, H.-J.; Han, P.; Sun, S.-Y.; Wang, L.-Y.; Yan, B.; Zhang, J.-H.; Zhang, W.; Yang, S.-Y.; Li, X.-J. Attenuated Associations between Increasing BMI and Unfavorable Lipid Profiles in Chinese Buddhist Vegetarians. Asia Pac. J. Clin. Nutr. 2013, 22, 249–256. [Google Scholar] [CrossRef]
- Dinu, M.; Abbate, R.; Gensini, G.F.; Casini, A.; Sofi, F. Vegetarian, Vegan Diets and Multiple Health Outcomes: A Systematic Review with Meta-Analysis of Observational Studies. Crit. Rev. Food Sci. Nutr. 2017, 57, 3640–3649. [Google Scholar] [CrossRef]
- Huang, Q.; Jiang, H.; Zhang, B.; Wang, H.; Jia, X.; Huang, F.; Wang, L.; Wang, Z. Threshold-Effect Association of Dietary Cholesterol Intake with Dyslipidemia in Chinese Adults: Results from the China Health and Nutrition Survey in 2015. Nutrients 2019, 11, 2885. [Google Scholar] [CrossRef]
- Wu, F.; Zhuang, P.; Zhang, Y.; Zhan, C.; Zhang, Y.; Jiao, J. Egg and Dietary Cholesterol Consumption and Mortality Among Hypertensive Patients: Results from a Population-Based Nationwide Study. Front. Nutr. 2021, 8, 739533. [Google Scholar] [CrossRef] [PubMed]
- Micha, R.; Khatibzadeh, S.; Shi, P.; Fahimi, S.; Lim, S.; Andrews, K.G.; Engell, R.E.; Powles, J.; Ezzati, M.; Mozaffarian, D. Global, Regional, and National Consumption Levels of Dietary Fats and Oils in 1990 and 2010: A Systematic Analysis Including 266 Country-Specific Nutrition Surveys. BMJ 2014, 348, g2272. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Su, C.; Wang, Z.; Wang, H.; Jiang, H.; Zhang, B. Evaluation of Dietary Cholesterol Intake in Elderly Chinese: A Longitudinal Study from the China Health and Nutrition Survey. BMJ Open 2016, 6, e011074. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhai, F.Y.; Du, S.F.; Popkin, B.M. The China Health and Nutrition Survey, 1989–2011. Obes. Rev. 2014, 15, 2–7. [Google Scholar] [CrossRef]
- Popkin, B.M.; Du, S.; Zhai, F.; Zhang, B. Cohort Profile: The China Health and Nutrition Survey--Monitoring and Understanding Socio-Economic and Health Change in China, 1989-2011. Int. J. Epidemiol. 2010, 39, 1435–1440. [Google Scholar] [CrossRef]
- Pang, S.-J.; Jia, S.-S.; Man, Q.-Q.; Song, S.; Li, Y.-Q.; Song, P.-K.; Zhao, W.-H.; Zhang, J. Dietary Cholesterol in the Elderly Chinese Population: An Analysis of CNHS 2010–2012. Nutrients 2017, 9, 934. [Google Scholar] [CrossRef] [PubMed]
- Zhai, F.Y.; Du, S.F.; Wang, Z.H.; Zhang, J.G.; Du, W.W.; Popkin, B.M. Dynamics of the Chinese Diet and the Role of Urbanicity, 1991–2011. Obes. Rev. 2014, 15, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Zhai, F.; Guo, X.; Popkin, B.M.; Ma, L.; Wang, Q.; Shuigao, W.Y.; Ge, J.A.K. Evaluation of the 24-Hour Individual Recall Method in China. Food Nutr. Bull. 1996, 17, 1–7. [Google Scholar] [CrossRef]
- Ray, W.D.; Collett, D. Modelling Survival Data in Medical Research. J. R. Stat. Soc. Ser. A 1995, 158, 188. [Google Scholar] [CrossRef]
- Questionnaires—China Health and Nutrition Survey (CHNS). Available online: https://www.cpc.unc.edu/projects/china/data/questionnaires (accessed on 18 October 2023).
- Annesi, I.; Moreau, T.; Lellouch, J. Efficiency of the Logistic Regression and Cox Proportional Hazards Models in Longitudinal Studies. Stat. Med. 1989, 8, 1515–1521. [Google Scholar] [CrossRef]
- Zhou, B.; Coorperative Meta-Analysis Group of China Obesity Task Force. Predictive Values of Body Mass Index and Waist Circumference to Risk Factors of Related Diseases in Chinese Adult Population. Zhonghua Liu Xing Bing Xue Za Zhi 2002, 23, 5–10. [Google Scholar]
- García-Maldonado, E.; Zapatera, B.; Alcorta, A.; Vaquero, M.P. Metabolic and Nutritional Biomarkers in Adults Consuming Lacto-Ovo Vegetarian, Vegan and Omnivorous Diets in Spain. A Cross-Sectional Study. Food Funct. 2023, 14, 1608–1616. [Google Scholar] [CrossRef]
- Koch, C.A.; Kjeldsen, E.W.; Frikke-Schmidt, R. Vegetarian or Vegan Diets and Blood Lipids: A Meta-Analysis of Randomized Trials. Eur. Heart J. 2023, 44, 2609–2622. [Google Scholar] [CrossRef]
- Xu, J.; Eilat-Adar, S.; Loria, C.; Goldbourt, U.; Howard, B.V.; Fabsitz, R.R.; Zephier, E.M.; Mattil, C.; Lee, E.T. Dietary Fat Intake and Risk of Coronary Heart Disease: The Strong Heart Study. Am. J. Clin. Nutr. 2006, 84, 894–902. [Google Scholar] [CrossRef]
- He, K.; Merchant, A.; Rimm, E.B.; Rosner, B.A.; Stampfer, M.J.; Willett, W.C.; Ascherio, A. Dietary Fat Intake and Risk of Stroke in Male US Healthcare Professionals: 14 Year Prospective Cohort Study. BMJ 2003, 327, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Yaemsiri, S.; Sen, S.; Tinker, L.; Rosamond, W.; Wassertheil-Smoller, S.; He, K. Trans Fat, Aspirin, and Ischemic Stroke in Postmenopausal Women. Ann. Neurol. 2012, 72, 704–715. [Google Scholar] [CrossRef]
- Ascherio, A.; Rimm, E.B.; Giovannucci, E.L.; Spiegelman, D.; Stampfer, M.; Willett, W.C. Dietary Fat and Risk of Coronary Heart Disease in Men: Cohort Follow up Study in the United States. BMJ 1996, 313, 84–90. [Google Scholar] [CrossRef]
- Iso, H.; Stampfer, M.J.; Manson, J.E.; Rexrode, K.; Hu, F.B.; Hennekens, C.H.; Colditz, G.A.; Speizer, F.E.; Willett, W.C. Prospective Study of Fat and Protein Intake and Risk of Intraparenchymal Hemorrhage in Women. Circulation 2001, 103, 856–863. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Laukkanen, J.A.; Virtanen, J.K. Egg and Cholesterol Intake, apoE4 Phenotype and Risk of Venous Thromboembolism: Findings from a Prospective Cohort Study. Br. J. Nutr. 2023, 129, 292–300. [Google Scholar] [CrossRef]
- Abdollahi, A.M.; Virtanen, H.E.; Voutilainen, S.; Kurl, S.; Tuomainen, T.-P.; Salonen, J.T.; Virtanen, J.K. Egg Consumption, Cholesterol Intake, and Risk of Incident Stroke in Men: The Kuopio Ischaemic Heart Disease Risk Factor Study. Am. J. Clin. Nutr. 2019, 110, 169–176. [Google Scholar] [CrossRef]
- Wang, K.; Xiang, Q.; Hu, L.; Wang, L.; Zhang, Y. Frequency of Egg Intake Associated with Mortality in Chinese Adults: An 8-Year Nationwide Cohort Study. IJERPH 2022, 19, 14777. [Google Scholar] [CrossRef]
- Wang, K.; Wang, L.; Liu, L.; Zhou, P.; Mo, S.; Luo, S.; Zhang, Y.; Wang, K.; Yuan, Y.; Yin, Z.; et al. Longitudinal Association of Egg Intake Frequency with Cardiovascular Disease in Chinese Adults. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 908–917. [Google Scholar] [CrossRef]
- Qin, C.; Lv, J.; Guo, Y.; Bian, Z.; Si, J.; Yang, L.; Chen, Y.; Zhou, Y.; Zhang, H.; Liu, J.; et al. Associations of Egg Consumption with Cardiovascular Disease in a Cohort Study of 0.5 Million Chinese Adults. Heart 2018, 104, 1756–1763. [Google Scholar] [CrossRef] [PubMed]
- Kapourchali, F.R.; Surendiran, G.; Goulet, A.; Moghadasian, M.H. The Role of Dietary Cholesterol in Lipoprotein Metabolism and Related Metabolic Abnormalities: A Mini-Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 2408–2415. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.-Y.; Chang, C.C.Y.; Ohgami, N.; Yamauchi, Y. Cholesterol Sensing, Trafficking, and Esterification. Annu. Rev. Cell Dev. Biol. 2006, 22, 129–157. [Google Scholar] [CrossRef] [PubMed]
- Vincent, M.J.; Allen, B.; Palacios, O.M.; Haber, L.T.; Maki, K.C. Meta-Regression Analysis of the Effects of Dietary Cholesterol Intake on LDL and HDL Cholesterol. Am. J. Clin. Nutr. 2019, 109, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.J.H. Dietary Cholesterol and the Risk of Cardiovascular Disease in Patients: A Review of the Harvard Egg Study and Other Data. Int. J. Clin. Pract. 2009, 63, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Li, J.; Chang, C.; Gu, L.; Xiong, W.; Su, Y.; Yang, Y. The Association of Dietary Cholesterol from Egg Consumption on Cardiovascular Diseases Risk Varies from Person to Person. J. Agric. Food Chem. 2022, 70, 14977–14988. [Google Scholar] [CrossRef]
- Hoffman, J.R.; Falvo, M.J. Protein—Which Is Best? J. Sports Sci. Med. 2004, 3, 118–130. [Google Scholar] [PubMed]
- Cardiovascular Diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 13 February 2024).
- Joint WHO/FAO/UNU Expert Consultation. Protein and Amino Acid Requirements in Human Nutrition. World Health Organ. Tech. Rep. Ser. 2007, 935, 1–265, back cover. [Google Scholar]
- Bernstein, A.M.; Sun, Q.; Hu, F.B.; Stampfer, M.J.; Manson, J.E.; Willett, W.C. Major Dietary Protein Sources and Risk of Coronary Heart Disease in Women. Circulation 2010, 122, 876–883. [Google Scholar] [CrossRef]
- Rohrmann, S.; Overvad, K.; Bueno-de-Mesquita, H.B.; Jakobsen, M.U.; Egeberg, R.; Tjønneland, A.; Nailler, L.; Boutron-Ruault, M.-C.; Clavel-Chapelon, F.; Krogh, V.; et al. Meat Consumption and Mortality—Results from the European Prospective Investigation into Cancer and Nutrition. BMC Med. 2013, 11, 63. [Google Scholar] [CrossRef] [PubMed]
- Burr, M.; Gilbert, J.; Holliday, R.; Elwood, P.; Fehily, A.; Rogers, S.; Sweetnam, P.; Deadman, N. Effects of Changes in Fat, Fish, and Fibre Intakes on Death and Myocardial Reinfarction: Diet and Reinfarction Trial (DART). Lancet 1989, 334, 757–761. [Google Scholar] [CrossRef]
- Keast, D.R.; Fulgoni, V.L.; Nicklas, T.A.; O’Neil, C.E. Food Sources of Energy and Nutrients among Children in the United States: National Health and Nutrition Examination Survey 2003–2006. Nutrients 2013, 5, 283–301. [Google Scholar] [CrossRef]
- O’Neil, C.E.; Keast, D.R.; Fulgoni, V.L.; Nicklas, T.A. Food Sources of Energy and Nutrients among Adults in the US: NHANES 2003–2006. Nutrients 2012, 4, 2097–2120. [Google Scholar] [CrossRef]

| Characteristic | Q1 (0–79.0) (mg/d) | Q2 (79.0–183.3) (mg/d) | Q3 (183.3–362.5) (mg/d) | Q4 (362.5+) (mg/d) | p Value |
|---|---|---|---|---|---|
| N | 976 | 975 | 976 | 976 | |
| Age (years) | 53.00 (45.00–63.00) | 52.00 (45.00–62.00) | 52.00 (45.00–62.00) | 53.00 (46.00–62.00) | 0.273 |
| Calorie intake (kcal/d) | 2302.93 (1859.83–2768.64) | 2324.01 (1901.29–2811.87) | 2263.85 (1861.39–2677.74) | 2348.70 (1916.19–2760.74) | 0.05 |
| Poultry (mg/d) * | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–1.47) | <0.001 |
| Eggs (mg/d) * | 0.00 (0.00–0.00) | 0.00 (0.00–97.50) | 168.08 (48.75–226.67) | 390.00 (283.33–565.42) | <0.001 |
| Red meats (mg/d) * | 35.67 (17.83–54.94) | 76.67 (27.00–107.00) | 66.67 (23.00–123.05) | 76.67 (30.67–131.08) | <0.001 |
| Seafood(mg/d) * | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–32.00) | 0.00 (0.00–57.79) | <0.001 |
| Smoking status, % | 0.005 | ||||
| Never | 627 (64.24%) | 587 (60.21%) | 633 (64.86%) | 663 (67.93%) | |
| Smoke | 349 (35.76%) | 388 (39.79%) | 343 (35.14%) | 313 (32.07%) | |
| BMI, % | <0.001 | ||||
| <18.50 | 127 (13.01%) | 90 (9.23%) | 69 (7.07%) | 48 (4.92%) | |
| 18.50–23.99 | 645 (66.09%) | 597 (61.23%) | 554 (56.76%) | 506 (51.84%) | |
| 24.00–27.99 | 167 (17.11%) | 213 (21.85%) | 260 (26.64%) | 322 (32.99%) | |
| 28.00+ | 37 (3.79%) | 75 (7.69%) | 93 (9.53%) | 100 (10.25%) | |
| Sex, % | 0.008 | ||||
| Male | 421 (43.14%) | 478 (49.03%) | 485 (49.69%) | 484 (49.59%) | |
| Female | 555 (56.86%) | 497 (50.97%) | 491 (50.31%) | 492 (50.41%) | |
| Hypertension, % | <0.001 | ||||
| No risk of hypertension | 775 (79.41%) | 737 (75.59%) | 725 (74.28%) | 665 (68.14%) | |
| Risk of hypertension | 201 (20.59%) | 238 (24.41%) | 251 (25.72%) | 311 (31.86%) | |
| Residence, % | <0.001 | ||||
| Urban Site | 267 (27.36%) | 369 (37.85%) | 499 (51.13%) | 634 (64.96%) | |
| Rural Site | 709 (72.64%) | 606 (62.15%) | 477 (48.87%) | 342 (35.04%) | |
| Geographic location, % | <0.001 | ||||
| North China | 320 (32.79%) | 314 (32.21%) | 408 (41.80%) | 518 (53.07%) | |
| South China | 656 (67.21%) | 661 (67.79%) | 568 (58.20%) | 458 (46.93%) | |
| CVD, % | 0.039 | ||||
| No CVD | 874 (89.55%) | 842 (86.36%) | 851 (87.19%) | 833 (85.35%) | |
| CVD | 102 (10.45%) | 133 (13.64%) | 125 (12.81%) | 143 (14.65%) | |
| Enrollment time | <0.001 | ||||
| Before 1999 | 841 (86.17%) | 765 (78.46%) | 640 (65.57%) | 570 (58.40%) | |
| After 1999 | 135 (13.83%) | 210 (21.54%) | 336 (34.43%) | 406 (41.60%) | |
| Alcohol consumption | 0.15 | ||||
| Never | 621 (64.35%) | 589 (61.10%) | 585 (60.31%) | 578 (59.65%) | |
| Drink | 344 (35.65%) | 375 (38.90%) | 385 (39.69%) | 391 (40.35%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Y.; Yu, Y. Associations between Cholesterol Intake, Food Sources and Cardiovascular Disease in Chinese Residents. Nutrients 2024, 16, 716. https://doi.org/10.3390/nu16050716
Cao Y, Yu Y. Associations between Cholesterol Intake, Food Sources and Cardiovascular Disease in Chinese Residents. Nutrients. 2024; 16(5):716. https://doi.org/10.3390/nu16050716
Chicago/Turabian StyleCao, Yuxue, and Yan Yu. 2024. "Associations between Cholesterol Intake, Food Sources and Cardiovascular Disease in Chinese Residents" Nutrients 16, no. 5: 716. https://doi.org/10.3390/nu16050716
APA StyleCao, Y., & Yu, Y. (2024). Associations between Cholesterol Intake, Food Sources and Cardiovascular Disease in Chinese Residents. Nutrients, 16(5), 716. https://doi.org/10.3390/nu16050716





