Targeting Metabolic Diseases: The Role of Nutraceuticals in Modulating Oxidative Stress and Inflammation
Abstract
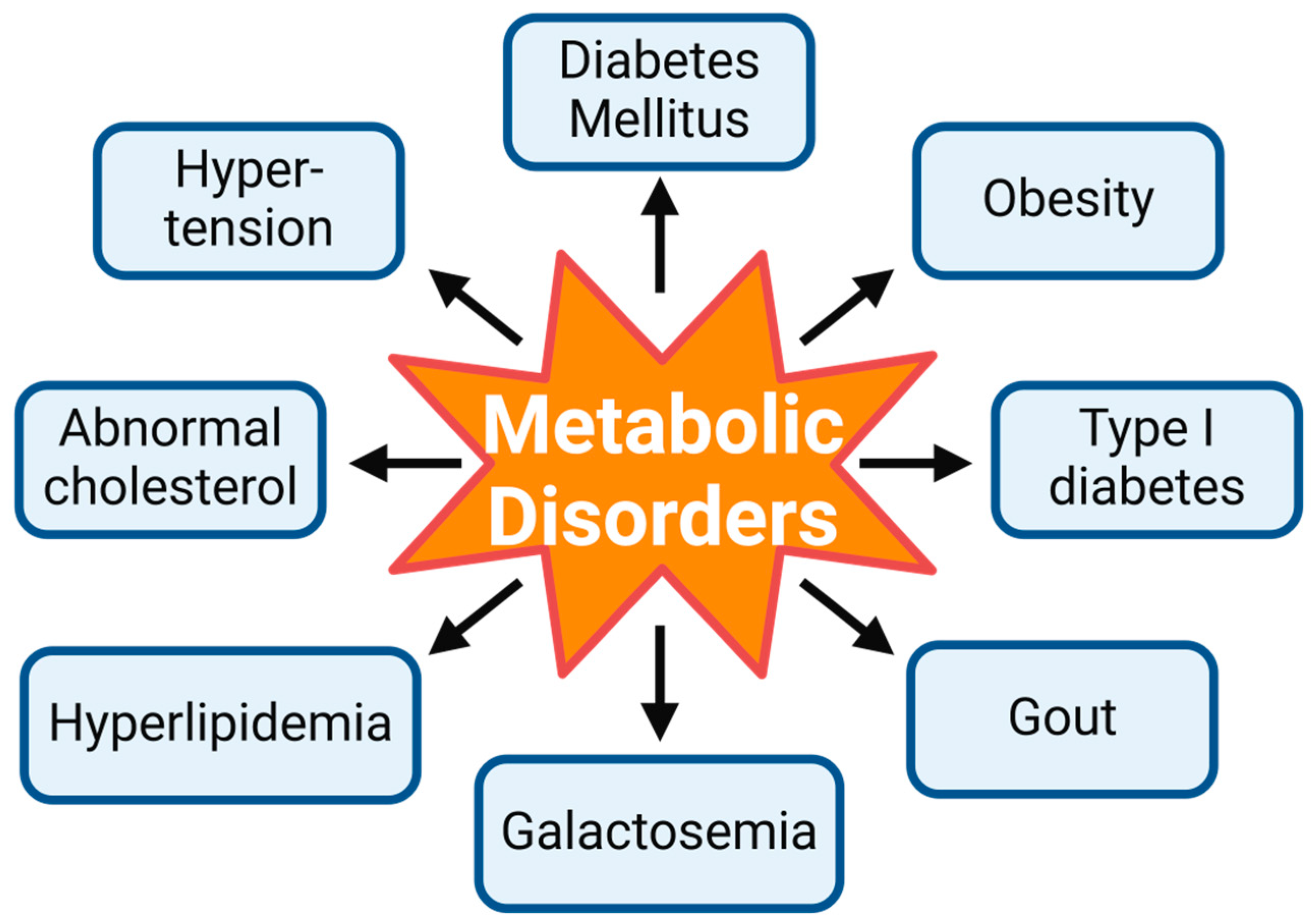
1. Introduction
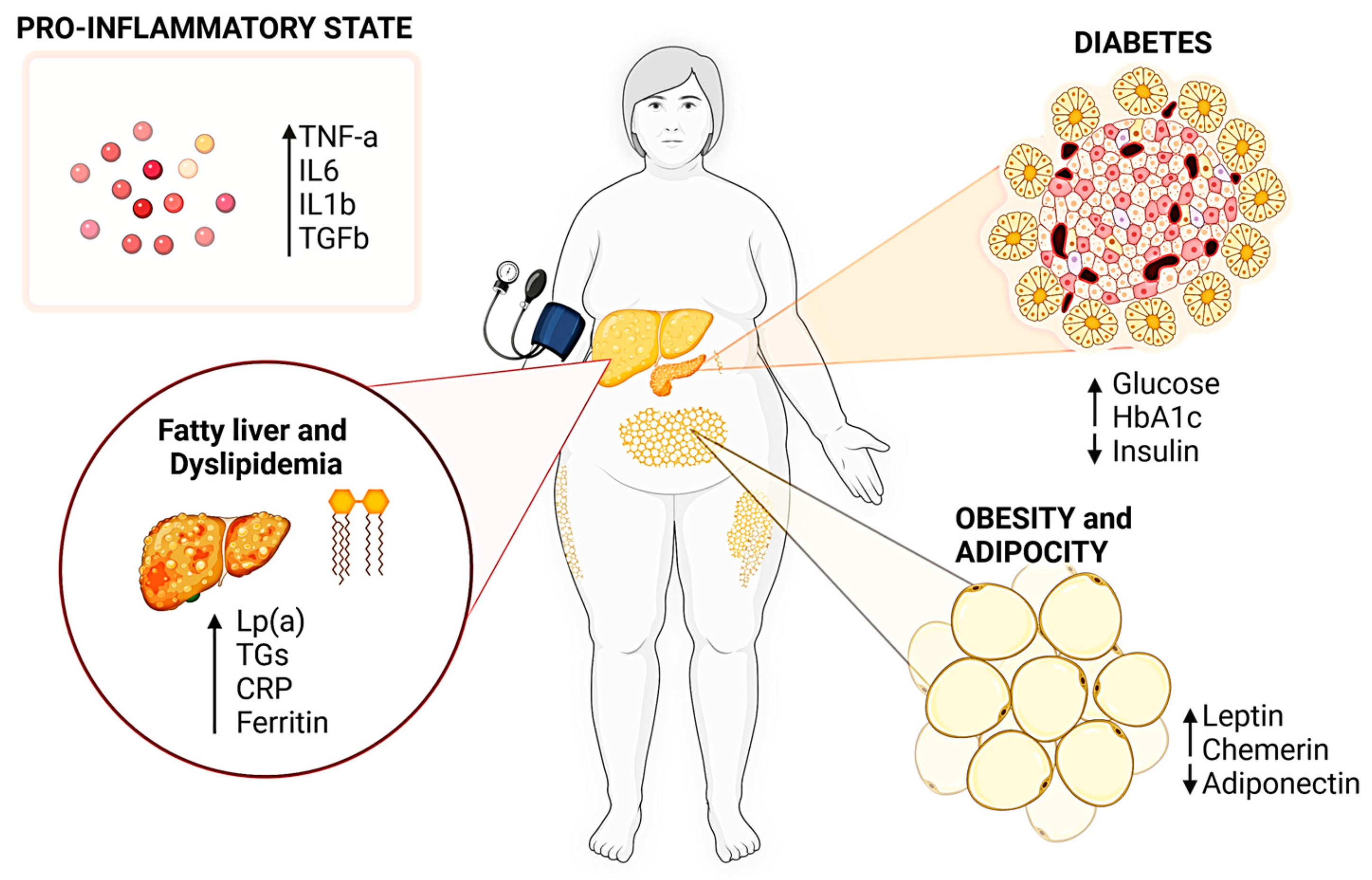
1.1. Molecular Pathways in Metabolic Disorders
1.2. Nutraceutical Interventions in Metabolic Disorders
2. Challenges in Metabolic Disorders and Nutraceuticals as a Novel Targeting Strategy
Nutraceuticals: A Novel and Promising Approach
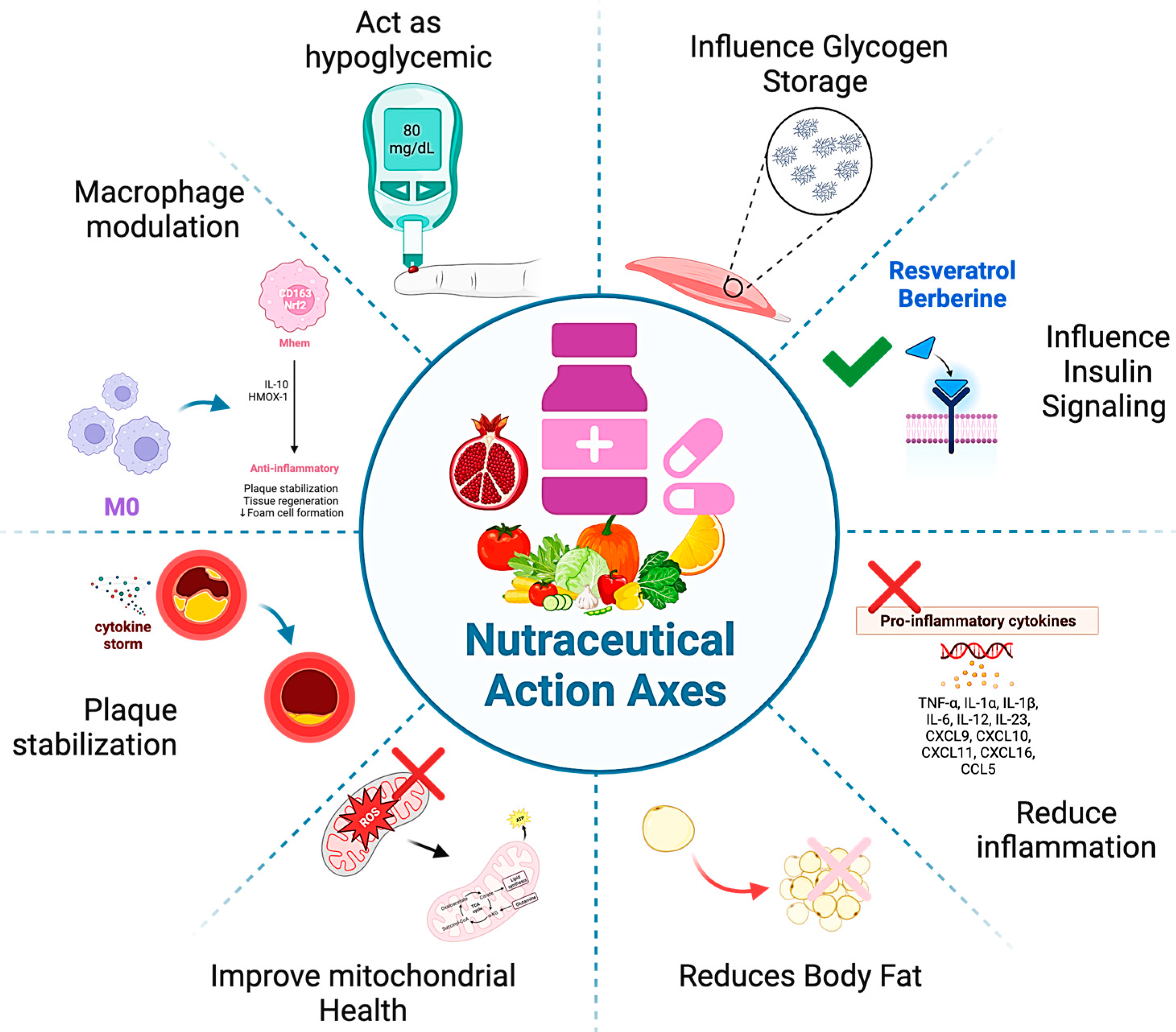
3. Nutraceuticals Usage in Addressing Oxidative Stress and Inflammation in Metabolic Disorders
3.1. Historical Perspective of Nutraceuticals
3.2. Nutraceuticals in Metabolic Syndrome and Cardiometabolic Disorders
3.3. Emerging Nutraceutical Compounds
3.4. Nutraceutical-Based Pharmacological Modulation
3.5. Potential Usage of Algae-Derived Nutraceuticals
4. Nutraceuticals as Novel Drug Targets
4.1. Interaction of Nutraceuticals with Oxidative Stress Pathways
4.2. Considerations for Clinical Translation and Challenges of Drug Formulations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Heindel, J.J.; Blumberg, B.; Cave, M.; Machtinger, R.; Mantovani, A.; Mendez, M.A.; Nadal, A.; Palanza, P.; Panzica, G.; Sargis, R.; et al. Metabolism disrupting chemicals and metabolic disorders. Reprod. Toxicol. 2017, 68, 3–33. [Google Scholar] [CrossRef]
- Sethi, J.K.; Hotamisligil, G.S. Metabolic Messengers: Tumor necrosis factor. Nat. Metab. 2021, 3, 1302–1312. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Suárez, V.J.; Martín-Rodríguez, A.; Redondo-Flórez, L.; López-Mora, C.; Yáñez-Sepúlveda, R.; Tornero-Aguilera, J.F. New Insights and Potential Therapeutic Interventions in Metabolic Diseases. Int. J. Mol. Sci. 2023, 24, 10672. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.L.; Chung, J.; Kim, K.J.; Kim, H.J.; Seo, W.W.; Jeon, K.H.; Cho, I.; Park, J.J.; Lee, M.H.; Suh, J.; et al. Lifestyle Modification in the Management of Metabolic Syndrome: Statement From Korean Society of CardioMetabolic Syndrome (KSCMS). Korean Circ. J. 2022, 52, 93–109. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, M.; Merchant, R.A.; Morley, J.E.; Anker, S.D.; Aprahamian, I.; Arai, H.; Aubertin-Leheudre, M.; Bernabei, R.; Cadore, E.L.; Cesari, M.; et al. International Exercise Recommendations in Older Adults (ICFSR): Expert Consensus Guidelines. J. Nutr. Health Aging 2021, 25, 824–853. [Google Scholar] [CrossRef] [PubMed]
- Prescott, J.A.; Mitchell, J.P.; Cook, S.J. Inhibitory feedback control of NF-κB signalling in health and disease. Biochem. J. 2021, 478, 2619–2664. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Bie, Y.; Feng, H.; Xie, B.; Liu, M.; Zhao, F. Inflammatory Factors Driving Atherosclerotic Plaque Progression New Insights. J. Transl. Intern. Med. 2022, 10, 36–47. [Google Scholar] [CrossRef]
- Yang, M.; Yao, X.; Xia, F.; Xiang, S.; Tang, W.; Zhou, B. Hugan Qingzhi tablets attenuates endoplasmic re-ticulum stress in nonalcoholic fatty liver disease rats by regulating PERK and ATF6 pathways. BMC Complement. Med. Ther. 2024, 24, 36. [Google Scholar] [CrossRef]
- Rochlani, Y.; Pothineni, N.V.; Kovelamudi, S.; Mehta, J.L. Metabolic syndrome: Pathophysiology, man-agement, and modulation by natural compounds. Ther. Adv. Cardiovasc. Dis. 2017, 11, 215–225. [Google Scholar] [CrossRef]
- Haque, S.; Kodidela, S.; Sinha, N.; Kumar, P.; Cory, T.J.; Kumar, S. Differential packaging of inflammatory cytokines/chemokines and oxidative stress modulators in U937 and U1 macrophages-derived extracellular vesicles upon exposure to tobacco constituents. PLoS ONE 2020, 15, e0233054. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Raghani, N.; Chorawala, M.; Bhattacharya, S.; Prajapati, B.G.; Elossaily, G.M.; Chaiyasut, C. NF-κB Pathway and Its Inhibitors: A Promising Frontier in the Management of Alzheimer’s Disease. Biomedicines 2023, 11, 2587. [Google Scholar] [CrossRef]
- Jia, Z.; Anandh Babu, P.V.; Chen, W.; Sun, X. Natural Products Targeting on Oxidative Stress and Inflammation: Mechanisms, Therapies, and Safety Assessment. Oxid. Med. Cell. Longev. 2018, 9, 6576093. [Google Scholar] [CrossRef]
- Yang, K.; Song, M. New Insights into the Pathogenesis of Metabolic-Associated Fatty Liver Disease (MAFLD): Gut-Liver-Heart Crosstalk. Nutrients 2023, 15, 3970. [Google Scholar] [CrossRef] [PubMed]
- Piché, M.E.; Tchernof, A.; Després, J.P. Obesity Phenotypes, Diabetes, and Cardiovascular Diseases. Circ. Res. 2020, 126, 1477–1500, Erratum in Circ. Res. 2020, 127, e107. [Google Scholar] [CrossRef]
- Grandl, G.; Wolfrum, C. Hemostasis, endothelial stress, inflammation, and the metabolic syndrome. Semin. Immunopathol. 2018, 40, 215–224. [Google Scholar] [CrossRef]
- Liu, L.; Jin, R.; Hao, J.; Zeng, J.; Yin, D.; Yi, Y.; Zhu, M.; Mandal, A.; Hua, Y.; Ng, C.K.; et al. Consumption of the Fish Oil High-Fat Diet Uncouples Obesity and Mammary Tumor Growth through Induction of Reactive Oxygen Species in Protumor Macrophages. Cancer Res. 2020, 80, 2564–2574. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Dec, K.; Kałduńska, J.; Kawczuga, D.; Kochman, J.; Janda, K. Reactive oxygen species—Sources, functions, oxidative damage. Pol. Merkur. Lek. 2020, 48, 124–127. [Google Scholar]
- Darenskaya, M.A.; Kolesnikova, L.I.; Kolesnikov, S.I. Oxidative Stress: Pathogenetic Role in Diabetes Mellitus and Its Complications and Therapeutic Approaches to Correction. Bull. Exp. Biol. Med. 2021, 1, 179–189. [Google Scholar] [CrossRef]
- Huang, X.; Liu, G.; Guo, J.; Su, Z. The PI3K/AKT pathway in obesity and type 2 diabetes. Int. J. Biol. Sci. 2018, 14, 1483–1496. [Google Scholar] [CrossRef]
- Lim, H.J.; Park, J.E.; Han, J.S. HM-chromanone alleviates hyperglycemia and inflammation in mice with endotoxin-induced insulin resistance. Toxicol. Res. 2023, 12, 665–674. [Google Scholar] [CrossRef]
- Yung, J.H.M.; Giacca, A. Role of c-Jun N-terminal Kinase (JNK) in Obesity and Type 2 Diabetes. Cells 2020, 9, 706. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Liu, T.; Qiao, Y.; Liu, D.; Yang, L.; Mao, H.; Ma, F.; Wang, Y.; Peng, L.; Zhan, Y. Oxidative stress and inflammation in diabetic nephropathy: Role of polyphenols. Front. Immunol. 2023, 14, 1185317. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Song, K.; Tu, B.; Lin, L.C.; Sun, H.; Zhou, Y.; Li, R.; Shi, Y.; Yang, J.J.; Zhang, Y.; et al. Crosstalk between oxidative stress and epigenetic marks: New roles and therapeutic implications in cardiac fibrosis. Redox Biol. 2023, 65, 102820. [Google Scholar] [CrossRef] [PubMed]
- Casper, E. The crosstalk between Nrf2 and NF-κB pathways in coronary artery disease: Can it be regulated by SIRT6? Life Sci. 2023, 330, 122007. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Wang, S.; Wang, H.; Zhang, T.; Yang, Y.; Zhao, T.; Chen, Z.; Xia, G.; Wang, C. SIRT1 reduction contributes to doxorubicin-induced oxidative stress and meiotic failure in mouse oocytes. Toxicol. Appl. Pharmacol. 2023, 476, 116671. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, M.; Wallert, M.; Lorkowski, S.; Peter, K. Cardiovascular and Metabolic Protection by Vitamin E: A Matter of Treatment Strategy? Antioxidants 2020, 9, 935. [Google Scholar] [CrossRef] [PubMed]
- Bešlo, D.; Golubić, N.; Rastija, V.; Agić, D.; Karnaš, M.; Šubarić, D.; Lučić, B. Antioxidant Activity, Metabolism, and Bioavailability of Polyphenols in the Diet of Animals. Antioxidants 2023, 12, 1141. [Google Scholar] [CrossRef] [PubMed]
- Nainu, F.; Frediansyah, A.; Mamada, S.S.; Permana, A.D.; Salampe, M.; Chandran, D.; Emran, T.B.; Simal-Gandara, J. Natural products targeting inflammation-related metabolic disorders: A comprehensive review. Heliyon 2023, 9, e16919. [Google Scholar] [CrossRef]
- Sahakyan, G.; Vejux, A.; Sahakyan, N. The Role of Oxidative Stress-Mediated Inflammation in the De-velopment of T2DM-Induced Diabetic Nephropathy: Possible Preventive Action of Tannins and Other Oligomeric Polyphenols. Molecules 2022, 27, 9035. [Google Scholar] [CrossRef]
- Maciejczyk, M.; Żebrowska, E.; Chabowski, A. Insulin Resistance and Oxidative Stress in the Brain: What’s New? Int. J. Mol. Sci. 2019, 20, 874. [Google Scholar] [CrossRef]
- Kim, S.Y.; Jeong, J.M.; Kim, S.J. Pro-inflammatory hepatic macrophages generate ROS through NADPH oxidase 2 via endocytosis of monomeric TLR4–MD2 complex. Nat. Commun. 2017, 8, 2247. [Google Scholar] [CrossRef]
- Li, X.; Ren, Y.; Chang, K.; Wu, W.; Griffiths, H.R.; Lu, S.; Gao, D. Adipose tissue macrophages as potential targets for obesity and metabolic diseases. Front. Immunol. 2023, 19, 1153915. [Google Scholar] [CrossRef]
- Mishra, B.; Upadhyay, M.; Kumar, A. Role of nutraceuticals in metabolic syndrome. In Drug Delivery Systems for Metabolic Disorders; Dureja, H., Narasimha Murthy, S., Wich, P.W., Dua, K., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: London, UK, 2022; Chapter 30; pp. 459–467. [Google Scholar] [CrossRef]
- Yang, G.; Chang, C.C.; Yang, Y.; Yuan, L.; Xu, L.; Ho, C.T.; Li, S. Resveratrol Alleviates Rheumatoid Arthritis via Reducing ROS and Inflammation, Inhibiting MAPK Signaling Pathways, and Suppressing Angiogenesis. J. Agric. Food Chem. 2018, 66, 12953–12960. [Google Scholar] [CrossRef]
- Ma, C.; Wang, Y.; Dong, L.; Li, M.; Cai, W. Anti-inflammatory effect of resveratrol through the suppression of NF-κB and JAK/STAT signaling pathways. Acta Biochim. Biophys. Sin. 2015, 47, 207–213. [Google Scholar] [CrossRef]
- Huang, S.M.; Wu, C.H.; Yen, G.C. Effects of flavonoids on the expression of the pro-inflammatory response in human monocytes induced by ligation of the receptor for AGEs. Mol. Nutr. Food Res. 2006, 50, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef] [PubMed]
- Chedea, V.S.; Tomoiagǎ, L.L.; Macovei, Ş.O.; Mǎgureanu, D.C.; Iliescu, M.L.; Bocsan, I.C.; Buzoianu, A.D.; Voşloban, C.M.; Pop, R.M. Antioxidant/Pro-Oxidant Actions of Polyphenols from Grapevine and Wine By-Products-Base for Complementary Therapy in Ischemic Heart Diseases. Front. Cardiovasc. Med. 2021, 8, 750508. [Google Scholar] [CrossRef] [PubMed]
- Martelli, A.; Flori, L.; Gorica, E.; Piragine, E.; Saviano, A.; Annunziata, G.; Di Minno, M.N.D.; Ciampaglia, R.; Calcaterra, I.; Maione, F.; et al. Vascular Effects of the Polyphenolic Nutraceutical Supplement Taurisolo®: Focus on the Protection of the Endothelial Function. Nutrients 2021, 13, 1540. [Google Scholar] [CrossRef] [PubMed]
- Ulusoy, H.G.; Sanlier, N. A minireview of quercetin: From its metabolism to possible mechanisms of its biological activities. Crit. Rev. Food Sci. Nutr. 2020, 60, 3290–3303. [Google Scholar] [CrossRef] [PubMed]
- Amakura, Y.; Tsutsumi, T.; Sasaki, K.; Nakamura, M.; Yoshida, T.; Maitani, T. Influence of food polyphenols on aryl hydrocarbon receptor-signaling pathway estimated by in vitro bioassay. Phytochemistry 2008, 69, 3117–3130. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Rayess, Y.E.; Rizk, A.A.; Sadaka, C.; Zgheib, R.; Zam, W.; Sestito, S.; Rapposelli, S.; Neffe-Skocińska, K.; Zielińska, D.; et al. Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications. Front. Pharmacol. 2020, 11, 01021. [Google Scholar] [CrossRef]
- Jena, A.B.; Samal, R.R.; Bhol, N.K.; Duttaroy, A.K. Cellular Red-Ox system in health and disease: The latest update. Biomed. Pharmacother. 2023, 162, 114606. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, X.; Hu, G.; Xu, C.; Jiang, H. Curcumin Attenuates Hydrogen Peroxide-Induced Premature Senescence via the Activation of SIRT1 in Human Umbilical Vein Endothelial Cells. Biol. Pharm. Bull. 2015, 38, 1134–1141. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Ahmadi, Z.; Mohammadinejad, R.; Farkhondeh, T.; Samarghandian, S. Curcumin Activates the Nrf2 Pathway and Induces Cellular Protection against Oxidative Injury. Curr. Mol. Med. 2020, 20, 116–133. [Google Scholar] [CrossRef]
- El-Far, A.H.; Elewa, Y.H.A.; Abdelfattah, E.A.; Alsenosy, A.A.; Atta, M.S.; Abou-Zeid, K.M.; Al Jaouni, S.K.; Mousa, S.A.; Noreldin, A.E. Thymoquinone and Curcumin Defeat Aging-Associated Oxidative Alterations Induced by D-Galactose in Rats’ Brain and Heart. Int. J. Mol. Sci. 2021, 22, 6839. [Google Scholar] [CrossRef]
- Kuroda, M.; Mimaki, Y.; Nishiyama, T.; Mae, T.; Kishida, H.; Tsukagawa, M.; Takahashi, K.; Kawada, T.; Nakagawa, K.; Kitahara, M. Hypoglycemic effects of turmeric (Curcuma longa L. rhizomes) on genetically diabetic KK-Ay mice. Biol. Pharm. Bull. 2005, 28, 937–939. [Google Scholar] [CrossRef]
- Weisberg, S.P.; Leibel, R.; Tortoriello, D.V. Dietary curcumin significantly improves obesity-associated inflammation and diabetes in mouse models of diabesity. Endocrinology 2008, 149, 3549–3558. [Google Scholar] [CrossRef]
- Peng, Y.; Ao, M.; Dong, B.; Jiang, Y.; Yu, L.; Chen, Z.; Hu, C.; Xu, R. Anti-Inflammatory Effects of Curcumin in the Inflammatory Diseases: Status, Limitations and Countermeasures. Drug Des. Dev. Ther. 2021, 15, 4503–4525. [Google Scholar] [CrossRef]
- Sahebkar, A. Curcuminoids for the management of hypertriglyceridaemia. Nat. Rev. Cardiol. 2014, 11, 123. [Google Scholar] [CrossRef]
- Friesen, J.A.; Rodwell, V.W. The 3-hydroxy-3-methylglutaryl coenzyme-A (HMG-CoA) reductases. Genome Biol. 2004, 5, 248. [Google Scholar] [CrossRef]
- Rachmawati, H.; Soraya, I.S.; Kurniati, N.F.; Rahma, A. In Vitro Study on Antihypertensive and Antihypercholesterolemic Effects of a Curcumin Nanoemulsion. Sci. Pharm. 2016, 84, 131–140. [Google Scholar] [CrossRef]
- Guariglia, M.; Saba, F.; Rosso, C.; Bugianesi, E. Molecular Mechanisms of Curcumin in the Pathogenesis of Metabolic Dysfunction Associated Steatotic Liver Disease. Nutrients 2023, 15, 5053. [Google Scholar] [CrossRef]
- Wu, L.Y.; Chen, C.W.; Chen, L.K.; Chou, H.Y.; Chang, C.L.; Juan, C.C. Curcumin Attenuates Adipogenesis by Inducing Preadipocyte Apoptosis and Inhibiting Adipocyte Differentiation. Nutrients 2019, 11, 2307. [Google Scholar] [CrossRef]
- Jakab, J.; Miškić, B.; Mikšić, Š.; Juranić, B.; Ćosić, V.; Schwarz, D.; Včev, A. Adipogenesis as a Potential Anti-Obesity Target: A Review of Pharmacological Treatment and Natural Products. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 67–83. [Google Scholar] [CrossRef]
- Dong, Y.; Zhu, H.; Chen, L.; Huang, Y.; Christen, W.; Cook, N.R.; Copeland, T.; Mora, S.; Buring, J.E.; Lee, I.M.; et al. Effects of Vitamin D3 and Marine Omega-3 Fatty Acids Supplementation on Biomarkers of Systemic Inflammation: 4-Year Findings from the VITAL Randomized Trial. Nutrients 2022, 14, 5307. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Y.; Yang, Z.; Liu, M.; Zhang, C.; Zhao, Y.; Song, C. ω-3 DPA Protected Neurons from Neuroinflammation by Balancing Microglia M1/M2 Polarizations through Inhibiting NF-κB/MAPK p38 Signaling and Activating Pathways. Mar. Drugs 2021, 19, 587. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, L.; Wang, D.; Yan, N.; Li, C.; Wu, M.; Wang, F.; Mi, B.; Chen, F.; Jia, W.; et al. Omega-3 polyunsaturated fatty acid biomarkers and risk of type 2 diabetes, cardiovascular disease, cancer, and mortality. Clin. Nutr. 2022, 41, 1798–1807. [Google Scholar] [CrossRef]
- Heshmati, J. Effect of omega-3 fatty acid supplementation on gene expression of inflammation, oxidative stress and cardiometabolic parameters: Systematic review and meta-analysis. J. Funct. Foods 2021, 85, 104619. [Google Scholar] [CrossRef]
- Frankhouser, D.E.; Steck, S.; Sovic, M.G.; Belury, M.A.; Wang, Q.; Clinton, S.K.; Bundschuh, R.; Yan, P.S.; Yee, L.D. Dietary omega-3 fatty acid intake impacts peripheral blood DNA methylation -anti-inflammatory effects and individual variability in a pilot study. J. Nutr. Biochem. 2022, 99, 108839. [Google Scholar] [CrossRef]
- Marion-Letellier, R.; Savoye, G.; Ghosh, S. Polyunsaturated fatty acids and inflammation. IUBMB Life 2015, 67, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Siriwardhana, N.; Kalupahana, N.S.; Fletcher, S.; Xin, W.; Claycombe, K.J.; Quignard-Boulange, A.; Zhao, L.; Saxton, A.M.; Moustaid-Moussa, N. n-3 and n-6 polyunsaturated fatty acids differentially regulate adipose angiotensinogen and other inflammatory adipokines in part via NF-κB-dependent mechanisms. J. Nutr. Biochem. 2012, 23, 1661–1667. [Google Scholar] [CrossRef]
- Korbecki, J.; Bobiński, R.; Dutka, M. Self-regulation of the inflammatory response by peroxisome proliferator-activated receptors. Inflamm. Res. 2019, 68, 443–458. [Google Scholar] [CrossRef]
- Heming, M.; Gran, S.; Jauch, S.L.; Fischer-Riepe, L.; Russo, A.; Klotz, L.; Hermann, S.; Schäfers, M.; Roth, J.; Barczyk-Kahlert, K. Peroxisome Proliferator-Activated Receptor-γ Modulates the Response of Macrophages to Lipopolysaccharide and Glucocorticoids. Front. Immunol. 2018, 9, 893. [Google Scholar] [CrossRef]
- Kalea, A.Z.; Drosatos, K.; Buxton, J.L. Nutriepigenetics and cardiovascular disease. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Caprara, G. Mediterranean-Type Dietary Pattern and Physical Activity: The Winning Combination to Counteract the Rising Burden of Non-Communicable Diseases (NCDs). Nutrients 2021, 13, 429. [Google Scholar] [CrossRef]
- Arca, M.; Borghi, C.; Pontremoli, R.; De Ferrari, G.M.; Colivicchi, F.; Desideri, G.; Temporelli, P.L. Hypertriglyceridemia and omega-3 fatty acids: Their often overlooked role in cardiovascular disease prevention. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 197–205. [Google Scholar] [CrossRef]
- Chen, C.; Yu, X.; Shao, S. Effects of Omega-3 Fatty Acid Supplementation on Glucose Control and Lipid Levels in Type 2 Diabetes: A Meta-Analysis. PLoS ONE 2015, 10, e0139565. [Google Scholar] [CrossRef]
- Jackson, K.H.; Polreis, J.M.; Tintle, N.L.; Kris-Etherton, P.M.; Harris, W.S. Association of reported fish intake and supplementation status with the omega-3 index. Prostaglandins Leukot. Essent. Fat. Acids 2019, 142, 4–10. [Google Scholar] [CrossRef]
- Elagizi, A.; Lavie, C.J.; O’Keefe, E.; Marshall, K.; O’Keefe, J.H.; Milani, R.V. An Update on Omega-3 Polyunsaturated Fatty Acids and Cardiovascular Health. Nutrients 2021, 13, 204. [Google Scholar] [CrossRef]
- Ciccone, V.; Piragine, E.; Gorica, E.; Citi, V.; Testai, L.; Pagnotta, E.; Matteo, R.; Pecchioni, N.; Montanaro, R.; Di Cesare Mannelli, L.; et al. Anti-Inflammatory Effect of the Natural H2S-Donor Erucin in Vascular Endothelium. Int. J. Mol. Sci. 2022, 23, 15593. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.P.; Sulaiman Rahman, H. Antioxidant and Oxidative Stress: A Mutual Interplay in Age-Related Diseases. Front. Pharmacol. 2018, 9, 1162. [Google Scholar] [CrossRef]
- Francque, S.M.; Marchesini, G.; Kautz, A.; Walmsley, M.; Dorner, R.; Lazarus, J.V.; Zelber-Sagi, S.; Hallsworth, K.; Busetto, L.; Frühbeck, G.; et al. Non-alcoholic fatty liver disease: A patient guideline. JHEP Rep. 2021, 3, 100322. [Google Scholar] [CrossRef]
- Federico, A.; Dallio, M.; Masarone, M.; Gravina, A.G.; Di Sarno, R.; Tuccillo, C.; Cossiga, V.; Lama, S.; Stiuso, P.; Morisco, F.; et al. Evaluation of the Effect Derived from Silybin with Vitamin D and Vitamin E Administration on Clinical, Metabolic, Endothelial Dysfunction, Oxidative Stress Parameters, and Serological Worsening Markers in Nonalcoholic Fatty Liver Disease Patients. Oxid. Med. Cell Longev. 2019, 2019, 8742075. [Google Scholar] [CrossRef] [PubMed]
- May, J.M.; Harrison, F.E. Role of vitamin C in the function of the vascular endothelium. Antioxid. Redox Signal. 2013, 19, 2068–2083. [Google Scholar] [CrossRef]
- Böhm, E.W.; Buonfiglio, F.; Voigt, A.M.; Bachmann, P.; Safi, T.; Pfeiffer, N.; Gericke, A. Oxidative stress in the eye and its role in the pathophysiology of ocular diseases. Redox Biol. 2023, 68, 102967. [Google Scholar] [CrossRef]
- Higashi, Y. Roles of Oxidative Stress and Inflammation in Vascular Endothelial Dysfunction-Related Disease. Antioxidants 2022, 11, 1958. [Google Scholar] [CrossRef]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vasc. Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef]
- Scioli, M.G.; Storti, G.; D’Amico, F.; Rodríguez Guzmán, R.; Centofanti, F.; Doldo, E.; Céspedes Miranda, E.M.; Orlandi, A. Oxidative Stress and New Pathogenetic Mechanisms in Endothelial Dysfunction: Potential Diagnostic Biomarkers and Therapeutic Targets. J. Clin. Med. 2020, 9, 1995. [Google Scholar] [CrossRef]
- Franca-Oliveira, G.; Martinez-Rodriguez, A.J.; Morato, E.; Hernández-Ledesma, B. Contribution of Proteins and Peptides to the Impact of a Soy Protein Isolate on Oxidative Stress and Inflammation-Associated Biomarkers in an Innate Immune Cell Model. Plants 2023, 12, 2011. [Google Scholar] [CrossRef]
- Testai, L.; Montanaro, R.; Flori, L.; Pagnotta, E.; Vellecco, V.; Gorica, E.; Ugolini, L.; Righetti, L.; Brancaleone, V.; Bucci, M.; et al. Persulfidation of mitoKv7.4 channels contributes to the cardioprotective effects of the H2S-donor Erucin against ischemia/reperfusion injury. Biochem. Pharmacol. 2023, 215, 115728. [Google Scholar] [CrossRef]
- Daliu, P.; Annunziata, G.; Tenore, G.C.; Santini, A. Abscisic acid identification in Okra, Abelmoschus esculentus L. (Moench): Perspective nutraceutical use for the treatment of diabetes. Nat. Prod. Res. 2020, 34, 3–9. [Google Scholar] [CrossRef]
- Petrarca, C.; Viola, D. Redox Remodeling by Nutraceuticals for Prevention and Treatment of Acute and Chronic Inflammation. Antioxidants 2023, 12, 132. [Google Scholar] [CrossRef]
- Farrokhi, M.; Taheri, F.; Jafari Khouzani, P.; Rahmani, E.; Tavakoli, R.; Moghadam Fard, A.; Rajabloo, Y.; Sadeghniiat-Haghighi, A.; Shahbazi, K.; Aghabababak Semnani, M.; et al. Role of Precision Medicine and Personalized Medicine in the Treatment of Diseases. Kindle 2023, 3, 1–164. Available online: https://preferpub.org/index.php/kindle/article/view/Book24 (accessed on 6 January 2023).
- Morya, G.C.; Tiwari, V. A conceptual study on nutraceuticals in ayurvedic perspectives w.s.r. to Rasayana. J. Glob. Biosci. 2018, 7, 5350–5357. [Google Scholar]
- DeFelice, S.L. The nutraceutical revolution: Its impact on food industry R&D. Trends Food Sci. Technol. 1995, 6, 59–61. [Google Scholar]
- Santini, A.; Cammarata, S.M.; Capone, G.; Ianaro, A.; Tenore, G.C.; Pani, L.; Novellino, E. Nutraceuticals: Opening the debate for a regulatory framework. Br. J. Clin. Pharmacol. 2018, 84, 659–672. [Google Scholar] [CrossRef]
- Fernandes, S.D.; Narayana, R.C.; Narayanan, A.V. The emergence of India as a blossoming market for nutraceutical supplements: An overview. Trends Food Sci. Technol. 2019, 86, 579–585. [Google Scholar] [CrossRef]
- Barrios, V.; Escobar, C.; Cicero, A.F.; Burke, D.; Fasching, P.; Banach, M.; Bruckert, E. A nutraceutical approach (Armolipid Plus) to reduce total and LDL cholesterol in individuals with mild to moderate dyslipidemia: Review of the clinical evidence. Atheroscler. Suppl. 2017, 24, 1–15. [Google Scholar] [CrossRef]
- Kastelein, J.J.; Maki, K.C.; Susekov, A.; Ezhov, M.; Nordestgaard, B.G.; Machielse, B.N.; Kling, D.; Da-vidson, M.H. Omega-3 free fatty acids for the treatment of severe hypertriglyceridemia: The EpanoVa fOr Lowering Very high triglyceridEs (EVOLVE) trial. J. Clin. Lipidol. 2014, 8, 94–106. [Google Scholar] [CrossRef]
- Ruscica, M.; Pavanello, C.; Gandini, S.; Macchi, C.; Botta, M.; Dall’Orto, D.; Del Puppo, M.; Bertolotti, M.; Bosisio, R.; Mombelli, G.; et al. Correction to: Nutraceutical approach for the management of cardiovascular risk—A combination containing the probiotic Bifidobacterium longum BB536 and red yeast rice extract: Results from a randomized, double-blind, placebo-controlled study. Nutr. J. 2019, 18, 54. [Google Scholar] [CrossRef]
- Tenore, G.C.; Caruso, D.; Buonom, G.; D’Avino, M.; Ciampaglia, R.; Novellino, E. Plasma lipid lowering effect by a novel chia seed based nutraceutical formulation. J. Funct. Foods 2018, 42, 38–46. [Google Scholar] [CrossRef]
- Annunziata, G.; Maisto, M.; Schisano, C.; Ciampaglia, R.; Narciso, V.; Tenore, G.C.; Novellino, E. Effects of Grape Pomace Polyphenolic Extract (Taurisolo®) in Reducing TMAO Serum Levels in Humans: Preliminary Results from a Randomized, Placebo-Controlled, Cross-Over Study. Nutrients 2019, 11, 139. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Qian, M.; Jiang, Q.; Tan, B.; Yin, Y.; Han, X. Evidence of Flavonoids on Disease Prevention. Antioxidants 2023, 12, 527. [Google Scholar] [CrossRef]
- Santos, L.R.; Neves, C.; Melo, M.; Soares, P. Selenium and Selenoproteins in Immune Medi-ated Thyroid Disorders. Diagnostics 2018, 8, 70. [Google Scholar] [CrossRef] [PubMed]
- Recinella, L.; Gorica, E.; Chiavaroli, A.; Fraschetti, C.; Filippi, A.; Cesa, S.; Cairone, F.; Martelli, A.; Calderone, V.; Veschi, S.; et al. Anti-Inflammatory and Antioxidant Effects Induced by Allium sati-vum L. Extracts on an Ex Vivo Experimental Model of Ulcerative Colitis. Foods 2022, 11, 3559. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Pan, J.; Hu, Y.; Ding, L.; Li, Y.; Cui, X.; Zhang, M.; Zhang, Z.; Li, C. Advances in the study of vascular relat-ed protective effect of garlic (Allium sativum) extract and compounds. J. Nutr. Biochem. 2023, 124, 109531. [Google Scholar] [CrossRef]
- Recinella, L.; Chiavaroli, A.; Masciulli, F.; Fraschetti, C.; Filippi, A.; Cesa, S.; Cairone, F.; Gorica, E.; De Leo, M.; Braca, A.; et al. Protective Effects Induced by a Hydroalcoholic Allium sativum Extract in Isolated Mouse Heart. Nutrients 2021, 13, 2332. [Google Scholar] [CrossRef]
- Zykin, P.A.; Andreeva, E.A.; Lykholay, A.N.; Tsvetkova, N.V.; Voylokov, A.V. Anthocyanin Composition and Content in Rye Plants with Different Grain Color. Molecules 2018, 23, 948. [Google Scholar] [CrossRef]
- Mucha, P.; Skoczyńska, A.; Małecka, M.; Hikisz, P.; Budzisz, E. Overview of the Antioxidant and Anti-Inflammatory Activities of Selected Plant Compounds and Their Metal Ions Complexes. Molecules 2021, 26, 4886. [Google Scholar] [CrossRef]
- Yu, J.; Bi, X.; Yu, B.; Chen, D. Isoflavones: Anti-Inflammatory Benefit and Possible Caveats. Nutrients 2016, 8, 361. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavo-noids as Potential Anti-Inflammatory Molecules: A Review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef] [PubMed]
- Ba, W.; Xu, W.; Deng, Z.; Zhang, B.; Zheng, L.; Li, H. The Antioxidant and Anti-Inflammatory Ef-fects of the Main Carotenoids from Tomatoes via Nrf2 and NF-κB Signaling Pathways. Nutrients 2023, 15, 4652. [Google Scholar] [CrossRef] [PubMed]
- Van Steenwijk, H.P.; Bast, A.; de Boer, A. The Role of Circulating Lycopene in Low-Grade Chronic Inflammation: A Systematic Review of the Literature. Molecules 2020, 25, 4378. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, S.; Fletcher, C. Trimethylamine N-oxide: Breathe new life. Br. J. Pharmacol. 2018, 175, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Truong, V.L.; Jun, M.; Jeong, W.S. Role of resveratrol in regulation of cellular defense systems against oxidative stress. Biofactors 2018, 44, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Matkawala, F.; Nighojkar, S.; Nighojkar, A. Next-generation nutraceuticals: Bioactive peptides from plant proteases. Biotechnologia 2022, 103, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Aluko, R.E. Antihypertensive peptides from food proteins. Annu. Rev. Food Sci. Technol. 2015, 6, 235–262. [Google Scholar] [CrossRef]
- LoVerme, J.; La Rana, G.; Russo, R.; Calignano, A.; Piomelli, D. The search for the palmitoylethanolamide receptor. Life Sci. 2005, 77, 1685–1698. [Google Scholar] [CrossRef]
- Gorąca, A.; Huk-Kolega, H.; Piechota, A.; Kleniewska, P.; Ciejka, E.; Skibska, B. Lipoic acid—Bio-logical activity and therapeutic potential. Pharmacol. Rep. 2011, 63, 849–858. [Google Scholar] [CrossRef]
- AlAli, M.; Alqubaisy, M.; Aljaafari, M.N.; AlAli, A.O.; Baqais, L.; Molouki, A.; Abushelaibi, A.; Lai, K.S.; Lim, S.E. Nutraceuticals: Transformation of Conventional Foods into Health Promoters/Disease Preventers and Safety Considerations. Molecules 2021, 26, 2540. [Google Scholar] [CrossRef]
- Drake, P.M.; Szeto, T.H.; Paul, M.J.; The, A.Y.; Ma, J.K. Recombinant biologic products versus nutraceuticals from plants—A regulatory choice? Br. J. Clin. Pharmacol. 2017, 83, 82–87. [Google Scholar] [CrossRef]
- Gertsch, J. Cannabimimetic phytochemicals in the diet—An evolutionary link to food selection and metabolic stress adaptation? Br. J. Pharmacol. 2017, 174, 1464–1483. [Google Scholar] [CrossRef]
- Pferschy-Wenzig, E.M.; Bauer, R. The relevance of pharmacognosy in pharmacological research on herbal medicinal products. Epilepsy Behav. 2015, 52 Pt B, 344–362. [Google Scholar] [CrossRef]
- Nasri, H.; Baradaran, A.; Shirzad, H.; Rafieian-Kopaei, M. New concepts in nutraceuticals as alternative for pharmaceuticals. Int. J. Prev. Med. 2014, 5, 1487–1499. [Google Scholar]
- Das, L.; Bhaumik, E.; Raychaudhuri, U.; Chakraborty, R. Role of nutraceuticals in human health. J. Food Sci. Technol. 2012, 49, 173–183. [Google Scholar] [CrossRef]
- Umen, J.; Herron, M.D. Green Algal Models for Multicellularity. Annu. Rev. Genet. 2021, 55, 603–632. [Google Scholar] [CrossRef] [PubMed]
- Chénais, B. Algae and Microalgae and Their Bioactive Molecules for Human Health. Molecules 2021, 26, 1185. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Factories 2018, 17, 36. [Google Scholar] [CrossRef]
- Wu, J.Y.; Tso, R.; Teo, H.S.; Haldar, S. The utility of algae as sources of high value nutritional ingredients, particularly for alternative/complementary proteins to improve human health. Front. Nutr. 2023, 10, 1277343. [Google Scholar] [CrossRef]
- Ampofo, J.; Abbey, L. Microalgae: Bioactive Composition, Health Benefits, Safety and Prospects as Potential High-Value Ingredients for the Functional Food Industry. Foods 2022, 11, 1744. [Google Scholar] [CrossRef] [PubMed]
- Babich, O.; Sukhikh, S.; Larina, V.; Kalashnikova, O.; Kashirskikh, E.; Prosekov, A.; Noskova, S.; Ivanova, S.; Fendri, I.; Smaoui, S.; et al. Algae: Study of Edible and Biologically Active Fractions, Their Properties and Applications. Plants 2022, 11, 780. [Google Scholar] [CrossRef]
- Ślusarczyk, J.; Adamska, E.; Czerwik-Marcinkowska, J. Fungi and Algae as Sources of Medicinal and Other Biologically Active Compounds: A Review. Nutrients 2021, 13, 3178. [Google Scholar] [CrossRef]
- Riccio, G.; Lauritano, C. Microalgae with Immunomodulatory Activities. Mar. Drugs 2019, 18, 2. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Prasad, P.; Sreedhar, R.V.; Akhilender Naidu, K.; Shang, X.; Keum, Y.S. Omega-3 Polyunsaturated Fatty Acids (PUFAs): Emerging Plant and Microbial Sources, Oxidative Stability, Bioavailability, and Health Benefits-A Review. Antioxidants 2021, 10, 1627. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Diao, J.; Bi, Y.; Zeng, L.; Wang, F.; Chen, L.; Zhang, W. Rewiring the Metabolic Network to Increase Docosahexaenoic Acid Productivity in Crypthecodinium cohnii by Fermentation Supernatant-Based Adaptive Laboratory Evolution. Front. Microbiol. 2022, 13, 824189. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.K.; Tian, Y.T.; Dai, Y.R.; Wang, D.; Liu, K.C.; Cui, Y.H. Development of an alternative medium via completely replaces the medium components by mixed wastewater and crude glycerol for efficient production of docosahexaenoic acid by Schizochytrium sp. Chemosphere 2022, 291 Pt 1, 132868. [Google Scholar] [CrossRef] [PubMed]
- Jiao, K.; Chang, J.; Zeng, X.; Ng, I.S.; Xiao, Z.; Sun, Y.; Tang, X.; Lin, L. 5-Aminolevulinic acid promotes arachidonic acid biosynthesis in the red microalga Porphyridium purpureum. Biotechnol. Biofuels 2017, 10, 168. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekhar, T.; Riazunnisa, K.; Vijaya Lakshmi, D.; Anu Prasanna, V.; Veera Bram-hachari, P. Exploration of Bioactive Functional Molecules from Marine Algae: Chal-lenges and Applications in Nutraceuticals. In Marine Bioactive Molecules for Biomedical and Pharmacotherapeutic Applications; Veera Bramhachari, P., Berde, C.V., Eds.; Springer: Singapore, 2023. [Google Scholar] [CrossRef]
- Minhas, L.A.; Kaleem, M.; Farooqi, H.M.U.; Kausar, F.; Waqar, R.; Bhatti, T.; Aziz, S.; Jung, D.W.; Mumtaz, A.S. Algae-derived bioactive compounds as potential pharmaceuticals for cancer therapy: A comprehensive review. Algal Res. 2024, 78, 103396. [Google Scholar] [CrossRef]
- Daliu, P.; Santini, A.; Novellino, E. From pharmaceuticals to nutraceuticals: Bridging disease prevention and management. Expert Rev. Clin. Pharmacol. 2019, 12, 1552135. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Santini, A. Nutraceuticals in Human Health. Foods 2020, 9, 370. [Google Scholar] [CrossRef]
- Durazzo, A.; Nazhand, A.; Lucarini, M.; Atanasov, A.G.; Souto, E.B.; Novellino, E.; Capasso, R.; Santini, A. An Updated Overview on Nanonutraceuticals: Focus on Nanoprebiotics and Nanoprobiotics. Int. J. Mol. Sci. 2020, 21, 2285. [Google Scholar] [CrossRef]
- Kim, M.; Kim, Y. Hypocholesterolemic effects of curcumin via up-regulation of cholesterol 7a-hydroxylase in rats fed a high fat diet. Nutr. Res. Pract. 2010, 4, 191–195. [Google Scholar] [CrossRef]
- Manocha, S.; Dhiman, S.; Grewal, A.S.; Guarve, K. Nanotechnology: An approach to overcome bioavailability challenges of nutraceuticals. J. Drug Deliv. Sci. Technol. 2022, 72, 103418. [Google Scholar] [CrossRef]
- Deponte, M. Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 3217–3266. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Fogacci, F.; Fogacci, S. Cardiovascular Risk Factors Management in Pregnancy: A Role for Nutraceuticals? In Nutraceuticals and Cardiovascular Disease; Cicero, A.F., Rizzo, M., Eds.; Contemporary Cardiology; Humana: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Sut, S.; Baldan, V.; Faggian, M.; Peron, G.; Dall Acqua, S. Nutraceuticals, A New Challenge for Medicinal Chemistry. Curr. Med. Chem. 2016, 23, 3198–3223. [Google Scholar] [CrossRef] [PubMed]
- Assadpour, E.; Mahdi Jafari, S. A systematic review on nanoencapsulation of food bioactive ingredients and nutraceuticals by various nanocarriers. Crit. Rev. Food Sci. Nutr. 2019, 59, 3129–3151. [Google Scholar] [CrossRef] [PubMed]
- Carrizzo, A.; Izzo, C.; Forte, M.; Sommella, E.; Di Pietro, P.; Venturini, E.; Ciccarelli, M.; Galasso, G.; Rubattu, S.; Campiglia, P.; et al. A Novel Promising Frontier for Human Health: The Beneficial Effects of Nutraceuticals in Cardiovascular Diseases. Int. J. Mol. Sci. 2020, 21, 8706. [Google Scholar] [CrossRef]
- Calella, P.; Cerullo, G.; Di Dio, M.; Liguori, F.; Di Onofrio, V.; Gallè, F.; Liguori, G. Antioxidant, anti-inflammatory and immunomodulatory effects of spirulina in exercise and sport: A systematic review. Front. Nutr. 2022, 9, 1048258. [Google Scholar] [CrossRef] [PubMed]
- Calella, P.; Di Dio, M.; Cerullo, G.; Di Onofrio, V.; Gallé, F.; Liguori, G. Antioxidant, immunomodulatory, and anti-inflammatory effects of Spirulina in disease conditions: A systematic review. Int. J. Food Sci. Nutr. 2022, 73, 1047–1056. [Google Scholar] [CrossRef]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.D.; Jha, N.K.; Jha, S.K.; Sadek, B.; Ojha, S. Pharmacological and Molecular Insight on the Cardioprotective Role of Apigenin. Nutrients 2023, 15, 385. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.K.; Song, I.S. Pharmacokinetic Drug-Drug Interactions and Herb-Drug Interac-tions. Pharmaceutics 2021, 13, 610. [Google Scholar] [CrossRef]
- Bushra, R.; Aslam, N.; Khan, A.Y. Food-drug interactions. Oman Med. J. 2011, 26, 77–83. [Google Scholar] [CrossRef] [PubMed]
| Nutraceutical | Primary Active Compound | Health Benefits |
|---|---|---|
| Omega-3 Fatty Acids [35,58,59] | EPA, DHA | Anti-inflammatory, cardiovascular health |
| Probiotics [91] | Various live bacteria | Gut health, immune support |
| Resveratrol [93] | Stilbenoids | Antioxidant, antiaging |
| Vitamin D [56,57] | Cholecalciferol, Ergocalciferol | Bone health, immune function |
| Flavonoids [36,94] | Quercetin, Kaempferol, Myricetin | Anti-inflammatory, cardiovascular health, anticarcinogenic, antioxidant |
| Curcumin [46,49] | Curcuminoids | Anti-inflammatory, antioxidant |
| Selenium [95] | Selenomethionine, Selenocysteine | Antioxidant, thyroid function, immune health |
| Coenzyme Q10 [89] | Ubiquinone, Ubiquinol | Antioxidant, heart health |
| Allicin (Garlic) [96,97,98] | Allicin | Anti-inflammatory, antioxidant |
| Anthocyanins [99,100] | Cyanidin, Delphinidin | Anti-inflammatory, antioxidant |
| Soy Isoflavones [101,102] | Genistein, Daidzein | Antioxidant, cardiovascular and bone health, menopausal symptom relief |
| Lycopene [103,104] | Lycopene | Antioxidant, anti-inflammatory, anticarcinogenic, heart health |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dama, A.; Shpati, K.; Daliu, P.; Dumur, S.; Gorica, E.; Santini, A. Targeting Metabolic Diseases: The Role of Nutraceuticals in Modulating Oxidative Stress and Inflammation. Nutrients 2024, 16, 507. https://doi.org/10.3390/nu16040507
Dama A, Shpati K, Daliu P, Dumur S, Gorica E, Santini A. Targeting Metabolic Diseases: The Role of Nutraceuticals in Modulating Oxidative Stress and Inflammation. Nutrients. 2024; 16(4):507. https://doi.org/10.3390/nu16040507
Chicago/Turabian StyleDama, Aida, Kleva Shpati, Patricia Daliu, Seyma Dumur, Era Gorica, and Antonello Santini. 2024. "Targeting Metabolic Diseases: The Role of Nutraceuticals in Modulating Oxidative Stress and Inflammation" Nutrients 16, no. 4: 507. https://doi.org/10.3390/nu16040507
APA StyleDama, A., Shpati, K., Daliu, P., Dumur, S., Gorica, E., & Santini, A. (2024). Targeting Metabolic Diseases: The Role of Nutraceuticals in Modulating Oxidative Stress and Inflammation. Nutrients, 16(4), 507. https://doi.org/10.3390/nu16040507







