Calcium Deficiency Decreases Bone Mass without Affecting Adiposity in Ovariectomized Rats Fed a High-Fat Diet
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Diet, and Treatments
2.2. Sample Collection and Preparation
2.3. Body Composition
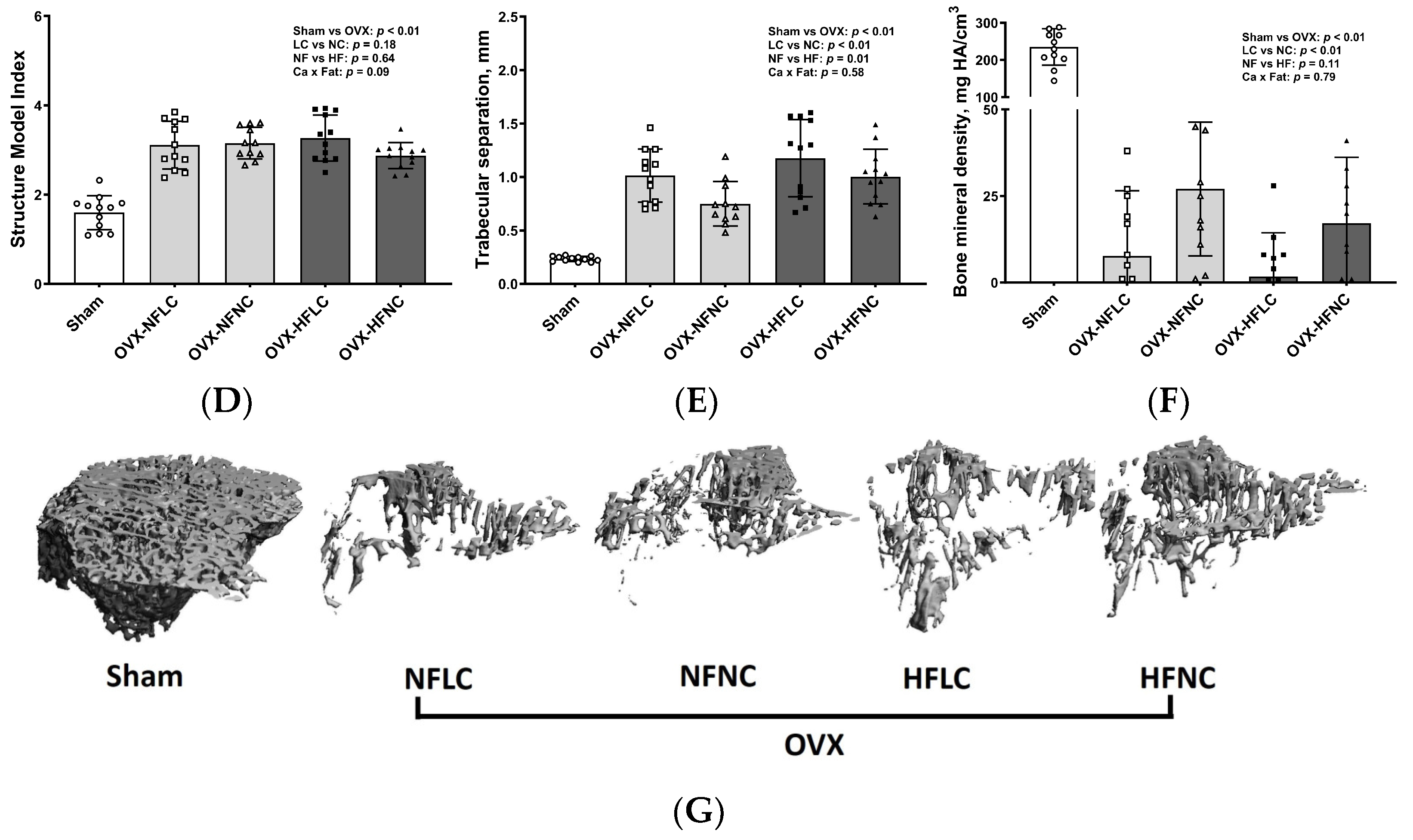
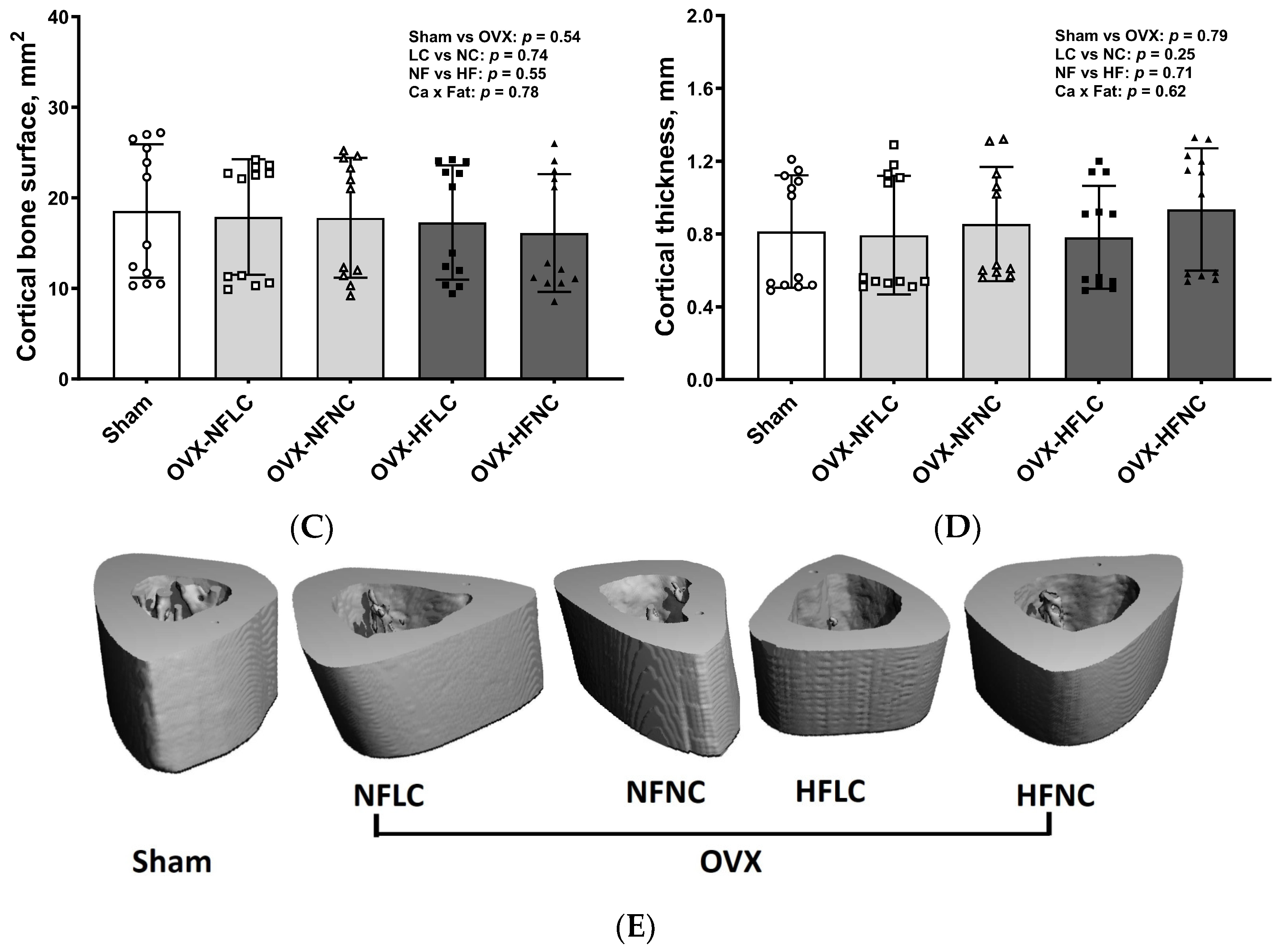
2.4. Bone Structural and Mechanical Evaluation with Micro Computed Tomography (µCT)
2.5. Femur Dry Weight and Mineral Analysis
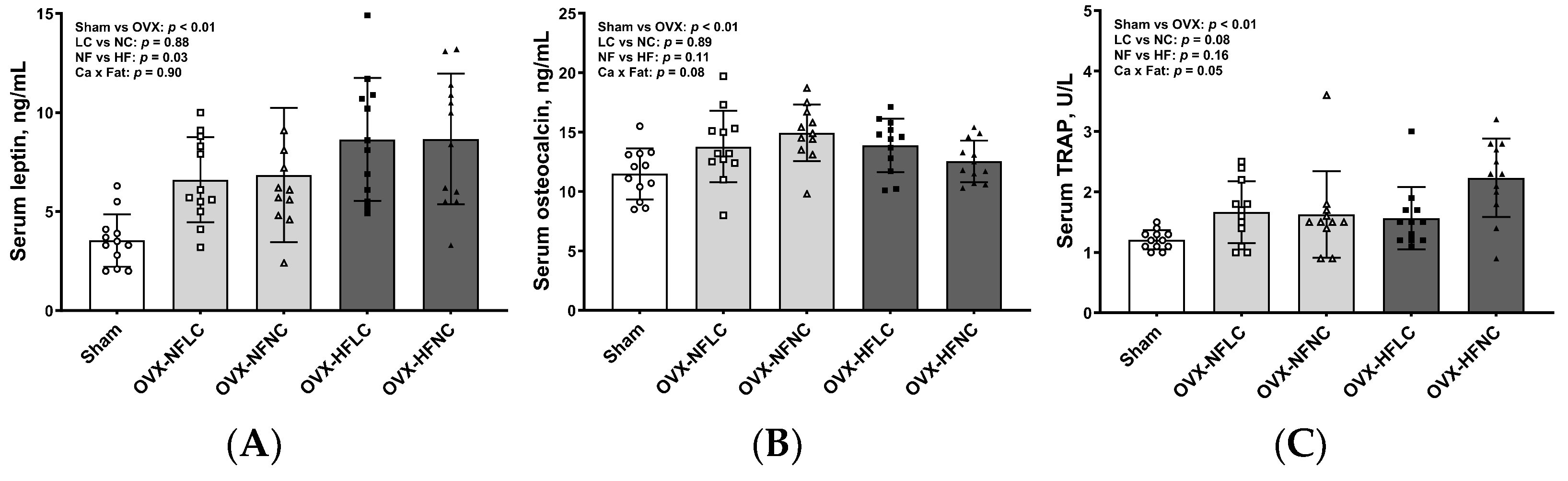
2.6. Measurements of Serum Biochemical Markers
2.7. Statistical Analyses
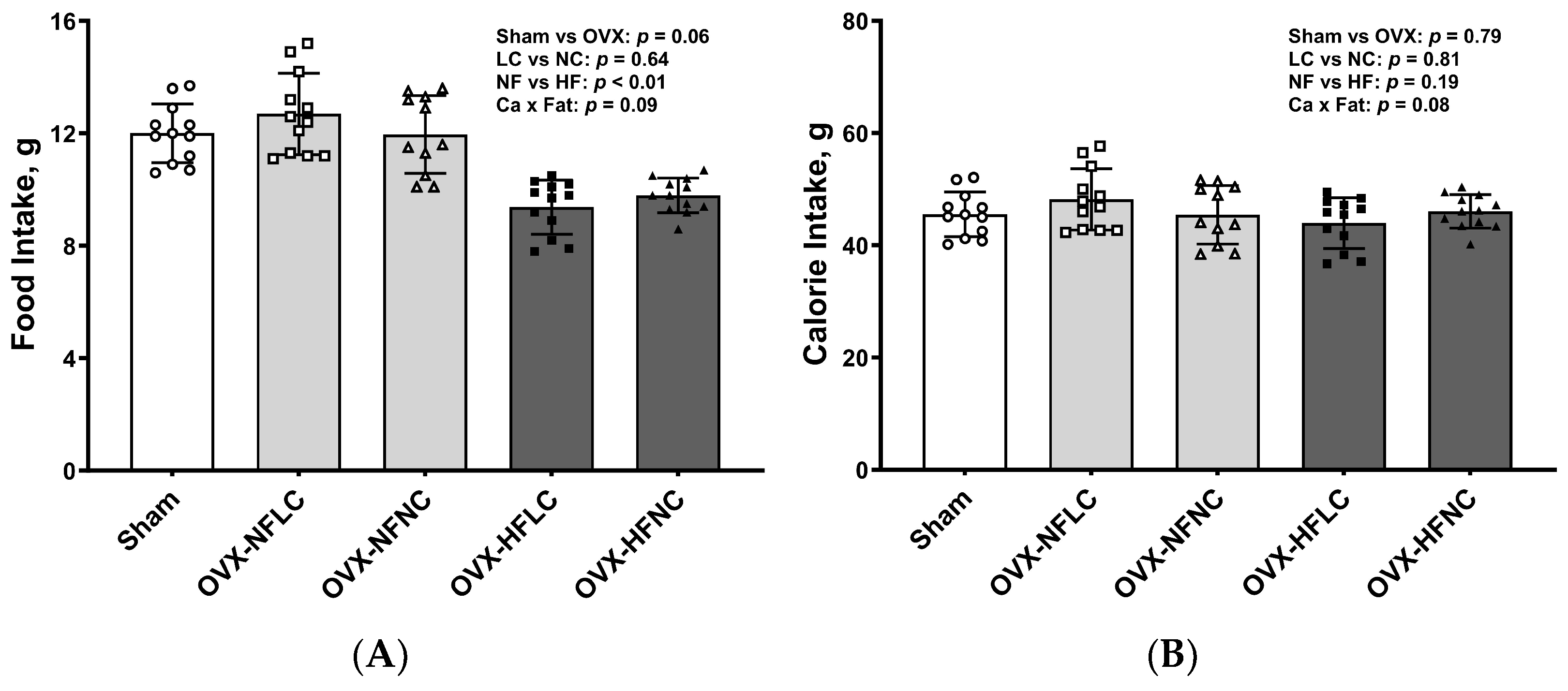
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weaver, C.M. The growing years and prevention of osteoporosis in later life. Proc. Nutr. Soc. 2000, 59, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Tanski, W.; Kosiorowska, J.; Szymanska-Chabowska, A. Osteoporosis—Risk factors, pharmaceutical and non-pharmaceutical treatment. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 3557–3566. [Google Scholar] [CrossRef] [PubMed]
- Persson, P.; Gagnemo-Persson, R.; Hakanson, R. The effect of high or low dietary calcium on bone and calcium homeostasis in young male rats. Calcif. Tissue Int. 1993, 52, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Seto, H.; Aoki, K.; Kasugai, S.; Ohya, K. Trabecular bone turnover, bone marrow cell development, and gene expression of bone matrix proteins after low calcium feeding in rats. Bone 1999, 25, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Shapses, S.A.; Sukumar, D. Bone metabolism in obesity and weight loss. Annu. Rev. Nutr. 2012, 32, 287–309. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.J.; Liu, Y.J.; Liu, P.Y.; Hamilton, J.; Recker, R.R.; Deng, H.W. Relationship of obesity with osteoporosis. J. Clin. Endocrinol. Metab. 2007, 92, 1640–1646. [Google Scholar] [CrossRef] [PubMed]
- Rosen, C.J.; Bouxsein, M.L. Mechanisms of disease: Is osteoporosis the obesity of bone? Nat. Clin. Pract. Rheumatol. 2006, 2, 35–43. [Google Scholar] [CrossRef]
- Kyung, T.W.; Lee, J.E.; Phan, T.V.; Yu, R.; Choi, H.S. Osteoclastogenesis by bone marrow-derived macrophages is enhanced in obese mice. J. Nutr. 2009, 139, 502–506. [Google Scholar] [CrossRef]
- Harahap, I.A.; Landrier, J.F.; Suliburska, J. Interrelationship between Vitamin D and Calcium in Obesity and Its Comorbid Conditions. Nutrients 2022, 14, 3187. [Google Scholar] [CrossRef]
- Pannu, P.K.; Calton, E.K.; Soares, M.J. Calcium and Vitamin D in Obesity and Related Chronic Disease. Adv. Food Nutr. Res. 2016, 77, 57–100. [Google Scholar] [CrossRef]
- Zemel, M.B.; Shi, H.; Greer, B.; Dirienzo, D.; Zemel, P.C. Regulation of adiposity by dietary calcium. FASEB J. 2000, 14, 1132–1138. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, J.T.; Rumbold, P.L.; Stevenson, E.J. Effect of calcium intake on fat oxidation in adults: A meta-analysis of randomized, controlled trials. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2012, 13, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.S.; Smith, D.L., Jr.; Nagy, T.R. Validation of quantitative magnetic resonance (QMR) for determination of body composition in rats. Int. J. Body Compos. Res. 2009, 7, 99–107. [Google Scholar] [PubMed]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Muller, R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef] [PubMed]
- Riggs, B.L.; Khosla, S.; Melton, L.J., 3rd. Sex steroids and the construction and conservation of the adult skeleton. Endocr. Rev. 2002, 23, 279–302. [Google Scholar] [CrossRef] [PubMed]
- Ley, C.J.; Lees, B.; Stevenson, J.C. Sex- and menopause-associated changes in body-fat distribution. Am. J. Clin. Nutr. 1992, 55, 950–954. [Google Scholar] [CrossRef]
- Chen, Y.; Heiman, M.L. Increased weight gain after ovariectomy is not a consequence of leptin resistance. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E315–E322. [Google Scholar] [CrossRef]
- McElroy, J.F.; Wade, G.N. Short- and long-term effects of ovariectomy on food intake, body weight, carcass composition, and brown adipose tissue in rats. Physiol. Behav. 1987, 39, 361–365. [Google Scholar] [CrossRef]
- Cao, J.J.; Gregoire, B.R. A high-fat diet increases body weight and circulating estradiol concentrations but does not improve bone structural properties in ovariectomized mice. Nutr. Res. 2016, 36, 320–327. [Google Scholar] [CrossRef]
- Brown, J.L.; Spicer, M.T.; Spicer, L.J. Effect of high-fat diet on body composition and hormone responses to glucose tolerance tests. Endocrine 2002, 19, 327–332. [Google Scholar] [CrossRef]
- Tortoriello, D.V.; McMinn, J.; Chua, S.C. Dietary-induced obesity and hypothalamic infertility in female DBA/2J mice. Endocrinology 2004, 145, 1238–1247. [Google Scholar] [CrossRef]
- Cao, J.J. Caloric restriction combined with exercise is effective in reducing adiposity and mitigating bone structural deterioration in obese rats. Ann. N. Y. Acad. Sci. 2018, 1433, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Shu, L.; Beier, E.; Sheu, T.; Zhang, H.; Zuscik, M.J.; Puzas, E.J.; Boyce, B.F.; Mooney, R.A.; Xing, L. High-fat diet causes bone loss in young mice by promoting osteoclastogenesis through alteration of the bone marrow environment. Calcif. Tissue Int. 2015, 96, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Sato, A.; Kawano, H.; Notsu, T.; Ohta, M.; Nakakuki, M.; Mizuguchi, K.; Itoh, M.; Suganami, T.; Ogawa, Y. Antiobesity effect of eicosapentaenoic acid in high-fat/high-sucrose diet-induced obesity: Importance of hepatic lipogenesis. Diabetes 2010, 59, 2495–2504. [Google Scholar] [CrossRef] [PubMed]
- Zemel, M.B.; Miller, S.L. Dietary calcium and dairy modulation of adiposity and obesity risk. Nutr. Rev. 2004, 62, 125–131. [Google Scholar] [CrossRef]
- Zemel, M.B.; Thompson, W.; Milstead, A.; Morris, K.; Campbell, P. Calcium and dairy acceleration of weight and fat loss during energy restriction in obese adults. Obes. Res. 2004, 12, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Kamycheva, E.; Joakimsen, R.M.; Jorde, R. Intakes of calcium and vitamin d predict body mass index in the population of Northern Norway. J. Nutr. 2003, 133, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Paradis, S.; Cabanac, M. Calcium deficiency cannot induce obesity in rats. Physiol. Behav. 2005, 85, 259–264. [Google Scholar] [CrossRef]
- Loos, R.J.; Rankinen, T.; Leon, A.S.; Skinner, J.S.; Wilmore, J.H.; Rao, D.C.; Bouchard, C. Calcium intake is associated with adiposity in Black and White men and White women of the HERITAGE Family Study. J. Nutr. 2004, 134, 1772–1778. [Google Scholar] [CrossRef]
- Shapses, S.A.; Heshka, S.; Heymsfield, S.B. Effect of calcium supplementation on weight and fat loss in women. J. Clin. Endocrinol. Metab. 2004, 89, 632–637. [Google Scholar] [CrossRef]
- Zhang, Q.; Tordoff, M.G. No effect of dietary calcium on body weight of lean and obese mice and rats. Am. J. Physiology. Regul. Integr. Comp. Physiol. 2004, 286, R669–R677. [Google Scholar] [CrossRef][Green Version]
- Parfitt, A.M. Misconceptions (2): Turnover is always higher in cancellous than in cortical bone. Bone 2002, 30, 807–809. [Google Scholar] [CrossRef]
- Morgan, E.F.; Barnes, G.L.; Einhorn, T.A. The bone organ system: Form and function. In Osteoporosis, 3rd ed.; Marcus, R., Feldman, D., Nelson, D.A., Rosen, C.J., Eds.; Elsevier Academic Press: Burlington, MA, USA, 2008; Volume 1, pp. 3–25. [Google Scholar]
- Ott, S.M. Cortical or Trabecular Bone: What’s the Difference? Am. J. Nephrol. 2018, 47, 373–375. [Google Scholar] [CrossRef]
- Thomas, T. Intermittent parathyroid hormone therapy to increase bone formation. Jt. Bone Spine 2006, 73, 262–269. [Google Scholar] [CrossRef]



| Ingredient | NFLC | NFNC | HFLC | HFNC |
|---|---|---|---|---|
| g | ||||
| Casein, 80 Mesh | 200 | 200 | 200 | 200 |
| L-Cystine | 3.0 | 3.0 | 3.0 | 3.0 |
| Corn starch | 315 | 315 | 72.8 | 72.8 |
| Maltodextrin 10 | 35 | 35 | 100 | 100 |
| Sucrose | 350 | 350 | 172.8 | 172.8 |
| Cellulose, BW200 | 50 | 50 | 50 | 50 |
| Soybean oil | 25.0 | 25.0 | 25.0 | 25.0 |
| Lard | 20 | 20 | 177.5 | 177.5 |
| Mineral mix 2 | 10.0 | 10.0 | 10.0 | 10.0 |
| Dicalcium phosphate | 3.4 | 13.0 | 3.4 | 13.0 |
| Calcium carbonate | 0 | 5.5 | 0 | 5.5 |
| Potassium citrate, H2O | 8.89 | 16.5 | 8.89 | 16.5 |
| Potassium phosphate | 9.6 | 0 | 9.6 | 0 |
| Vitamin mix 3 | 10.0 | 10.0 | 11.1 | 12.5 |
| Choline bitartrate | 2.0 | 2.0 | 2.2 | 2.5 |
| Total weight, g | 1042 | 1055 | 845 | 858 |
| Energy, kcal/g diet | 3.89 | 3.85 | 4.80 | 4.73 |
| % Energy | ||||
| Carbohydrate | 70 | 70 | 35 | 35 |
| Protein | 20 | 20 | 22 | 25 |
| Fat | 10 | 10 | 45 | 45 |
| Ca, g/4057 kcal | 1.0 | 6.0 | 1.0 | 6.0 |
| Weight (mg) | Femur Weight/BW (%) | Calcium (mg/g) | Phosphorus (mg/g) | Ca:P | Magnesium (mg/g) | Copper (µg/g) | Iron (µg/g) | Zinc (µg/g) | Manganese (µg/g) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Sham | 690 ± 73 | 0.24 ± 0.01 | 220 ± 19 | 89.1 ± 7.4 | 2.47 ± 0.21 | 2.14 ± 0.16 | 1.70 ± 0.48 | 57.3 ± 11.2 | 216 ± 15 | 0.80 ± 0.04 |
| OVX-NFLC | 653 ± 35 | 0.20 ± 0.02 | 194 ± 21 | 90.1 ± 4.7 | 2.16 ± 0.28 | 2.15 ± 0.10 | 1.13 ± 0.09 | 52.2 ± 5.1 | 219 ± 17 | 0.76 ± 0.05 |
| OVX-NFNC | 682 ± 62 | 0.21 ± 0.02 | 210 ± 18 | 90.4 ± 6.9 | 2.33 ± 0.20 | 2.13 ± 0.11 | 1.14 ± 0.10 | 48.8 ± 8.8 | 212.5 ± 11.5 | 0.74 ± 0.06 |
| OVX-HFLC | 657 ± 38 | 0.19 ± 0.01 | 221 ± 8 | 90.8 ± 5.2 | 2.44 ± 0.11 | 2.11 ± 0.05 | 1.17 ± 0.17 | 54.1 ± 9.8 | 227.3 ± 9.5 | 0.74 ± 0.06 |
| OVX-HFNC | 699 ± 38 | 0.20 ± 0.02 | 238 ± 21 | 92.2 ± 2.6 | 2.59 ± 0.24 | 2.19 ± 0.07 | 1.26 ± 0.07 | 56.3 ± 10.4 | 234.0 ± 10.6 | 0.78 ± 0.05 |
| Ca | <0.01 | 0.36 | <0.01 | 0.57 | 0.02 | 0.30 | 0.22 | 0.82 | 0.93 | 0.47 |
| Fat | 0.41 | 0.04 | <0.01 | 0.38 | <0.01 | 0.67 | 0.04 | 0.07 | <0.01 | 0.37 |
| Ca × Fat | 0.57 | 0.85 | 0.93 | 0.70 | 0.84 | 0.06 | 0.37 | 0.28 | 0.09 | 0.09 |
| Sham vs. OVX | 0.28 | <0.01 | 0.63 | 0.33 | 0.27 | 0.87 | <0.01 | 0.16 | 0.13 | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, J.J.; Gregoire, B.R. Calcium Deficiency Decreases Bone Mass without Affecting Adiposity in Ovariectomized Rats Fed a High-Fat Diet. Nutrients 2024, 16, 478. https://doi.org/10.3390/nu16040478
Cao JJ, Gregoire BR. Calcium Deficiency Decreases Bone Mass without Affecting Adiposity in Ovariectomized Rats Fed a High-Fat Diet. Nutrients. 2024; 16(4):478. https://doi.org/10.3390/nu16040478
Chicago/Turabian StyleCao, Jay J., and Brian R. Gregoire. 2024. "Calcium Deficiency Decreases Bone Mass without Affecting Adiposity in Ovariectomized Rats Fed a High-Fat Diet" Nutrients 16, no. 4: 478. https://doi.org/10.3390/nu16040478
APA StyleCao, J. J., & Gregoire, B. R. (2024). Calcium Deficiency Decreases Bone Mass without Affecting Adiposity in Ovariectomized Rats Fed a High-Fat Diet. Nutrients, 16(4), 478. https://doi.org/10.3390/nu16040478





