Anti-Glycation Properties of Zinc-Enriched Arthrospira platensis (Spirulina) Contribute to Prevention of Metaflammation in a Diet-Induced Obese Mouse Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Zinc-Enriched Spirulina (Zn-SP)
2.2. Animal Model
2.3. Oral Glucose Tolerance Test
2.4. Blood Insulin
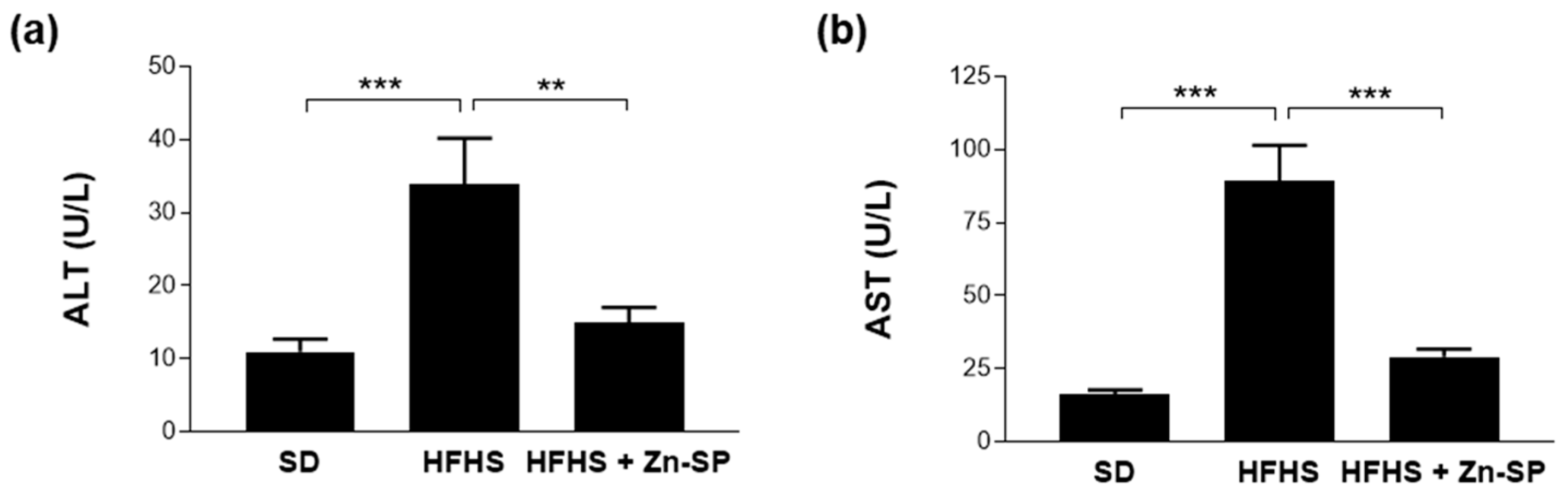
2.5. Hepatic Transaminases
2.6. Cytokines Immunoassay
2.7. Liver Tissue Extraction
2.8. Advanced Glycation End Product (AGE) Quantification
2.9. Western Blotting Analysis
2.10. Glyoxalase-1 (Glo-1) Activity Assay
2.11. Glutathione Assay
2.12. Microbiota Composition Analysis
2.13. Statistical Analysis
2.14. Chemical Reagents
3. Results
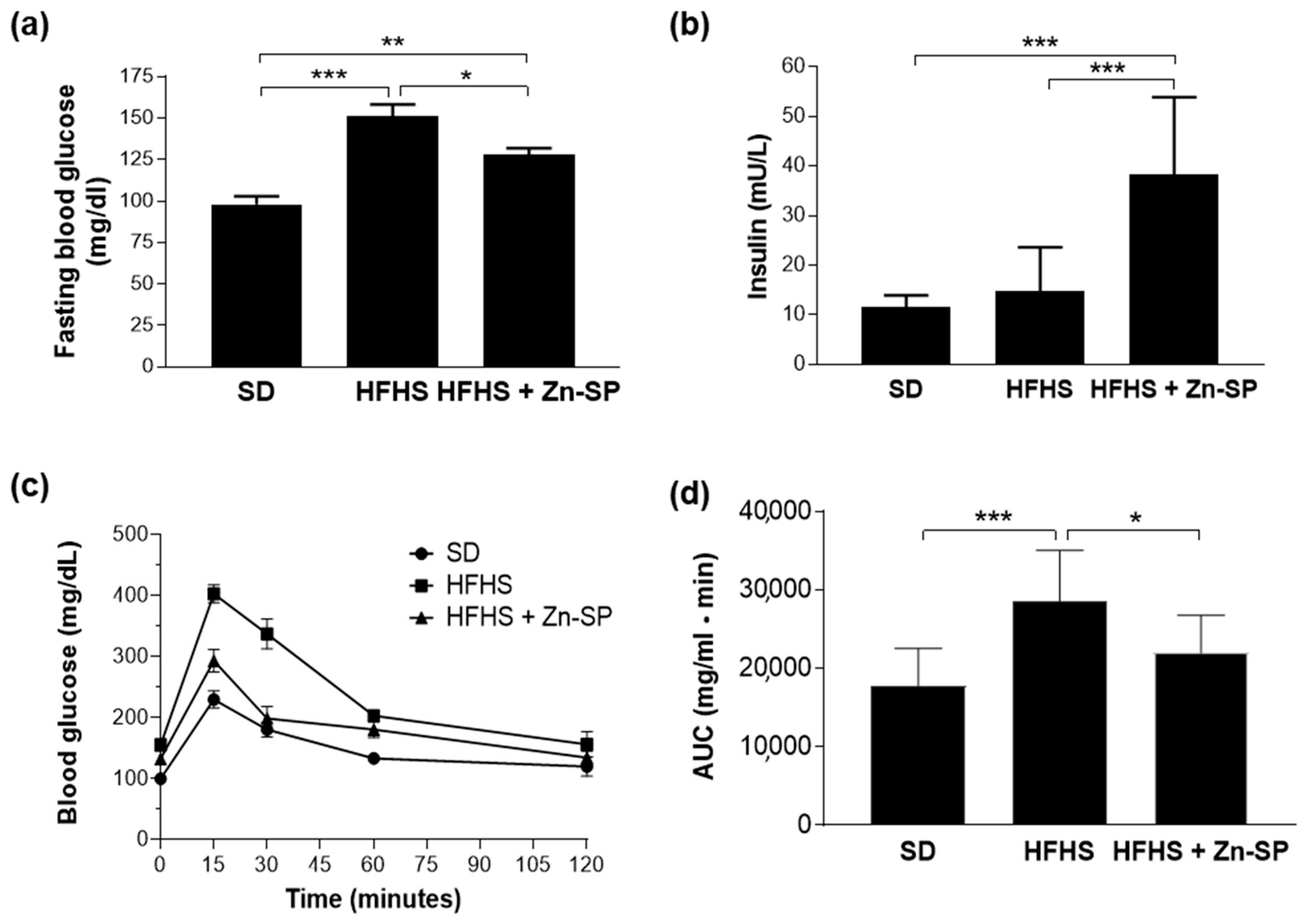
3.1. Effects of Dietary Manipulation and Zn-SP Supplementation on Systemic Metabolic Parameters
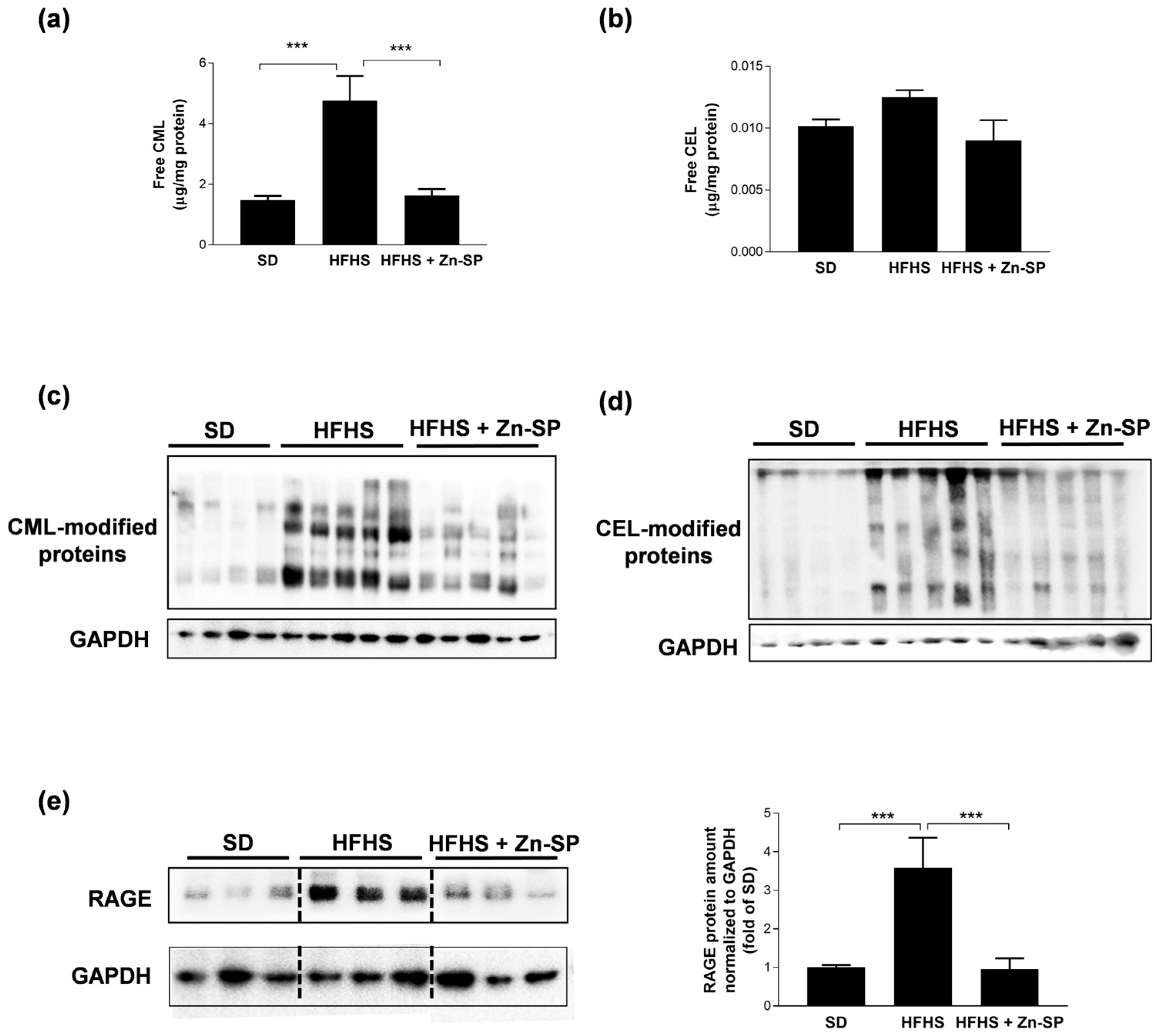
3.2. Zn-SP Prevents AGE Accumulation in Liver
3.3. Zn-SP Enhances Antioxidant and Anti-Glycation Defenses in Liver
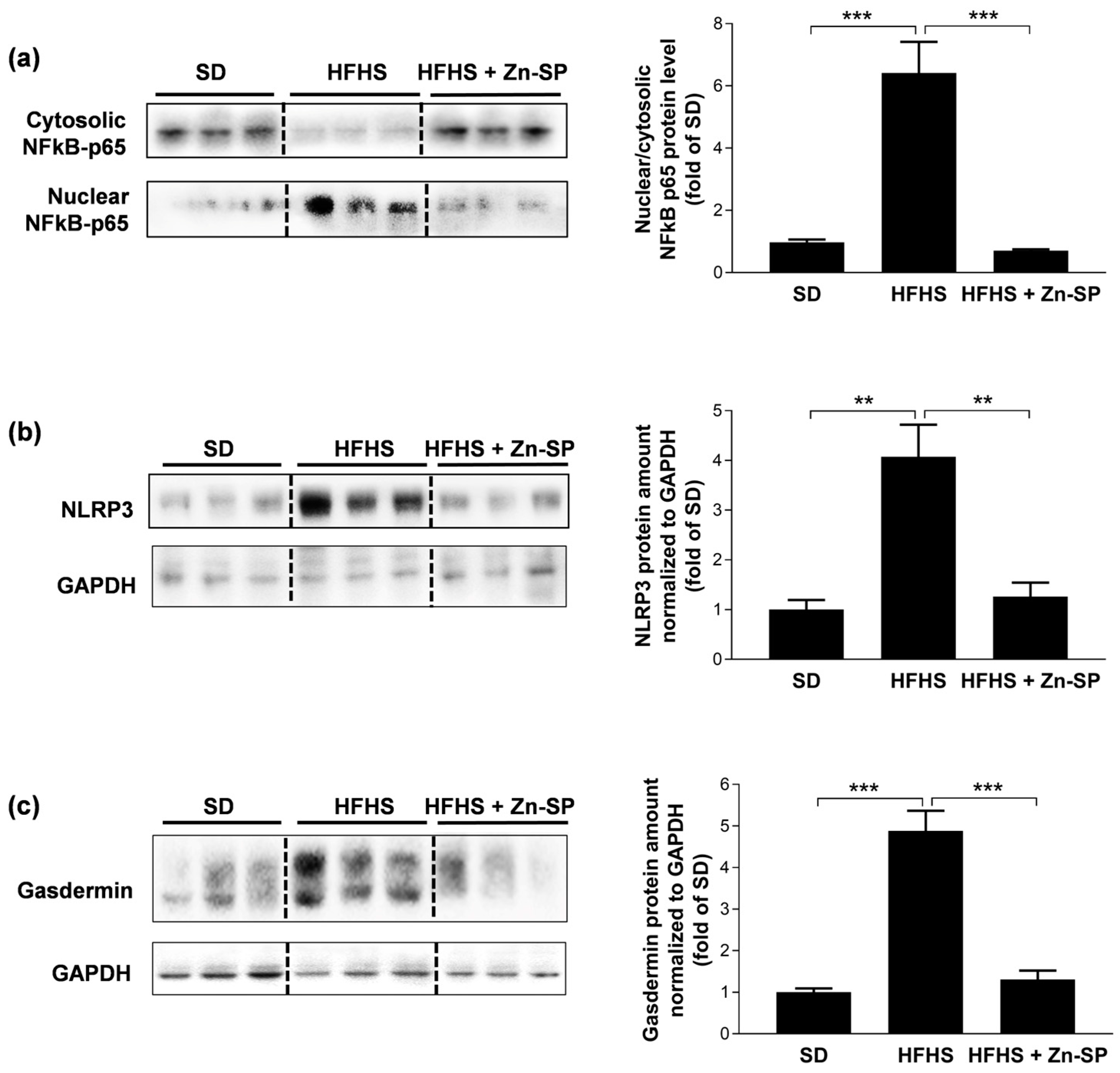
3.4. Zn-SP Inhibits Diet-Induced Activation of Pro-Inflammatory Signaling in Liver
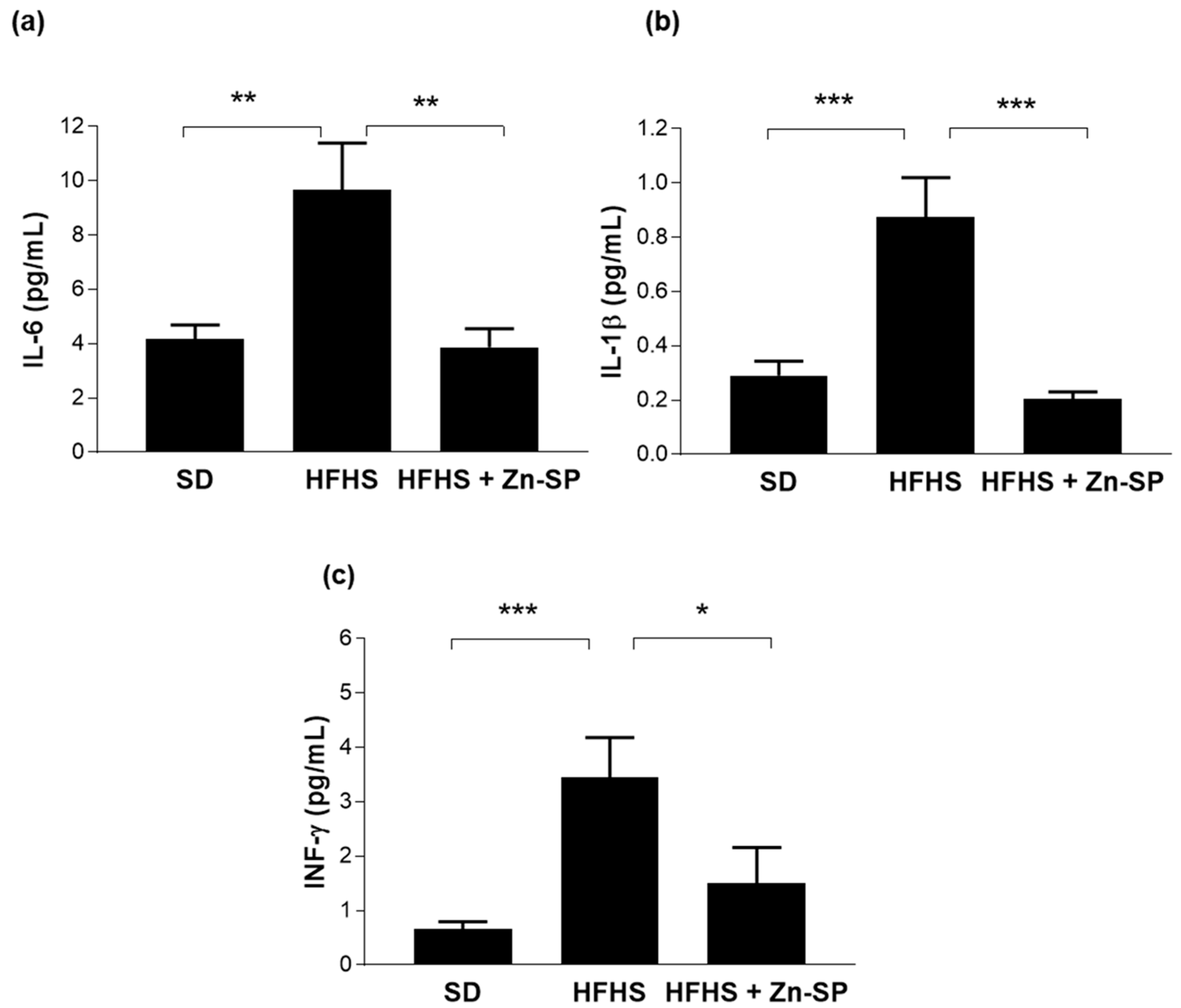
3.5. Zn-SP Supplementation Counteracts Diet-Induced Increases in Blood Markers of Inflammation and Liver Injury
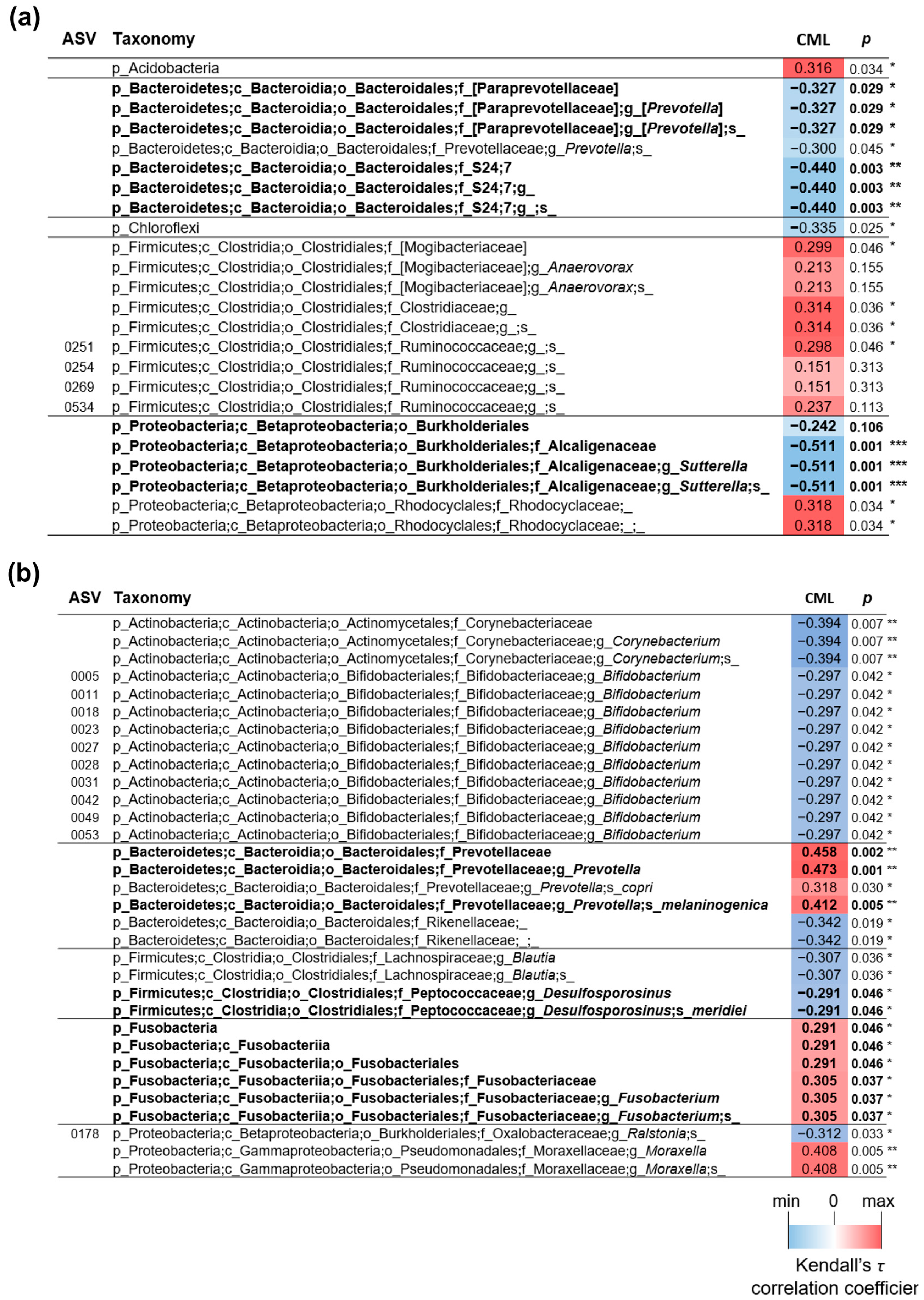
3.6. Effects of Dietary Manipulation and Zn-SP Supplementation on Intestinal Microbiota
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Popkin, B.M.; Adair, L.S.; Ng, S.W. Global Nutrition Transition and the Pandemic of Obesity in Developing Countries. Nutr. Rev. 2012, 70, 3–21. [Google Scholar] [CrossRef]
- Blüher, M. Obesity: Global Epidemiology and Pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef]
- Santini, A.; Novellino, E. Nutraceuticals in hypercholesterolaemia: An overview. Br. J. Pharmacol. 2017, 174, 1450–1463. [Google Scholar] [CrossRef]
- Rochlani, Y.; Pothineni, N.V.; Kovelamudi, S.; Mehta, J.L. Metabolic Syndrome: Pathophysiology, Management, and Modulation by Natural Compounds. Ther. Adv. Cardiovasc. Dis. 2017, 11, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Green, M.; Arora, K.; Prakash, S. Microbial Medicine: Prebiotic and Probiotic Functional Foods to Target Obesity and Metabolic Syndrome. Int. J. Mol. Sci. 2020, 21, 2890. [Google Scholar] [CrossRef] [PubMed]
- Aragno, M.; Mastrocola, R. Dietary Sugars and Endogenous Formation of Advanced Glycation Endproducts: Emerging Mechanisms of Disease. Nutrients 2017, 9, 385. [Google Scholar] [CrossRef] [PubMed]
- Abate, G.; Marziano, M.; Rungratanawanich, W.; Memo, M.; Uberti, D. Nutrition and AGE-Ing: Focusing on Alzheimer’s Disease. Oxid. Med. Cell Longev. 2017, 2017, 7039816. [Google Scholar] [CrossRef] [PubMed]
- Twarda-clapa, A.; Olczak, A.; Białkowska, A.M.; Koziołkiewicz, M. Advanced Glycation End-Products (AGEs): Formation, Chemistry, Classification, Receptors, and Diseases Related to AGEs. Cells 2022, 11, 1312. [Google Scholar] [CrossRef] [PubMed]
- Mastrocola, R.; Nigro, D.; Chiazza, F.; Medana, C.; Dal Bello, F.; Boccuzzi, G.; Collino, M.; Aragno, M. Fructose-Derived Advanced Glycation End-Products Drive Lipogenesis and Skeletal Muscle Reprogramming via SREBP-1c Dysregulation in Mice. Free Radic. Biol. Med. 2016, 91, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Mastrocola, R.; Dal Bello, F.; Cento, A.S.; Gaens, K.; Collotta, D.; Aragno, M.; Medana, C.; Collino, M.; Wouters, K.; Schalkwijk, C.G. Altered Hepatic Sphingolipid Metabolism in Insulin Resistant Mice: Role of Advanced Glycation Endproducts. Free Radic. Biol. Med. 2021, 169, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Mastrocola, R.; Collotta, D.; Gaudioso, G.; Le Berre, M.; Cento, A.S.; Ferreira, G.A.; Chiazza, F.; Verta, R.; Bertocchi, I.; Manig, F.; et al. Effects of Exogenous Dietary Advanced Glycation End Products on the Cross-Talk Mechanisms Linking Microbiota to Metabolic Inflammation. Nutrients 2020, 12, 2497. [Google Scholar] [CrossRef]
- Sarmah, S.; Roy, A.S. A Review on Prevention of Glycation of Proteins: Potential Therapeutic Substances to Mitigate the Severity of Diabetes Complications. Int. J. Biol. Macromol. 2022, 195, 565–588. [Google Scholar] [CrossRef] [PubMed]
- Zawada, A.; Machowiak, A.; Rychter, A.M.; Ratajczak, A.E.; Szymczak-Tomczak, A.; Dobrowolska, A.; Krela-Kaźmierczak, I. Accumulation of Advanced Glycation End-Products in the Body and Dietary Habits. Nutrients 2022, 14, 3982. [Google Scholar] [CrossRef]
- Maessen, D.E.; Brouwers, O.; Gaens, K.H.; Wouters, K.; Cleutjens, J.P.; Janssen, B.J.; Miyata, T.; Stehouwer, C.D.; Schalkwijk, C.G. Delayed Intervention with Pyridoxamine Improves Metabolic Function and Prevents Adipose Tissue Inflammation and Insulin Resistance in High-Fat Diet-Induced Obese Mice. Diabetes 2016, 65, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Tao, H.; Yancey, P.G.; Leuthner, Z.; May-Zhang, L.S.; Jung, J.Y.; Zhang, Y.; Ding, L.; Amarnath, V.; Liu, D.; et al. Scavenging Dicarbonyls with 5′-O-Pentyl-Pyridoxamine Increases HDL Net Cholesterol Efflux Capacity and Attenuates Atherosclerosis and Insulin Resistance. Mol. Metab. 2023, 67, 101651. [Google Scholar] [CrossRef]
- Hamedifard, Z.; Milajerdi, A.; Reiner, Ž.; Taghizadeh, M.; Kolahdooz, F.; Asemi, Z. The Effects of Spirulina on Glycemic Control and Serum Lipoproteins in Patients with Metabolic Syndrome and Related Disorders: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Phytother. Res. 2019, 33, 2609–2621. [Google Scholar] [CrossRef]
- Yousefi, R.; Saidpour, A.; Mottaghi, A. The Effects of Spirulina Supplementation on Metabolic Syndrome Components, Its Liver Manifestation and Related Inflammatory Markers: A Systematic Review. Complement. Ther. Med. 2019, 42, 137–144. [Google Scholar] [CrossRef]
- Lee, E.H.; Park, J.-E.; Choi, Y.-J.; Huh, K.-B.; Kim, W.-Y. A Randomized Study to Establish the Effects of Spirulina in Type 2 Diabetes Mellitus Patients. Nutr. Res. Pract. 2008, 2, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Deng, R.; Chow, T.J. Hypolipidemic, Antioxidant, and Antiinflammatory Activities of Microalgae Spirulina. Cardiovasc. Ther. 2010, 28, e33–e45. [Google Scholar] [CrossRef]
- Serban, M.C.; Sahebkar, A.; Dragan, S.; Stoichescu-Hogea, G.; Ursoniu, S.; Andrica, F.; Banach, M. A Systematic Review and Meta-Analysis of the Impact of Spirulina Supplementation on Plasma Lipid Concentrations. Clin. Nutr. 2016, 35, 842–851. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Colletti, A.; Bajraktari, G.; Descamps, O.; Djuric, D.M.; Ezhov, M.; Fras, Z.; Katsiki, N.; Langlois, M.; Latkovskis, G.; et al. Lipid-Lowering Nutraceuticals in Clinical Practice: Position Paper from an International Lipid Expert Panel. Nutr. Rev. 2017, 75, 731–767. [Google Scholar] [CrossRef]
- Nasirian, F.; Dadkhah, M.; Moradi-Kor, N.; Obeidavi, Z. Effects of Spirulina Platensis Microalgae on Antioxidant and Anti-Inflammatory Factors in Diabetic Rats. Diabetes Metab. Syndr. Obes. 2018, 11, 375–380. [Google Scholar] [CrossRef]
- Nawrocka, D.; Kornicka, K.; Śmieszek, A.; Marycz, K. Spirulina Platensis Improves Mitochondrial Function Impaired by Elevated Oxidative Stress in Adipose-Derived Mesenchymal Stromal Cells (ASCs) and Intestinal Epithelial Cells (IECs), and Enhances Insulin Sensitivity in Equine Metabolic Syndrome (EMS) Horses. Mar. Drugs 2017, 15, 237. [Google Scholar] [CrossRef]
- Finamore, A.; Palmery, M.; Bensehaila, S.; Peluso, I. Antioxidant, Immunomodulating, and Microbial-Modulating Activities of the Sustainable and Ecofriendly Spirulina. Oxid. Med. Cell Longev. 2017, 2017, 3247528. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, L.; Miron, A.; Klímová, B.; Wan, D.; Kuča, K. The Antioxidant, Immunomodulatory, and Anti-Inflammatory Activities of Spirulina: An Overview. Arch. Toxicol. 2016, 90, 1817–1840. [Google Scholar] [CrossRef]
- Cepoi, L.; Zinicovscaia, I.; Rudi, L.; Chiriac, T.; Miscu, V.; Djur, S.; Strelkova, L.; Vergel, K.; Nekhoroshkov, P. Growth and Heavy Metals Accumulation by Spirulina Platensis Biomass from Multicomponent Copper Containing Synthetic Effluents during Repeated Cultivation Cycles. Ecol. Eng. 2020, 142, 105637. [Google Scholar] [CrossRef]
- Prasad, A.S. Zinc: A Miracle Element. Its Discovery and Impact on Human Health. JSM Clin. Oncol. Res. 2014, 2, 1–7. [Google Scholar]
- Rios-Lugo, M.J.; Madrigal-Arellano, C.; Gaytán-Hernández, D.; Hernández-Mendoza, H.; Teresita Romero-Guzmán, E. Association of Serum Zinc Levels in Overweight and Obesity. Biol. Trace Elem. Res. 2020, 198, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Zinicovscaia, I.; Cepoi, L.; Rudi, L.; Chiriac, T.; Grozdov, D.; Vergel, K. Effect of Zinc-Containing Systems on Spirulina Platensis Bioaccumulation Capacity and Biochemical Composition. Environ. Sci. Pollut. Res. 2021, 28, 52216–52224. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, M.; Tsuneyama, K.; Fujimoto, T.; Selmi, C.; Gershwin, M.E.; Shimada, Y. Spirulina Improves Non-Alcoholic Steatohepatitis, Visceral Fat Macrophage Aggregation, and Serum Leptin in a Mouse Model of Metabolic Syndrome. Dig. Liver Dis. 2012, 44, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Sadek, K.M.; Lebda, M.A.; Nasr, S.M.; Shoukry, M. Spirulina Platensis Prevents Hyperglycemia in Rats by Modulating Gluconeogenesis and Apoptosis via Modification of Oxidative Stress and MAPK-Pathways. Biomed. Pharmacother. 2017, 92, 1085–1094. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, K.; Zhou, L.; He, J.; Zheng, X.; Zhang, L.; Zhong, X.; Wang, T. Evaluation of Long-Term Toxicity of Oral Zinc Oxide Nanoparticles and Zinc Sulfate in Mice. Biol. Trace Elem. Res. 2017, 178, 276–282. [Google Scholar] [CrossRef]
- Mastrocola, R.; Aimaretti, E.; Ferreira Alves, G.; Cento, A.S.; Fornelli, C.; Dal Bello, F.; Ferraris, C.; Goitre, L.; Perrelli, A.; Retta, S.F. Heterozygous Loss of KRIT1 in Mice Affects Metabolic Functions of the Liver, Promoting Hepatic Oxidative and Glycative Stress. Int. J. Mol. Sci. 2022, 23, 11151. [Google Scholar] [CrossRef]
- Mclellan, A.C.; Phillips, S.A.; Thornalley, P.J. The Assay of S-D-Lactoylglutathione in Biological Systems. Anal. Biochem. 1993, 211, 37–43. [Google Scholar] [CrossRef]
- Akerboom, T.P.M.; Sies, H. Assay of Glutathione, Glutathione Disulfide, and Glutathione Mixed Disulfides in Biological Samples. Methods Enzymol. 1981, 77, 373–382. [Google Scholar]
- Taverniti, V.; Cesari, V.; Gargari, G.; Rossi, U.; Biddau, C.; Lecchi, C.; Fiore, W.; Arioli, S.; Toschi, I.; Guglielmetti, S. Probiotics Modulate Mouse Gut Microbiota and Influence Intestinal Immune and Serotonergic Gene Expression in a Site-Specific Fashion. Front. Microbiol. 2021, 12, 706135. [Google Scholar] [CrossRef]
- Mantegazza, G.; Duncan, R.; Telesca, N.; Gargari, G.; Perotti, S.; Riso, P.; Guglielmetti, S. Lactic Acid Bacteria Naturally Associated with Ready-to-Eat Rocket Salad Can Survive the Human Gastrointestinal Transit. Food Microbiol. 2024, 118, 104418. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Kulshreshtha, A.; Anish Zacharia, J.; Jarouliya, U.; Bhadauriya, P.; Prasad, G.; Bisen, P. Spirulina in Health Care Management. Curr. Pharm. Biotechnol. 2008, 9, 400–405. [Google Scholar] [CrossRef] [PubMed]
- AlFadhly, N.K.Z.; Alhelfi, N.; Altemimi, A.B.; Verma, D.K.; Cacciola, F.; Narayanankutty, A. Trends and Technological Advancements in the Possible Food Applications of Spirulina and Their Health Benefits: A Review. Molecules 2022, 27, 5584. [Google Scholar] [CrossRef] [PubMed]
- Zeinalian, R.; Farhangi, M.A.; Shariat, A.; Saghafi-Asl, M. The Effects of Spirulina Platensis on Anthropometric Indices, Appetite, Lipid Profile and Serum Vascular Endothelial Growth Factor (VEGF) in Obese Individuals: A Randomized Double Blinded Placebo Controlled Trial. BMC Complement. Altern. Med. 2017, 17, 225. [Google Scholar] [CrossRef]
- Dinicolantonio, J.J.; Bhat, A.G.; Okeefe, J. Effects of Spirulina on Weight Loss and Blood Lipids: A Review. Open Heart 2020, 7, e001003. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Du, L.; Hosokawa, M.; Miyashita, K. Spirulina Lipids Alleviate Oxidative Stress and Inflammation in Mice Fed a High-Fat and High-Sucrose Diet. Mar. Drugs 2020, 18, 148. [Google Scholar] [CrossRef]
- He, F.; Antonucci, L.; Karin, M. NRF2 as a Regulator of Cell Metabolism and Inflammation in Cancer. Carcinogenesis 2020, 41, 405–416. [Google Scholar] [CrossRef]
- Do Perpétuo Socorro Carvalho Martins, M.; da Silva Santos Oliveira, A.S.; do Carmo de Carvalho e Martins, M.; de Carvalho, V.B.L.; Rodrigues, L.A.R.L.; Arcanjo, D.D.R.; Dos Santos, M.A.P.; Machado, J.S.R.; de Moura Rocha, M. Effects of Zinc Supplementation on Glycemic Control and Oxidative Stress in Experimental Diabetes: A Systematic Review. Clin. Nutr. ESPEN 2022, 51, 28–36. [Google Scholar]
- Olechnowicz, J.; Tinkov, A.; Skalny, A.; Suliburska, J. Zinc Status Is Associated with Inflammation, Oxidative Stress, Lipid, and Glucose Metabolism. J. Physiol. Sci. 2018, 68, 19–31. [Google Scholar] [CrossRef]
- Fukunaka, A.; Fujitani, Y. Role of Zinc Homeostasis in the Pathogenesis of Diabetes and Obesity. Int. J. Mol. Sci. 2018, 19, 476. [Google Scholar] [CrossRef]
- Sakamoto, S.; Ishikawa, Y.; Kudo, H.; Kagawa, Y. Increased Plasma Levels of Zinc in Obese Adult Females on a Weight-Loss Program Based on a Hypocaloric Balanced Diet. In Vivo 2005, 19, 1035–1037. [Google Scholar]
- Kelishadi, R.; Hashemipour, M.; Adeli, K.; Tavakoli, N.; Movahedian-Attar, A.; Shapouri, J.; Poursafa, P.; Rouzbahani, A. Effect of Zinc Supplementation on Markers of Insulin Resistance, Oxidative Stress, and Inflammation among Prepubescent Children with Metabolic Syndrome. Metab. Syndr. Relat. Disord. 2010, 8, 505–510. [Google Scholar] [CrossRef]
- Payahoo, L.; Ostadrahimi, A.; Mobasseri, M.; Bishak, Y.K.; Farrin, N.; Jafarabadi, M.A.; Mahluji, S. Effects of Zinc Supplementation on the Anthropometric Measurements, Lipid Profiles and Fasting Blood Glucose in the Healthy Obese Adults. Adv. Pharm. Bull. 2013, 3, 161–165. [Google Scholar] [CrossRef]
- Attia, J.R.; Holliday, E.; Weaver, N.; Peel, R.; Fleming, K.C.; Hure, A.; Wiggers, J.; McEvoy, M.; Searles, A.; Reeves, P.; et al. The Effect of Zinc Supplementation on Glucose Homeostasis: A Randomised Double-Blind Placebo-Controlled Trial. Acta Diabetol. 2022, 59, 965–975. [Google Scholar] [CrossRef]
- Zavros, A.; Giannaki, C.D.; Aphamis, G.; Roupa, Z.; Andreou, E. The Effects of Zinc and Selenium Supplementation on Body Composition and Thyroid Function in Individuals with Overweight or Obesity: A Systematic Review. J. Diet. Suppl. 2023, 20, 643–671. [Google Scholar] [CrossRef]
- Hannan, J.M.A.; Ansari, P.; Azam, S.; Flatt, P.R.; Abdel Wahab, Y.H.A. Effects of Spirulina Platensis on Insulin Secretion, Dipeptidyl Peptidase IV Activity and Both Carbohydrate Digestion and Absorption Indicate Potential as an Adjunctive Therapy for Diabetes. Br. J. Nutr. 2020, 124, 1021–1034. [Google Scholar] [CrossRef]
- Tamaki, M.; Fujitani, Y.; Hara, A.; Uchida, T.; Tamura, Y.; Takeno, K.; Kawaguchi, M.; Watanabe, T.; Ogihara, T.; Fukunaka, A.; et al. The Diabetes-Susceptible Gene SLC30A8/ZnT8 Regulates Hepatic Insulin Clearance. J. Clin. Investig. 2013, 123, 4513–4524. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Cui, W.; Tan, Y.; Luo, P.; Chen, Q.; Zhang, C.; Qu, W.; Miao, L.; Cai, L. Zinc Is Essential for the Transcription Function of Nrf2 in Human Renal Tubule Cells in Vitro and Mouse Kidney in Vivo under the Diabetic Condition. J. Cell Mol. Med. 2014, 18, 895–906. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.F.; DiNicolantonio, J.J.; O’Keefe, J.H. Nutraceutical Prevention of Diabetic Complications—Focus on Dicarbonyl and Oxidative Stress. Curr. Issues Mol. Biol. 2022, 44, 4314–4338. [Google Scholar] [CrossRef]
- McCarty, M.F.; Lujan, L.L.; Iloki Assanga, S. Targeting Sirt1, AMPK, Nrf2, CK2 and Soluble Guanylate Cyclase with Nutraceuticals: A Practical Strategy for Preserving Bone Mass. Int. J. Mol. Sci. 2022, 23, 4776. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.F.; Dinicolantonio, J.J.; Lerner, A. Review—Nutraceuticals Can Target Asthmatic Bronchoconstriction: NADPH Oxidase-Dependent Oxidative Stress, RhoA and Calcium Dynamics. J. Asthma Allergy 2021, 14, 685–701. [Google Scholar] [CrossRef]
- Reynaert, N.L.; Gopal, P.; Rutten, E.P.A.; Wouters, E.F.M.; Schalkwijk, C.G. Advanced Glycation End Products and Their Receptor in Age-Related, Non-Communicable Chronic Inflammatory Diseases; Overview of Clinical Evidence and Potential Contributions to Disease. Int. J. Biochem. Cell Biol. 2016, 81, 403–418. [Google Scholar] [CrossRef]
- Chavakis, T.; Bierhaus, A.; Al-Fakhri, N.; Schneider, D.; Witte, S.; Linn, T.; Nagashima, M.; Morser, J.; Arnold, B.; Preissner, K.T.; et al. The Pattern Recognition Receptor (RAGE) Is a Counterreceptor for Leukocyte Integrins: A Novel Pathway for Inflammatory Cell Recruitment. J. Exp. Med. 2003, 198, 1507–1515. [Google Scholar] [CrossRef]
- Hendawy, N.; Salaheldin, T.H.; Abuelezz, S.A. PCSK9 Inhibition Reduces Depressive like Behavior in CUMS-Exposed Rats: Highlights on HMGB1/RAGE/TLR4 Pathway, NLRP3 Inflammasome Complex and IDO-1. J. Neuroimmune Pharmacol. 2023, 18, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Chei, S.; Oh, H.J.; Song, J.H.; Seo, Y.J.; Lee, K.; Kim, K.J.; Lee, B.Y. Spirulina Maxima Extract Prevents Activation of the NLRP3 Inflammasome by Inhibiting ERK Signaling. Sci. Rep. 2020, 10, 2075. [Google Scholar] [CrossRef] [PubMed]
- Mastrocola, R.; Aragno, M.; Alloatti, G.; Collino, M.; Penna, C.; Pagliaro, P. Metaflammation: Tissue-Specific Alterations of the NLRP3 Inflammasome Platform in Metabolic Syndrome. Curr. Med. Chem. 2018, 25, 1294–1310. [Google Scholar] [CrossRef] [PubMed]
- Husain, A.; Alouffi, S.; Khanam, A.; Akasha, R.; Farooqui, A.; Ahmad, S. Therapeutic Efficacy of Natural Product ‘C-Phycocyanin’ in Alleviating Streptozotocin-Induced Diabetes via the Inhibition of Glycation Reaction in Rats. Int. J. Mol. Sci. 2022, 23, 14235. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Weickert, M.O.; Qureshi, S.; Kandala, N.B.; Anwar, A.; Waldron, M.; Shafie, A.; Messenger, D.; Fowler, M.; Jenkins, G.; et al. Improved Glycemic Control and Vascular Function in Overweight and Obese Subjects by Glyoxalase 1 Inducer Formulation. Diabetes 2016, 65, 2282–2294. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, L.; Della Posta, S.; Fanali, C.; Dugo, L.; De Gara, L.; Gugliucci, A. Antioxidant and Antiglycation Effects of Polyphenol Compounds Extracted from Hazelnut Skin on Advanced Glycation End-Products (AGEs) Formation. Antioxidants 2021, 10, 424. [Google Scholar] [CrossRef]
- Gugliucci, A.; Bastos, D.H.M.; Schulze, J.; Souza, M.F.F. Caffeic and Chlorogenic Acids in Ilex Paraguariensis Extracts Are the Main Inhibitors of AGE Generation by Methylglyoxal in Model Proteins. Fitoterapia 2009, 80, 339–344. [Google Scholar] [CrossRef]
- Aschner, M.; Skalny, A.V.; Gritsenko, V.A.; Kartashova, O.L.; Santamaria, A.; Rocha, J.B.T.; Spandidos, D.A.; Zaitseva, I.P.; Tsatsakis, A.; Tinkov, A.A. Role of Gut Microbiota in the Modulation of the Health Effects of Advanced Glycation End-products (Review). Int. J. Mol. Med. 2023, 51, 44. [Google Scholar] [CrossRef]
- Van Dongen, K.C.W.; Linkens, A.M.A.; Wetzels, S.M.W.; Wouters, K.; Vanmierlo, T.; van de Waarenburg, M.P.H.; Scheijen, J.L.J.M.; de Vos, W.M.; Belzer, C.; Schalkwijk, C.G. Dietary Advanced Glycation Endproducts (AGEs) Increase Their Concentration in Plasma and Tissues, Result in Inflammation and Modulate Gut Microbial Composition in Mice; Evidence for Reversibility. Food Res. Int. 2021, 147, 110547. [Google Scholar] [CrossRef]
- Gandhi, N.N.; Cobra, P.F.; Steele, J.L.; Markley, J.L.; Rankin, S.A. Lactobacillus Demonstrate Thiol-Independent Metabolism of Methylglyoxal: Implications toward Browning Prevention in Parmesan Cheese. J. Dairy. Sci. 2018, 101, 968–978. [Google Scholar] [CrossRef]
- Li, Q.; Wu, T.; Zhang, M.; Chen, H.; Liu, R. Induction of the Glycolysis Product Methylglyoxal on Trimethylamine Lyase Synthesis in the Intestinal Microbiota from Mice Fed with Choline and Dietary Fiber. Food Funct. 2021, 12, 9880–9893. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Chen, H.; Zhang, M.; Wu, T.; Liu, R.; Zhang, Z. Potential Correlation between Dietary Fiber-Suppressed Microbial Conversion of Choline to Trimethylamine and Formation of Methylglyoxal. J. Agric. Food Chem. 2019, 67, 13247–13257. [Google Scholar] [CrossRef] [PubMed]


| Calories (Kcal/100 g) (Kj/100 g) | 348 1480 |
| Total carbohydrate (g/100 g) Dietary fiber Sugar | 24.2 <1.0 <1.0 |
| Total fat (g/100 g) Saturated fat | 1.22 0.56 |
| Protein (g/100 g) | 60.05 |
| Salt (g/100 g) | 2.09 |
| Iron (Fe) (mg/100 g) | 27 |
| Potassium (K) (mg/100 g) | 1640 |
| Magnesium (Mg) (mg/100 g) | 304 |
| Phosphorus (P) (mg/100 g) | 830 |
| Zinc (Zn) (mg/100 g) | 200 |
| SD | HFHS | HFHS + Zn-SP | |
|---|---|---|---|
| Diet energy supply (kcal/g) | 3.85 | 5.56 | 5.56 |
| Body weight (g) | 28.37 ± 0.18 | 40.46 ± 0.76 * | 40.86 ± 0.25 * |
| Food intake (g/day/mouse) | 2.89 ± 0.05 | 3.10 ± 0.09 | 3.18 ± 0.15 |
| Total calorie intake (kcal/day/mouse) | 11.12 ± 0.20 | 17.26 ± 0.49 * | 17.70 ± 0.85 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aimaretti, E.; Porchietto, E.; Mantegazza, G.; Gargari, G.; Collotta, D.; Einaudi, G.; Ferreira Alves, G.; Marzani, E.; Algeri, A.; Dal Bello, F.; et al. Anti-Glycation Properties of Zinc-Enriched Arthrospira platensis (Spirulina) Contribute to Prevention of Metaflammation in a Diet-Induced Obese Mouse Model. Nutrients 2024, 16, 552. https://doi.org/10.3390/nu16040552
Aimaretti E, Porchietto E, Mantegazza G, Gargari G, Collotta D, Einaudi G, Ferreira Alves G, Marzani E, Algeri A, Dal Bello F, et al. Anti-Glycation Properties of Zinc-Enriched Arthrospira platensis (Spirulina) Contribute to Prevention of Metaflammation in a Diet-Induced Obese Mouse Model. Nutrients. 2024; 16(4):552. https://doi.org/10.3390/nu16040552
Chicago/Turabian StyleAimaretti, Eleonora, Elisa Porchietto, Giacomo Mantegazza, Giorgio Gargari, Debora Collotta, Giacomo Einaudi, Gustavo Ferreira Alves, Enrica Marzani, Alessandro Algeri, Federica Dal Bello, and et al. 2024. "Anti-Glycation Properties of Zinc-Enriched Arthrospira platensis (Spirulina) Contribute to Prevention of Metaflammation in a Diet-Induced Obese Mouse Model" Nutrients 16, no. 4: 552. https://doi.org/10.3390/nu16040552
APA StyleAimaretti, E., Porchietto, E., Mantegazza, G., Gargari, G., Collotta, D., Einaudi, G., Ferreira Alves, G., Marzani, E., Algeri, A., Dal Bello, F., Aragno, M., Cifani, C., Guglielmetti, S., Mastrocola, R., & Collino, M. (2024). Anti-Glycation Properties of Zinc-Enriched Arthrospira platensis (Spirulina) Contribute to Prevention of Metaflammation in a Diet-Induced Obese Mouse Model. Nutrients, 16(4), 552. https://doi.org/10.3390/nu16040552











