Gut Microbiome and Metabolome Modulation by High-Hydrostatic-Pressure-Processed Tomato Juice
Abstract
1. Introduction
2. Materials and Methods
2.1. Tomato Juice Preparation
2.2. Animal Study
2.3. Blood Biochemical Assay
2.4. Gut Microbiota Analysis
2.5. Analysis of Short-Chain Fatty Acids (SCFAs) by GC
2.6. Metabolomics Analysis
2.7. Statistical Analysis
3. Results
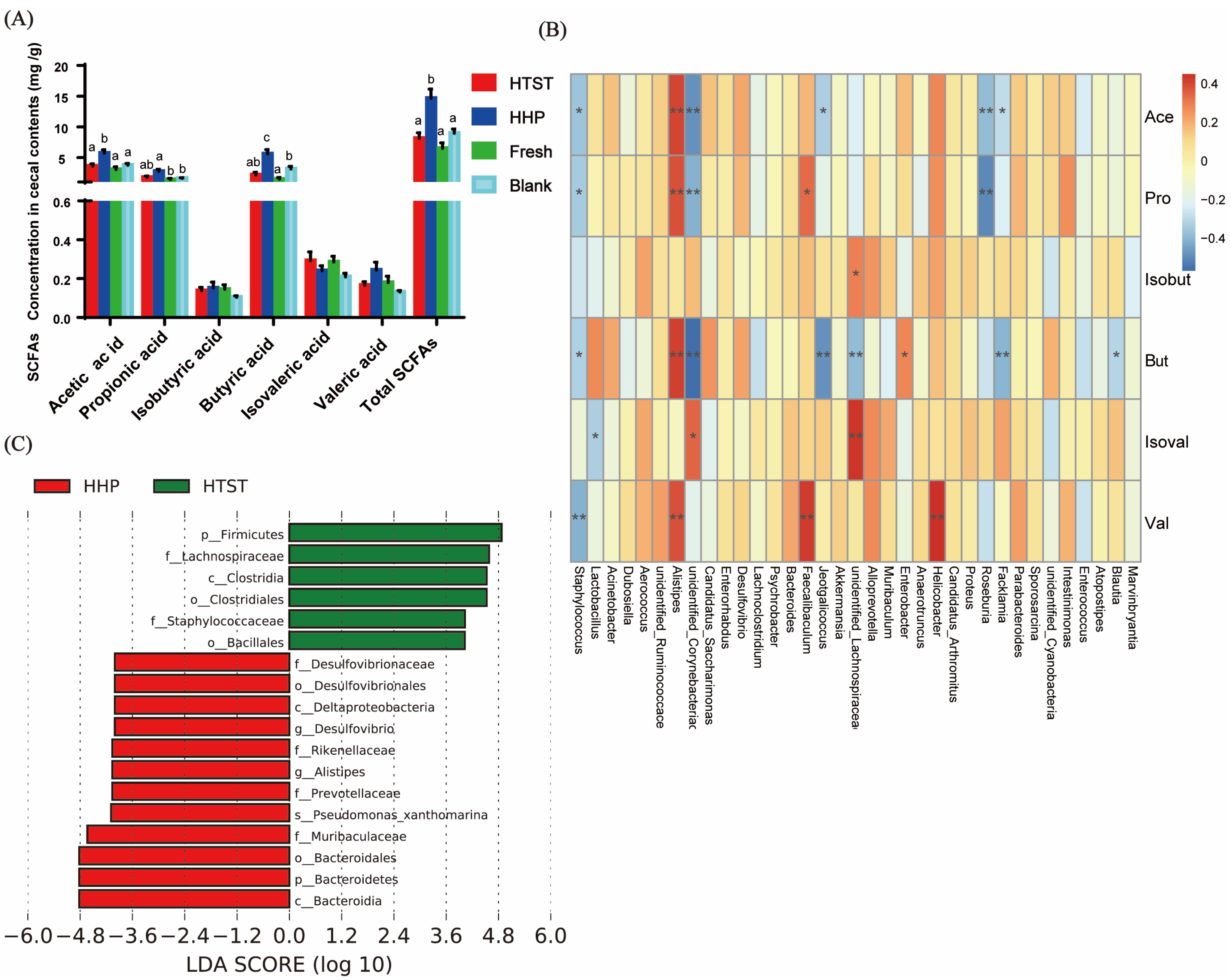
3.1. Effect of Tomato Juice on Biochemical Parameters
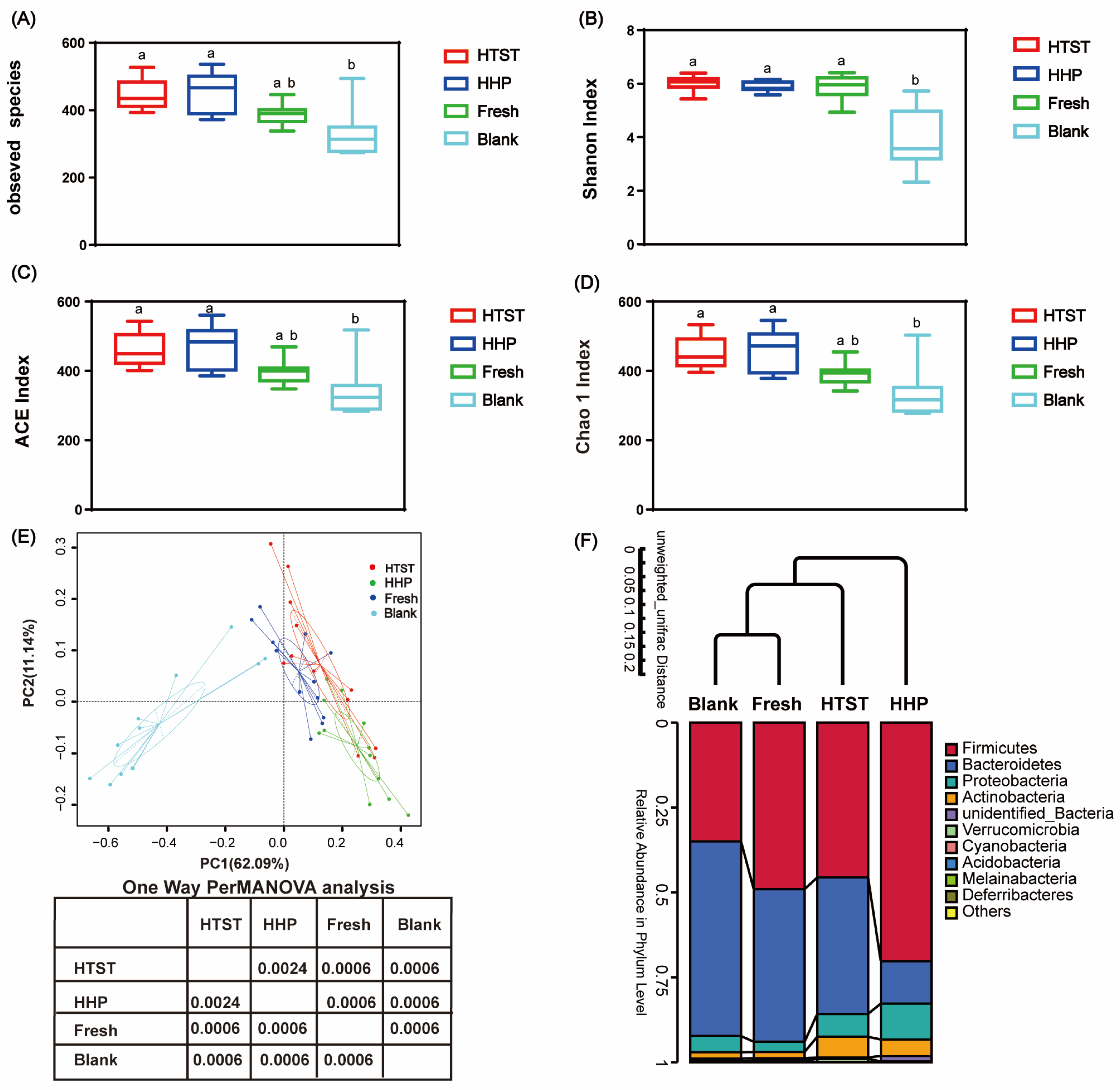
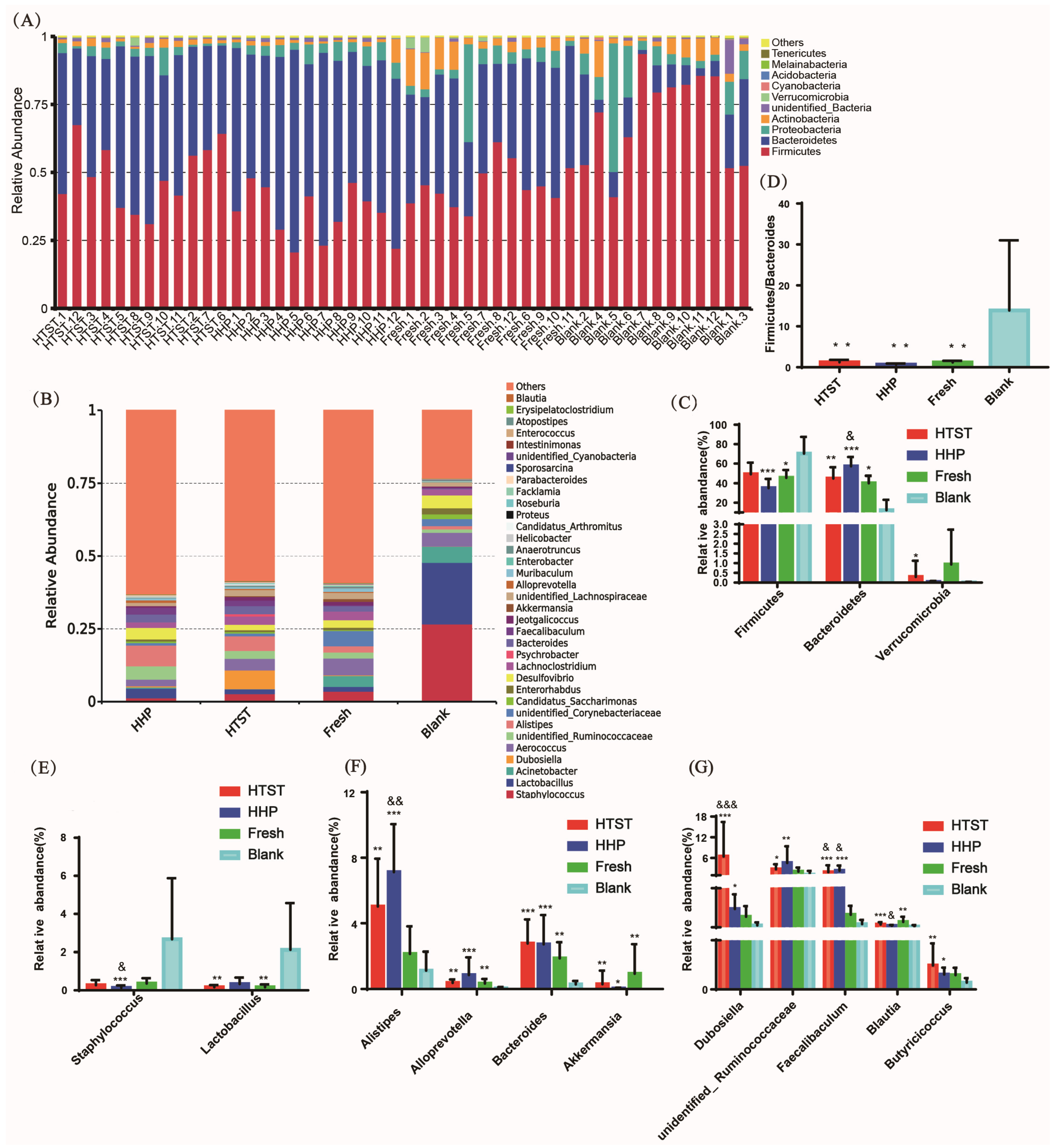
3.2. Effect of Tomato Juice on Gut Microbiota Composition
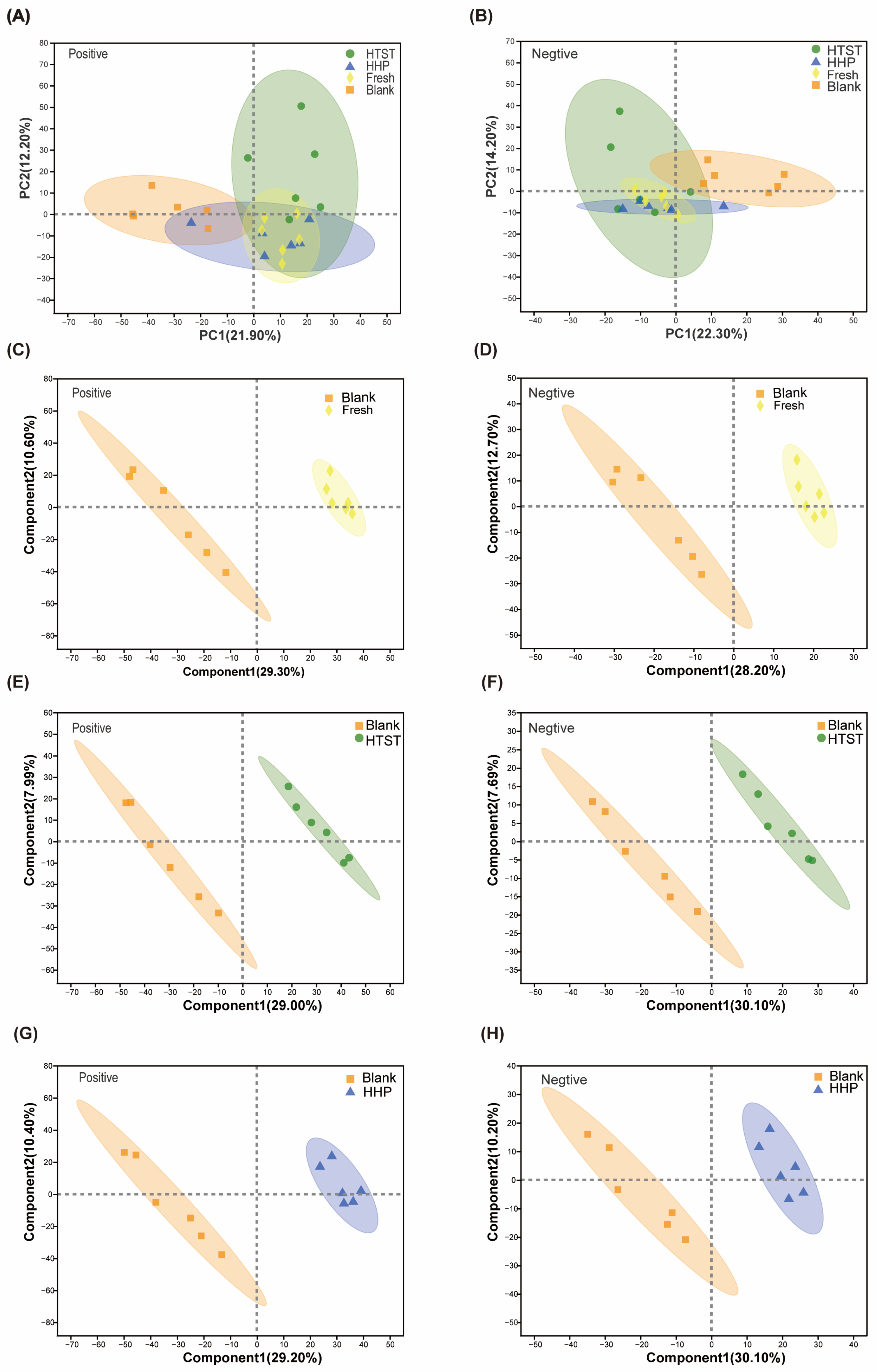
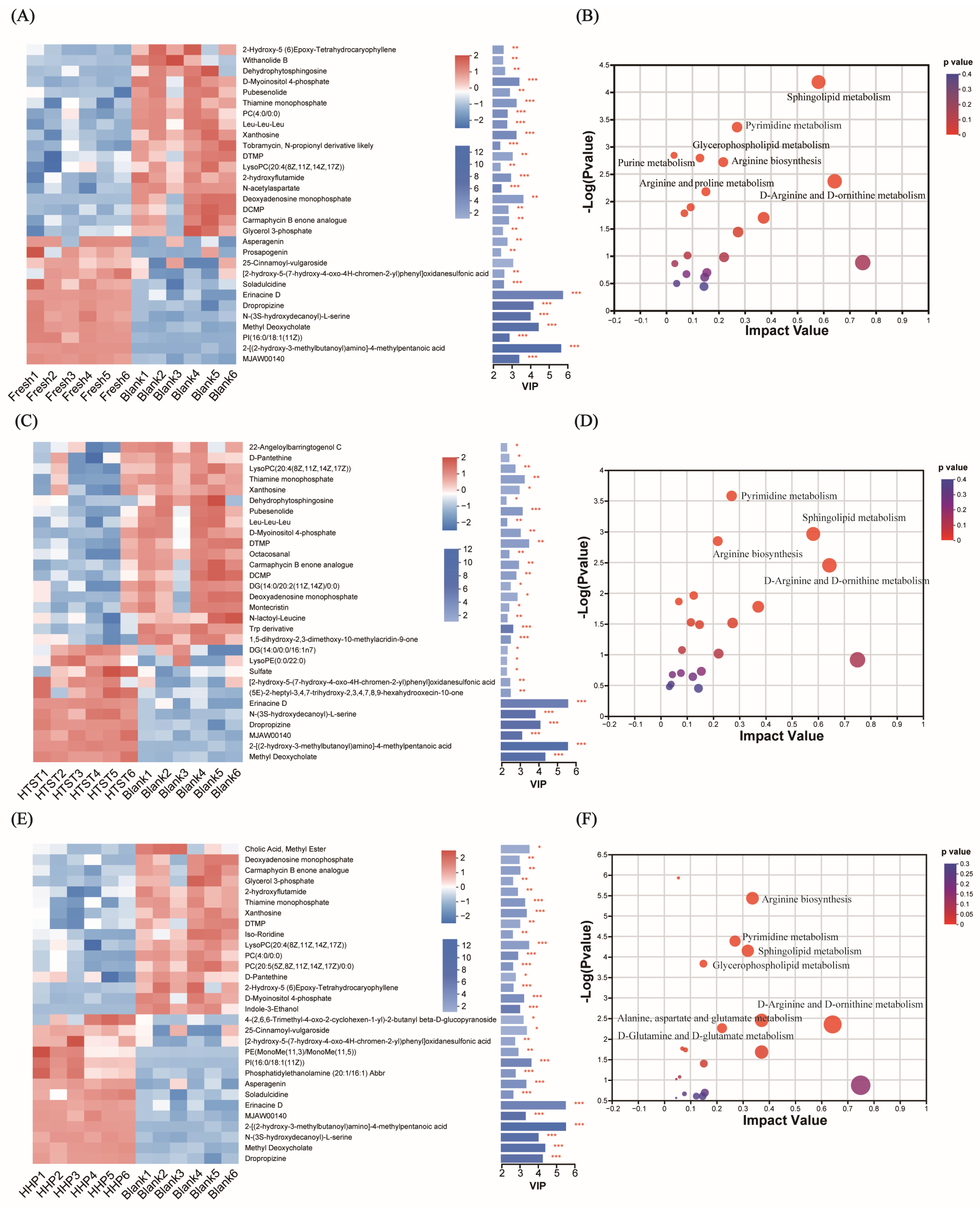
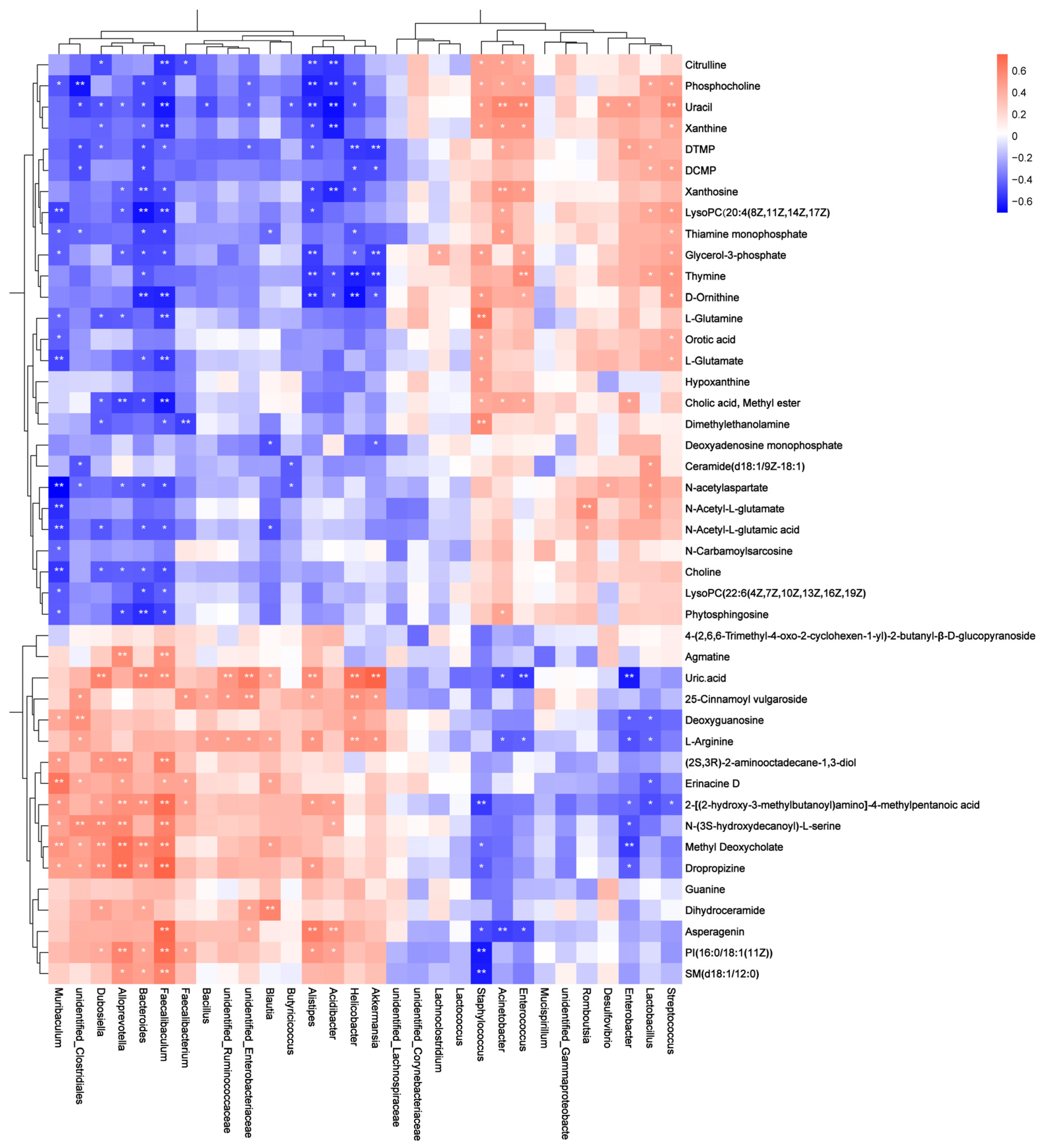
3.3. Effect of Tomato Juice on Metabolic Profiles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balasubramaniam, V.M.; Martinez-Monteagudo, S.I.; Gupta, R. Principles and application of high pressure-based technologies in the food industry. Annu. Rev. Food Sci. Technol. 2015, 6, 435–462. [Google Scholar] [CrossRef] [PubMed]
- Shahbaz, H.M.; Kim, J.U.; Kim, S.-H.; Park, J. Advances in Nonthermal Processing Technologies for Enhanced Microbiological Safety and Quality of Fresh Fruit and Juice Products. In Food Processing for Increased Quality and Consumption; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: New York, NY, USA, 2018; pp. 179–217. [Google Scholar]
- Wang, C.Y.; Wang, Y.T.; Wu, S.J.; Shyu, Y.T. Quality changes in high hydrostatic pressure and thermal pasteurized grapefruit juice during cold storage. J. Food Sci. Technol. 2018, 55, 5115–5122. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Du, B.L.; Cui, Z.W.; Xu, L.P.; Li, C.Y. Effects of high hydrostatic pressure and thermal processing on bioactive compounds, antioxidant activity, and volatile profile of mulberry juice. Food Sci. Technol. Int. 2017, 23, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Elizondo-Montemayor, L.; Hernandez-Brenes, C.; Ramos-Parra, P.A.; Moreno-Sanchez, D.; Nieblas, B.; Rosas-Perez, A.M.; Lamadrid-Zertuche, A.C. High hydrostatic pressure processing reduces the glycemic index of fresh mango puree in healthy subjects. Food Funct. 2015, 6, 1352–1360. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, P.; Wang, P.; Hu, X.; Chen, F. Targeting the gut microbiota by dietary nutrients: A new avenue for human health. Crit. Rev. Food Sci. Nutr. 2019, 59, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Carmody, R.N.; Gerber, G.K.; Luevano, J.M., Jr.; Gatti, D.M.; Somes, L.; Svenson, K.L.; Turnbaugh, P.J. Diet dominates host genotype in shaping the murine gut microbiota. Cell Host Microbe 2015, 17, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Zmora, N.; Suez, J.; Elinav, E. You are what you eat: Diet, health and the gut microbiota. Nat. Rev. Gastro. Hepat. 2019, 16, 35–56. [Google Scholar] [CrossRef]
- Shanmugam, H.; Ganguly, S.; Priya, B. Plant food bioactives and its effects on gut microbiota profile modulation for better brain health and functioning in Autism Spectrum Disorder individuals: A review. Food Front. 2022, 3, 124–141. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, D. Thermal processing of food reduces gut microbiota diversity of the host and triggers adaptation of the microbiota: Evidence from two vertebrates. Microbiome 2018, 6, 99. [Google Scholar] [CrossRef]
- Perez-Burillo, S.; Pastoriza, S.; Jimenez-Hernandez, N.; D’Auria, G.; Francino, M.P.; Rufian-Henares, J.A. Effect of food thermal processing on the composition of the gut microbiota. J. Agric. Food Chem. 2018, 66, 11500–11509. [Google Scholar] [CrossRef] [PubMed]
- FAO Tomato Production. Available online: https://www.fao.org/faostat/en/#data (accessed on 21 March 2023).
- Salehi, B.; Sharifi-Rad, R.; Sharopov, F.; Namiesnik, J.; Roointan, A.; Kamle, M.; Kumar, P.; Martins, N.; Sharifi-Rad, J. Beneficial effects and potential risks of tomato consumption for human health: An overview. Nutrition 2019, 62, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Liso, M.; De Santis, S.; Scarano, A.; Verna, G.; Dicarlo, M.; Galleggiante, V.; Campiglia, P.; Mastronardi, M.; Lippolis, A.; Vacca, M.; et al. A Bronze-Tomato Enriched Diet Affects the Intestinal Microbiome under Homeostatic and Inflammatory Conditions. Nutrients 2018, 10, 1862. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Alonso, F.J.; Gonzalez-Barrio, R.; Martin-Pozuelo, G.; Hidalgo, N.; Navarro-Gonzalez, I.; Masuero, D.; Soini, E.; Vrhovsek, U.; Periago, M.J. A study of the prebiotic-like effects of tomato juice consumption in rats with diet-induced non-alcoholic fatty liver disease (NAFLD). Food Funct. 2017, 8, 3542–3552. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, F.; Ma, L.; Liao, X.; Hu, X. Non-volatile and volatile metabolic profiling of tomato juice processed by high-hydrostatic-pressure and high-temperature short-time. Food Chem. 2021, 371, 131161. [Google Scholar] [CrossRef]
- Ruxton, C.H.S.; Myers, M. Fruit juices: Are they helpful or harmful? An evidence review. Nutrients 2021, 13, 1815. [Google Scholar] [CrossRef] [PubMed]
- He, W.S.; Li, L.; Rui, J.; Li, J.; Sun, Y.; Cui, D.; Xu, B. Tomato seed oil attenuates hyperlipidemia and modulates gut microbiota in C57BL/6J mice. Food Funct. 2020, 11, 4275–4290. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Krajmalnik-Brown, R.; Ilhan, Z.E.; Kang, D.W.; DiBaise, J.K. Effects of gut microbes on nutrient absorption and energy regulation. Nutr. Clin. Pract. 2012, 27, 201–214. [Google Scholar] [CrossRef]
- McKee, A.M.; Kirkup, B.M.; Madgwick, M.; Fowler, W.J.; Price, C.A.; Dreger, S.A.; Ansorge, R.; Makin, K.A.; Caim, S.; Le Gall, G.; et al. Antibiotic-induced disturbances of the gut microbiota result in accelerated breast tumor growth. iScience 2021, 24, 103012. [Google Scholar] [CrossRef]
- Downes, J.; Dewhirst, F.E.; Tanner, A.C.R.; Wade, W.G. Description of Alloprevotella rava gen. nov., sp. nov., isolated from the human oral cavity, and reclassification of Prevotella tannerae Moore et al. 1994 as Alloprevotella tannerae gen. nov., comb. nov. Int. J. Syst. Evol. Microbiol. 2013, 63, 1214–1218. [Google Scholar] [CrossRef] [PubMed]
- Eilam, O.; Zarecki, R.; Oberhardt, M.; Ursell, L.K.; Kupiec, M.; Knight, R.; Gophna, U.; Ruppin, E. Glycan degradation (GlyDeR) analysis predicts mammalian gut microbiota abundance and host diet-specific adaptations. mBio 2014, 5, e01526-14. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Mao, B.; Gu, J.; Wu, J.; Cui, S.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Blautia-a new functional genus with potential probiotic properties? Gut Microbes 2021, 13, 1875796. [Google Scholar] [CrossRef] [PubMed]
- Shang, Q.; Shan, X.; Cai, C.; Hao, J.; Li, G.; Yu, G. Dietary fucoidan modulates the gut microbiota in mice by increasing the abundance of Lactobacillus and Ruminococcaceae. Food Funct. 2016, 7, 3224–3232. [Google Scholar] [CrossRef] [PubMed]
- Eeckhaut, V.; Machiels, K.; Perrier, C.; Romero, C.; Maes, S.; Flahou, B.; Steppe, M.; Haesebrouck, F.; Sas, B.; Ducatelle, R.; et al. Butyricicoccus pullicaecorum in inflammatory bowel disease. Gut 2013, 62, 1745–1752. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, Q.; Cheng, L.; Buch, H.; Zhang, F. Akkermansia muciniphila is a promising probiotic. Microb. Biotechnol. 2019, 12, 1109–1125. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, C.M.; Chen, L.; Shaiber, A.; Eren, A.M.; Alegre, M.L. Gut microbes contribute to variation in solid organ transplant outcomes in mice. Microbiome 2018, 6, 96. [Google Scholar] [CrossRef] [PubMed]
- Tong, A.; Wu, W.; Chen, Z.; Wen, J.; Jia, R.; Liu, B.; Cao, H.; Zhao, C. Modulation of gut microbiota and lipid metabolism in rats fed high-fat diets by Ganoderma lucidum triterpenoids. Curr. Res. Food Sci. 2023, 6, 100427. [Google Scholar] [CrossRef]
- Liu, T.H.; Tao, W.C.; Liang, Q.E.; Tu, W.Q.; Xiao, Y.; Chen, L.G. Gut microbiota-related evidence provides new insights into the association between activating transcription factor 4 and development of salt-induced hypertension in mice. Front Cell Dev. Biol. 2020, 8, 585995. [Google Scholar] [CrossRef]
- Dziedzic, K.; Gorecka, D.; Szwengiel, A.; Michniewicz, J.; Drozdzynska, A.; Walkowiak, J. Interactions between fecal bacteria, bile acids and components of tomato pomace. Food Sci. Biotechnol. 2019, 28, 649–655. [Google Scholar] [CrossRef]
- Wu, T.; Gao, Y.; Hao, J.; Yin, J.; Li, W.; Geng, J.; Liu, R.; Sui, W.; Zhang, M. Lycopene, amaranth, and sorghum red pigments counteract obesity and modulate the gut microbiota in high-fat diet fed C57BL/6 mice. J. Funct. Foods 2019, 60, 103437. [Google Scholar] [CrossRef]
- Johnson, E.L.; Heaver, S.L.; Waters, J.L.; Kim, B.I.; Bretin, A.; Goodman, A.L.; Gewirtz, A.T.; Worgall, T.S.; Ley, R.E. Sphingolipids produced by gut bacteria enter host metabolic pathways impacting ceramide levels. Nat. Commun. 2020, 11, 2471. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.M.; Ke, X.; Hitchcock, D.; Jeanfavre, S.; Avila-Pacheco, J.; Nakata, T.; Arthur, T.D.; Fornelos, N.; Heim, C.; Franzosa, E.A.; et al. Bacteroides-derived sphingolipids are critical for maintaining intestinal homeostasis and symbiosis. Cell Host Microbe 2019, 25, 668–680. [Google Scholar] [CrossRef] [PubMed]
- Farooqui, A.A.; Horrocks, L.A.; Farooqui, T. Interactions between neural membrane glycerophospholipid and sphingolipid mediators: A recipe for neural cell survival or suicide. J. Neurosci. Res. 2007, 85, 1834–1850. [Google Scholar] [CrossRef] [PubMed]
- Neis, E.P.; Dejong, C.H.; Rensen, S.S. The role of microbial amino acid metabolism in host metabolism. Nutrients 2015, 7, 2930–2946. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, Y. Corynebacterium glutamicum mechanosensing: From osmoregulation to L-glutamate secretion for the avian microbiota-gut-brain axis. Microorganisms 2021, 9, 201. [Google Scholar] [CrossRef] [PubMed]
- Hibberd, M.C.; Wu, M.; Rodionov, D.A.; Li, X.; Cheng, J.; Griffin, N.W.; Barratt, M.J.; Giannone, R.J.; Osterman, O.A.; Hettich, R.L. The effects of micronutrient deficiencies on bacterial species from the human gut microbiota. Sci. Transl. Med. 2017, 9, eaal4069. [Google Scholar] [CrossRef] [PubMed]
- Gomez, V.P.B.S.; Martin-Pozuelo, G.; Santaella, M.; Periago, M.J. Effect of tomato juice intake on lycopene and short chain fatty acid content in faeces of Sprague Dawley rats. Ann. Vet. Anim. Sci. 2011, 27, 51–64. [Google Scholar]
- Elizondo-Montemayor, L.; Ramos-Parra, P.A.; Jacobo-Velázquez, D.A.; Treviño-Saldaña, N.; Marín-Obispo, L.M.; Ibarra-Garza, I.P.; Garcia-Amezquita, L.E.; Del Follo-Martínez, A.; Welti-Chanes, J.; Hernández-Brenes, C. High hydrostatic pressure stabilized micronutrients and shifted dietary fibers, from insoluble to soluble, producing a low-glycemic index mango pulp. CyTA J. Food 2020, 18, 203–215. [Google Scholar] [CrossRef]
- Holscher, H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef]
- Huang, W.; Fang, Q.; Fan, L.; Hong, T.; Tan, H.; Nie, S. Pectin with various degrees of esterification differentially alters gut microbiota and metabolome of healthy adults. eFood 2022, 3, e5. [Google Scholar] [CrossRef]
- Parker, B.J.; Wearsch, P.A.; Veloo, A.C.M.; Rodriguez-Palacios, A. The genus Alistipes: Gut bacteria with emerging implications to inflammation, cancer, and mental health. Front Immunol. 2020, 11, 906. [Google Scholar] [CrossRef] [PubMed]
- Salem, F.; Kindt, N.; Marchesi, J.R.; Netter, P.; Lopez, A.; Kokten, T.; Danese, S.; Jouzeau, J.Y.; Peyrin-Biroulet, L.; Moulin, D. Gut microbiome in chronic rheumatic and inflammatory bowel diseases: Similarities and differences. United Eur. Gastroent. 2019, 7, 1008–1032. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.; Naqvi, S.U.; Liu, Q.; Pan, H.; Ma, Z.; Kong, N.; Chen, Y.; Shi, D.; Kulyar, M.F.; Khan, J.A.; et al. Short chain fatty acids (SCFAs) are the potential immunomodulatory metabolites in controlling Staphylococcus aureus-mediated mastitis. Nutrients 2022, 14, 3687. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Li, D.; Ma, C.; Hu, X.; Chen, F. Gut Microbiome and Metabolome Modulation by High-Hydrostatic-Pressure-Processed Tomato Juice. Nutrients 2024, 16, 710. https://doi.org/10.3390/nu16050710
Wang X, Li D, Ma C, Hu X, Chen F. Gut Microbiome and Metabolome Modulation by High-Hydrostatic-Pressure-Processed Tomato Juice. Nutrients. 2024; 16(5):710. https://doi.org/10.3390/nu16050710
Chicago/Turabian StyleWang, Xuehua, Daotong Li, Chen Ma, Xiaosong Hu, and Fang Chen. 2024. "Gut Microbiome and Metabolome Modulation by High-Hydrostatic-Pressure-Processed Tomato Juice" Nutrients 16, no. 5: 710. https://doi.org/10.3390/nu16050710
APA StyleWang, X., Li, D., Ma, C., Hu, X., & Chen, F. (2024). Gut Microbiome and Metabolome Modulation by High-Hydrostatic-Pressure-Processed Tomato Juice. Nutrients, 16(5), 710. https://doi.org/10.3390/nu16050710





