Weight Categories, Trajectories, Eating Behavior, and Metabolic Consequences during Pregnancy and Postpartum in Women with GDM
Abstract
1. Introduction
2. Materials and Methods
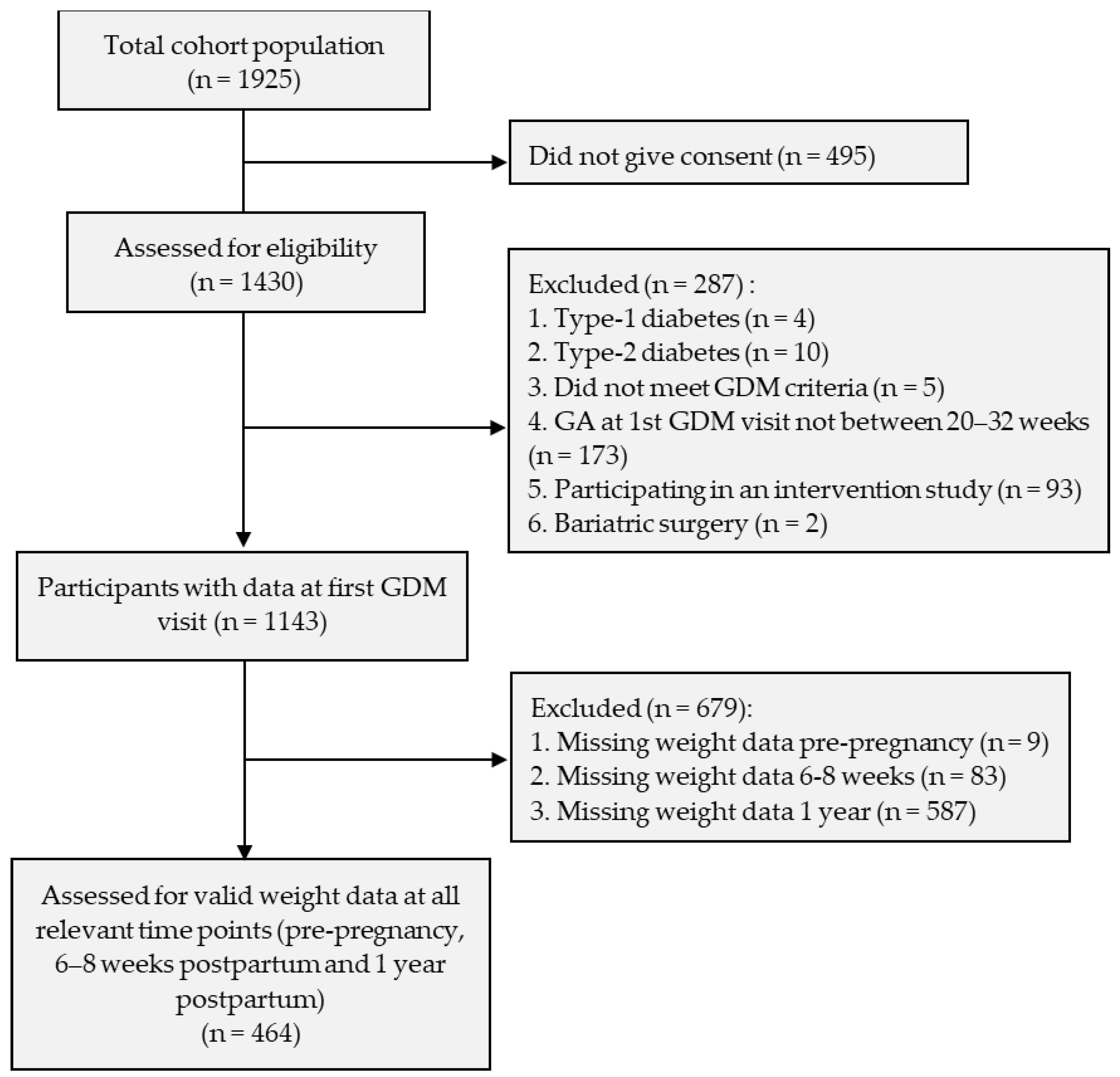
2.1. Participant Consent and Recruitment
2.2. Exclusion and Inclusion Criteria
2.3. GDM Diagnosis and Patient Follow-Up
2.4. Measures
2.4.1. Sociodemographic and Medical Characteristics
2.4.2. Intuitive Eating Behavior
2.4.3. Anthropometric Measures
2.4.4. Assessment of Glycemic Control Variables
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lobstein, T.; Brinsden, H.; Neveux, M.; Cavalcanti, B.O.; Barquera, S.; Baur, L.; Busch, V.; Buse, K.; Dietz, B.; French, A.; et al. World Obesity Atlas 2022. 2022. Available online: www.worldobesity.org#worldobesityatlas (accessed on 4 October 2023).
- Berg, C.; Forslund, H.B. The Influence of Portion Size and Timing of Meals on Weight Balance and Obesity. Curr. Obes. Rep. 2015, 4, 11–18. [Google Scholar] [CrossRef]
- LaCaille, L. Eating Behavior. In Encyclopedia of Behavioral Medicine; Gellman, M.D., Turner, J.R., Eds.; Springer: New York, NY, USA, 2013. [Google Scholar]
- Baz, B.; Riveline, J.-P.; Gautier, J.-F. Gestational diabetes mellitus: Definition, aetiological and clinical aspects. Eur. J. Endocrinol. 2016, 174, R43–R51. [Google Scholar] [CrossRef]
- Plows, J.F.; Stanley, J.L.; Baker, P.N.; Reynolds, C.M.; Vickers, M.H. The pathophysiology of gestational diabetes mellitus. Int. J. Mol. Sci. 2018, 19, 3342. [Google Scholar] [CrossRef]
- Voerman, E.; Santos, S.; Inskip, H.; Amiano, P.; Barros, H.; Charles, M.A.; Chatzi, L.; Chrousos, G.P.; Corpeleijn, E.; Crozier, S.; et al. Association of Gestational Weight Gain with Adverse Maternal and Infant Outcomes. JAMA 2019, 321, 1702–1715. [Google Scholar] [PubMed]
- Elsayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 15. Management of Diabetes in Pregnancy: Standards of Care in Diabetes—2023. Diabetes Care 2023, 46, S254–S266. [Google Scholar] [CrossRef] [PubMed]
- Hedderson, M.M.; Gunderson, E.P.; Ferrara, A. Gestational weight gain and risk of gestational diabetes mellitus. Obstet. Gynecol. 2010, 115, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Kominiarek, M.A.; Peaceman, A.M. Gestational weight gain. Am. J. Obstet. Gynecol. 2017, 217, 642–651. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Weight Gain during Pregnancy: Reexamining the Guidelines (2009); Committee to Reexamine IOM Pregnancy Weight Guidelines, Food and Nutrition Board, Board on Children Y and F, Institute of Medicine, National Research Council, Eds.; National Academies Press: Washington, DC, USA, 2009; Available online: http://www.nap.edu/catalog/12584 (accessed on 10 October 2023).
- Rong, K.; Yu, K.; Han, X.; Szeto, I.M.; Qin, X.; Wang, J.; Ning, Y.; Wang, P.; Ma, D. Pre-pregnancy BMI, gestational weight gain and postpartum weight retention: A meta-analysis of observational studies. Public Health Nutr. 2015, 18, 2172–2182. [Google Scholar] [CrossRef] [PubMed]
- Parrettini, S.; Caroli, A.; Torlone, E. Nutrition and Metabolic Adaptations in Physiological and Complicated Pregnancy: Focus on Obesity and Gestational Diabetes. Front. Endocrinol. 2020, 11, 611929. [Google Scholar] [CrossRef] [PubMed]
- Makama, M.; Skouteris, H.; Moran, L.J.; Lim, S. Reducing postpartum weight retention: A review of the implementation challenges of postpartum lifestyle interventions. J. Clin. Med. 2021, 10, 1891. [Google Scholar] [CrossRef]
- Monteiro, S.S.; Santos, T.S.; Fonseca, L.; Saraiva, M.; Pichel, F.; Pinto, C.; Pereira, M.T.; Vilaverde, J.; Almeida, M.C.; Dores, J. Inappropriate gestational weight gain impact on maternofetal outcomes in gestational diabetes. Ann. Med. 2022, 55, 207–214. [Google Scholar] [CrossRef]
- Minschart, C.; Myngheer, N.; Maes, T.; De Block, C.; Van Pottelbergh, I.; Abrams, P.; Vinck, W.; Leuridan, L.; Driessens, S.; Mathieu, C.; et al. Weight retention and glucose intolerance in early postpartum after gestational diabetes. Eur. J. Endocrinol. 2023, 188, 438–447. [Google Scholar] [CrossRef]
- Minschart, C.; Lammertyn, A.; Van Crombrugge, P.; Moyson, C.; Verhaeghe, J.; Vandeginste, S.; Verlaenen, H.; Vercammen, C.; Maes, T.; Dufraimont, E.; et al. Low Gestational Weight Gain in Women with Gestational Diabetes Is Safe with Better Metabolic Profile Postpartum. J. Clin. Endocrinol. Metab. 2023, 108, 665–679. [Google Scholar] [CrossRef]
- Quansah, D.Y.; Gross, J.; Gilbert, L.; Arhab, A.; Horsch, A.; Puder, J.J. Predictors and consequences of weight retention in the early and late postpartum period in women with gestational diabetes. Diabetes Res. Clin. Pr. 2020, 165, 108238. [Google Scholar] [CrossRef]
- Hamman, R.F.; Wing, R.R.; Edelstein, S.L.; Lachin, J.M.; Bray, G.A.; Delahanty, L.; Hoskin, M.; Kriska, A.M.; Mayer-Davis, E.J.; Pi-Sunyer, X.; et al. Effect of Weight Loss with Lifestyle Intervention on Risk of Diabetes. Diabetes Care 2006, 29, 2102–2107. [Google Scholar] [CrossRef] [PubMed]
- Newby, P.K. Are dietary intakes and eating behaviors related to childhood obesity? A comprehensive review of the evidence. J. Law Med. Ethic 2007, 35, 35–60. [Google Scholar] [CrossRef]
- Blundell, J.; Stubbs, R.; Golding, C.; Croden, F.; Alam, R.; Whybrow, S.; Lenoury, J.; Lawton, C. Resistance and susceptibility to weight gain: Individual variability in response to a high-fat diet. Physiol. Behav. 2005, 86, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Campos, M.B.; Menezes, I.H.C.F.; Peixoto, M.d.R.G.; Schincaglia, R.M. Intuitive eating in general aspects of eating behaviors in individuals with obesity: Randomized clinical trial. Clin. Nutr. ESPEN 2022, 50, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Tylka, T.L. Development and psychometric evaluation of a measure of intuitive eating. J. Couns. Psychol. 2006, 53, 226–240. [Google Scholar] [CrossRef]
- Tylka, T.L.; Ashley, M.K.V.D. Supplemental Material for The Intuitive Eating Scale–2: Item Refinement and Psychometric Evaluation with College Women and Men. J. Couns. Psychol. 2013, 60, 137–153. [Google Scholar] [CrossRef]
- Saunders, J.F.; Nichols-Lopez, K.A.; Frazier, L.D. Psychometric properties of the intuitive eating scale-2 (IES-2) in a culturally diverse Hispanic American sample. Eat. Behav. 2018, 28, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Schoenefeld, S.J.; Webb, J.B. Self-compassion and intuitive eating in college women: Examining the contributions of distress tolerance and body image acceptance and action. Eat Behav. 2013, 14, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Quansah, D.Y.; Gross, J.; Gilbert, L.; Helbling, C.; Horsch, A.; Puder, J.J. Intuitive eating is associated with weight and glucose control during pregnancy and in the early postpartum period in women with gestational diabetes mellitus (GDM): A clinical cohort study. Eat. Behav. 2019, 34, 101304. [Google Scholar] [CrossRef] [PubMed]
- Quansah, D.Y.; Gilbert, L.; Gross, J.; Horsch, A.; Puder, J.J. Intuitive eating is associated with improved health indicators at 1-year postpartum in women with gestational diabetes mellitus. J. Heal. Psychol. 2021, 26, 1168–1184. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.C.R.; Elliott, S.A.; Mccargar, L.J.; Bell, R.C.; Robson, P.J.; Prado, C.M.; The ENRICH Study Team. Associations of appetite sensations and metabolic characteristics with weight retention in postpartum women. Appl. Physiol. Nutr. Metab. 2020, 45, 875–885. Available online: www.nrcresearchpress.com (accessed on 4 October 2023). [CrossRef] [PubMed]
- Dakin, C.; Beaulieu, K.; Hopkins, M.; Gibbons, C.; Finlayson, G.; Stubbs, R.J. Do eating behavior traits predict energy intake and body mass index? A systematic review and meta-analysis. Obes. Rev. 2023, 24, e13515. [Google Scholar] [CrossRef]
- Leonard, S.A.; Rasmussen, K.M.; King, J.C.; Abrams, B. Trajectories of maternal weight from before pregnancy through postpartum and associations with childhood obesity. Am. J. Clin. Nutr. 2017, 106, 1295–1301. [Google Scholar] [CrossRef]
- Zhou, M.; Peng, X.; Yi, H.; Tang, S.; You, H. Determinants of excessive gestational weight gain: A systematic review and meta-analysis. Arch. Public Health 2022, 80, 129. [Google Scholar] [CrossRef]
- Gunderson, E.P.; Abrams, B.; Selvin, S. Does the pattern of postpartum weight change differ according to pregravid body size? Int. J. Obes. 2001, 25, 853–862. [Google Scholar] [CrossRef]
- Davis, E.M.; Stange, K.C.; Horwitz, R.I. Childbearing, stress and obesity disparities in women: A public health perspective. Matern. Child Health J. 2012, 16, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, L.A.; Klempel-Donchenko, M.; Redman, L.M. Pregnancy as a window to future health: Excessive gestational weight gain and obesity. Semin. Perinatol. 2015, 39, 296–303. [Google Scholar] [CrossRef]
- Bello, J.K.; Bauer, V.; Plunkett, B.A.; Poston, L.; Solomonides, A.; Endres, L. Pregnancy Weight Gain, Postpartum Weight Retention, and Obesity. Curr. Cardiovasc. Risk Rep. 2016, 10, 4. [Google Scholar] [CrossRef]
- Lund, J.; Clemmensen, C. Physiological protection against weight gain: Evidence from overfeeding studies and future directions. Philos. Trans. R. Soc. B Biol. Sci. 2023, 378, 20220229. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A.; Bouchard, C. The biology of human overfeeding: A systematic review. Obes. Rev. 2020, 21, e13040. [Google Scholar] [CrossRef] [PubMed]
- Ravussin, Y.; Edwin, E.; Gallop, M.; Xu, L.; Bartolomé, A.; Kraakman, M.J.; LeDuc, C.A.; Ferrante, A.W. Evidence for a Non-leptin System that Defends against Weight Gain in Overfeeding. Cell Metab. 2018, 28, 289–299.e5. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, J.; Evers, N.; Awazawa, M.; Nicholls, H.; Brönneke, H.; Dietrich, A.; Mauer, J.; Blüher, M.; Brüning, J. Obesogenic memory can confer long-term increases in adipose tissue but not liver inflammation and insulin resistance after weight loss. Mol. Metab. 2016, 5, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Metzger, B.E. International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Arditi, C.; Puder, J.; Vial, Y.; Hagon-Traub, I.; Burnand, B. Grossesse et diabète: Prise en charge multidisciplinaire du diabète: Recommandations pour la pratique clinique. Rev. Medicale Suisse 2018, 14, 2085. [Google Scholar]
- Lehmann, R.; Troendle, A.; Brändle, M. Neue erkenntnisse zur diagnostik und management des gestationsdiabetes: Empfehlungen der Schweizerischen Gesellschaft für Endokrinologie und Diabetologie (SGED). Ther. Umsch. 2009, 66, 695–706. [Google Scholar] [CrossRef]
- Camilleri, G.M.; Méjean, C.; Bellisle, F.; Andreeva, V.A.; Sautron, V.; Hercberg, S.; Péneau, S. Cross-cultural validity of the Intuitive Eating Scale-2. Psychometric evaluation in a sample of the general French population. Appetite 2015, 84, 6–14. [Google Scholar] [CrossRef]
- Quansah, D.Y.; Schenk, S.; Gilbert, L.; Arhab, A.; Gross, J.; Marques-Vidal, P.-M.; Rodriguez, E.G.; Hans, D.; Horsch, A.; Puder, J.J. Intuitive Eating Behavior, Diet Quality and Metabolic Health in the Postpartum in Women with Gestational Diabetes. Nutrients 2022, 14, 4272. [Google Scholar] [CrossRef]
- Zheng, Q.-X.; Wang, H.-W.; Jiang, X.-M.; Lin, Y.; Liu, G.-H.; Pan, M.; Ge, L.; Chen, X.-Q.; Wu, J.-L.; Zhang, X.-Y.; et al. Prepregnancy body mass index and gestational weight gain are associated with maternal and infant adverse outcomes in Chinese women with gestational diabetes. Sci Rep. 2022, 12, 2749. [Google Scholar] [CrossRef]
- Wong, T.; Barnes, R.A.; Ross, G.P.; Cheung, N.W.; Flack, J.R. Are the Institute of Medicine weight gain targets applicable in women with gestational diabetes mellitus? Diabetologia 2017, 60, 416–423. [Google Scholar] [CrossRef]
- Power, M.L.; Lott, M.L.; Mackeen, A.D.; DiBari, J.; Schulkin, J. A retrospective study of gestational weight gain in relation to the Institute of Medicine’s recommendations by maternal body mass index in rural Pennsylvania from 2006 to 2015. BMC Pregnancy Childbirth 2018, 18, 239. [Google Scholar] [CrossRef]
- Gallagher, K.; Ralph, J.; Petros, T.; Qualls, C.; Leeman, L.; Rogers, R.G. Postpartum Weight Retention in Primiparous Women and Weight Outcomes in Their Offspring. J. Midwifery Women’s Health 2019, 64, 427–434. [Google Scholar] [CrossRef]
- He, X.; Hu, C.; Chen, L.; Wang, Q.; Qin, F. The association between gestational weight gain and substantial weight retention 1-year postpartum. Arch. Gynecol. Obstet. 2014, 290, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Van Ha, A.V.; Zhao, Y.; Pham, N.M.; Nguyen, C.L.; Nguyen, P.T.H.; Chu, T.K.; Tang, H.K.; Binns, C.W.; Lee, A.H. Postpartum weight retention in relation to gestational weight gain and pre-pregnancy body mass index: A prospective cohort study in Vietnam. Obes. Res. Clin. Pract. 2019, 13, 143–149. [Google Scholar]
- Sha, T.; Cheng, G.; Li, C.; Gao, X.; Li, L.; Chen, C.; Yan, Y. Patterns of women’s postpartum weight retention and its associations with maternal obesity-related factors and parity. Int. J. Environ. Res. Public Health 2019, 16, 4510. [Google Scholar] [CrossRef] [PubMed]
- Soria-Contreras, D.C.; Rifas-Shiman, S.L.; Aris, I.M.; Perng, W.; Switkowski, K.M.; Téllez-Rojo, M.M.; Trejo-Valdivia, B.; López-Ridaura, R.; Oken, E. Weight Trajectories after Delivery are Associated with Adiposity and Cardiometabolic Markers at 3 Years Postpartum among Women in Project Viva. J. Nutr. 2020, 150, 1889–1898. [Google Scholar] [CrossRef]
- Abebe, D.S.; Von Soest, T.; Von Holle, A.; Zerwas, S.C.; Torgersen, L.; Bulik, C.M. Developmental Trajectories of Postpartum Weight 3 Years After Birth: Norwegian Mother and Child Cohort Study. Matern. Child Health J. 2015, 19, 917–925. [Google Scholar] [CrossRef][Green Version]
- Muñoz-Manrique, C.; Trejo-Valdivia, B.; Hernández-Cordero, S.; Cantoral, A.; Deierlein, A.L.; Colicino, E.; Niedzwiecki, M.M.; Wright, R.O.; Baccarelli, A.A.; Téllez-Rojo, M.M. Weight gain trajectories patterns from pregnancy to early postpartum: Identifying women at risk and timing to prevent weight regain. BMC Pregnancy Childbirth 2022, 22, 811. [Google Scholar] [CrossRef]
- Careau, V.; Halsey, L.G.; Pontzer, H.; Ainslie, P.N.; Andersen, L.F.; Anderson, L.J.; Arab, L.; Baddou, I.; Bedu-Addo, K.; Blaak, E.E.; et al. Energy compensation and adiposity in humans. Curr. Biol. 2021, 31, 4659–4666.e2. [Google Scholar] [CrossRef] [PubMed]
- Kullmann, S.; Valenta, V.; Wagner, R.; Tschritter, O.; Machann, J.; Häring, H.U.; Preissl, H.; Fritsche, A.; Heni, M. Brain insulin sensitivity is linked to adiposity and body fat distribution. Nat. Commun. 2020, 11, 1841. [Google Scholar] [CrossRef] [PubMed]
- Kullmann, S.; Heni, M.; Veit, R.; Scheffler, K.; Machann, J.; Haring, H.U.; Fritsche, A.; Preissl, H. Selective insulin resistance in homeostatic and cognitivecontrol brain areas in overweight and obese adults. Diabetes Care 2015, 38, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Paterson, H.; Treharne, G.J.; Horwath, C.; Haszard, J.J.; Herbison, P.; Hay-Smith, E.J.C. Intuitive eating and gestational weight gain. Eat. Behav. 2019, 34, 101311. [Google Scholar] [CrossRef] [PubMed]
- Kew, S.; Ye, C.; Hanley, A.J.; Connelly, P.W.; Sermer, M.; Zinman, B.; Retnakaran, R. Cardiometabolic implications of postpartum weight changes in the first year after delivery. Diabetes Care 2014, 37, 1998–2006. [Google Scholar] [CrossRef]
- Fan, Y.; Li, W.; Liu, H.; Wang, L.; Zhang, S.; Li, W.; Liu, H.; Leng, J.; Shen, Y.; Tuomilehto, J.; et al. Effects of obesity and a history of gestational diabetes on the risk of postpartum diabetes and hyperglycemia in Chinese women: Obesity, GDM and diabetes risk. Diabetes Res. Clin. Pract. 2019, 156, 107828. [Google Scholar] [CrossRef]
- Vounzoulaki, E.; Khunti, K.; Abner, S.C.; Tan, B.K.; Davies, M.J.; Gillies, C.L. Progression to type 2 diabetes in women with a known history of gestational diabetes: Systematic review and meta-analysis. BMJ 2020, 369, m1361. [Google Scholar] [CrossRef]

| Variables | Mean ± SD | n (%) |
|---|---|---|
| Age (years) Gestational age at 1st GDM visit (weeks) | 33.21 ± 5.12 28.71 ± 1.90 | |
| Gestational age at delivery (weeks) | 38.75 ± 1.90 | |
| Pre-pregnancy BMI (kg/m2) a | 26.26 ± 5.69 | |
| Educational level (n = 345) b | ||
| Lack of schooling | 5 (1.45) | |
| Compulsory school unachieved | 28 (8.12) | |
| Compulsory school achieved | 66 (19.13) | |
| High school | 33 (9.57) | |
| General and vocational education | 77 (22.32) | |
| University | 136 (39.42) | |
| Ethnicity | ||
| Switzerland | 130 (28.02) | |
| Europe + North America | 164 (35.34) | |
| Africa | 84 (18.10) | |
| Asia + Oceania | 66 (14.22) | |
| Latin America | 12 (2.59) | |
| Others | 8 (1.72) | |
| Social support c | ||
| No | 46 (9.91) | |
| Yes | 418 (90.09) | |
| Smoking during pregnancy (n = 446) d | ||
| No | 386 (86.55) | |
| Yes | 60 (13.45) | |
| Alcohol intake during pregnancy (n = 394) e | ||
| No | 367 (93.15) | |
| Yes | 27 (6.85) | |
| Previous history of GDM | ||
| No | 446 (96.12) | |
| Yes | 18 (3.88) | |
| Family history of diabetes (n = 441) f | ||
| First degree | 167 (37.87) | |
| Second degree | 100 (22.68) | |
| No | 174 (39.45) | |
| Gravida | ||
| 1 | 151 (32.54) | |
| 2 | 140 (30.17) | |
| ≥3 | 173 (37.28) | |
| Parity | ||
| 0 | 229 (49.35) | |
| 1 | 141 (30.39) | |
| ≥2 | 94 (20.26) | |
| Breastfeeding at hospital discharge (n = 439) g | ||
| No | 22 (5.01) | |
| Yes | 417 (94.99) | |
| Breastfeeding at 6-8 weeks postpartum (n = 437) h | ||
| No | 83 (18.99) | |
| Yes | 354 (81.01) | |
| Breastfeeding at 1-year postpartum (n = 401) i | ||
| No | 276 (68.83) | |
| Yes | 125 (31.17) |
| Variables | Total (n = 464) | (A) WNW (n = 230) | (B) WOW (n = 138) | (C) WOB (n = 96) | Overall p-Value | p-Value B vs. A | p-Value C vs. A | p-Value B vs. C |
|---|---|---|---|---|---|---|---|---|
| Pre-pregnancy BMI | 26.26 ± 5.69 | 22.02 ± 1.99 | 27.07 ± 1.31 | 35.27 ± 4.40 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| GWG | 12.39 ± 6.39 | 13.58 ± 5.64 | 13.15 ± 5.63 | 8.29 ± 7.58 | <0.0001 | 1.000 | <0.0001 | <0.0001 |
| EGWG (yes: n, %) | 160 (34.48) | 55 (23.91) | 71 (51.45) | 34 (35.42) | <0.0001 | N/A | N/A | N/A |
| ∆ Weight between end of pregnancy and 6–8 weeks postpartum | 8.17 ± 3.13 | 7.99 ± 2.84 | 8.50 ± 3.21 | 8.10 ± 3.69 | 0.354 | 0.463 | 1.000 | 1.000 |
| PPWR at 6–8 weeks postpartum | 4.16 ± 6.04 | 5.57 ± 5.37 | 4.54 ± 5.22 | 0.25 ± 6.96 | <0.0001 | 0.288 | <0.0001 | <0.0001 |
| PPWR at 1-year postpartum | 3.54 ± 6.46 | 3.26 ± 5.03 | 3.88 ± 7.06 | 3.76 ± 8.38 | 0.629 | 1.000 | 1.000 | 1.000 |
| Participants | Weight Changes between 6–8 Weeks and 1-Year Postpartum | p-Values for Differences between Groups | ||
|---|---|---|---|---|
| Presence of PPWR at 1-Year Postpartum | Absence of PPWR at 1-Year Postpartum | Differences between Groups * | ||
| Total, kg (n = 464) | 0.76 ± 5.78 | −4.49 ± 5.79 | −5.25 ± 0.61 | <0.0001 |
| WNW, kg (n = 230) | −1.43 ± 4.68 | −4.85 ± 4.69 | −3.41 ± 0.71 | <0.0001 |
| WOW, kg (n = 138) | 0.93 ± 4.50 | −5.37 ± 5.95 | −6.30 ± 0.96 | <0.0001 |
| WOB, kg (n = 96) | 6.04 ± 6.55 | −2.64 ± 7.33 | −8.67 ± 1.52 | <0.0001 |
| Variables | Total (n = 245) | (A) WNW (n = 134) | (B) WOW (n = 64) | (C) WOB (n = 47) | Overall p-Values | p-Value B vs. A | p-Value C vs. A | p-Value B vs. C |
|---|---|---|---|---|---|---|---|---|
| IE in pregnancy EPR score | 3.85 ± 0.86 | 3.98 ± 0.84 | 3.77 ± 0.88 | 3.60 ± 0.84 | 0.024 | 0.332 | 0.028 | 0.897 |
| IE in pregnancy RHSC score | 3.51 ± 0.85 | 3.60 ± 0.83 | 3.41 ± 0.87 | 3.40 ± 0.87 | 0.207 | 0.414 | 0.517 | 1.000 |
| IE at 1-year pp EPR score | 3.73 ± 0.95 | 3.92 ± 0.85 | 3.62 ± 1.04 | 3.29 ± 0.95 | 0.004 | 0.144 | 0.005 | 0.355 |
| IE at 1-year pp RHSC score | 3.47 ± 0.97 | 3.64 ± 0.96 | 3.27 ± 0.95 | 3.28 ± 0.91 | 0.028 | 0.047 | 0.220 | 1.000 |
| Model 1 | Model 2 | |||||
|---|---|---|---|---|---|---|
| Variables | OR | 95% CI | p-Value | OR | 95% CI | p-Value |
| GWG | 0.98 | 0.95–1.01 | 0.192 | 0.99 | 0.97–1.03 | 0.952 |
| PPWR at 1-year postpartum | 2.32 | 1.46–3.68 | <0.001 | 2.47 | 1.54–3.95 | <0.001 |
| Weight gain between 6-8 weeks and 1-year postpartum | 1.74 | 1.19–2.54 | 0.004 | 1.35 | 0.90–2.03 | 0.152 |
| PPWR at 1-year and weight gain between 6–8 weeks to 1-year postpartum VS all others | 2.07 | 1.41–3.06 | <0.001 | 1.71 | 1.14–2.57 | 0.009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schenk, S.; Ravussin, Y.; Lacroix, A.; Quansah, D.Y.; Puder, J.J. Weight Categories, Trajectories, Eating Behavior, and Metabolic Consequences during Pregnancy and Postpartum in Women with GDM. Nutrients 2024, 16, 560. https://doi.org/10.3390/nu16040560
Schenk S, Ravussin Y, Lacroix A, Quansah DY, Puder JJ. Weight Categories, Trajectories, Eating Behavior, and Metabolic Consequences during Pregnancy and Postpartum in Women with GDM. Nutrients. 2024; 16(4):560. https://doi.org/10.3390/nu16040560
Chicago/Turabian StyleSchenk, Sybille, Yann Ravussin, Alain Lacroix, Dan Yedu Quansah, and Jardena J. Puder. 2024. "Weight Categories, Trajectories, Eating Behavior, and Metabolic Consequences during Pregnancy and Postpartum in Women with GDM" Nutrients 16, no. 4: 560. https://doi.org/10.3390/nu16040560
APA StyleSchenk, S., Ravussin, Y., Lacroix, A., Quansah, D. Y., & Puder, J. J. (2024). Weight Categories, Trajectories, Eating Behavior, and Metabolic Consequences during Pregnancy and Postpartum in Women with GDM. Nutrients, 16(4), 560. https://doi.org/10.3390/nu16040560






