Incidence of Urinary Infections and Behavioral Risk Factors
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample Size Determination
2.3. Sample Size Pilot Study
2.4. Questionnaire Validation
2.5. Statistical Analysis
3. Results
3.1. Socio-Demographic Characteristics
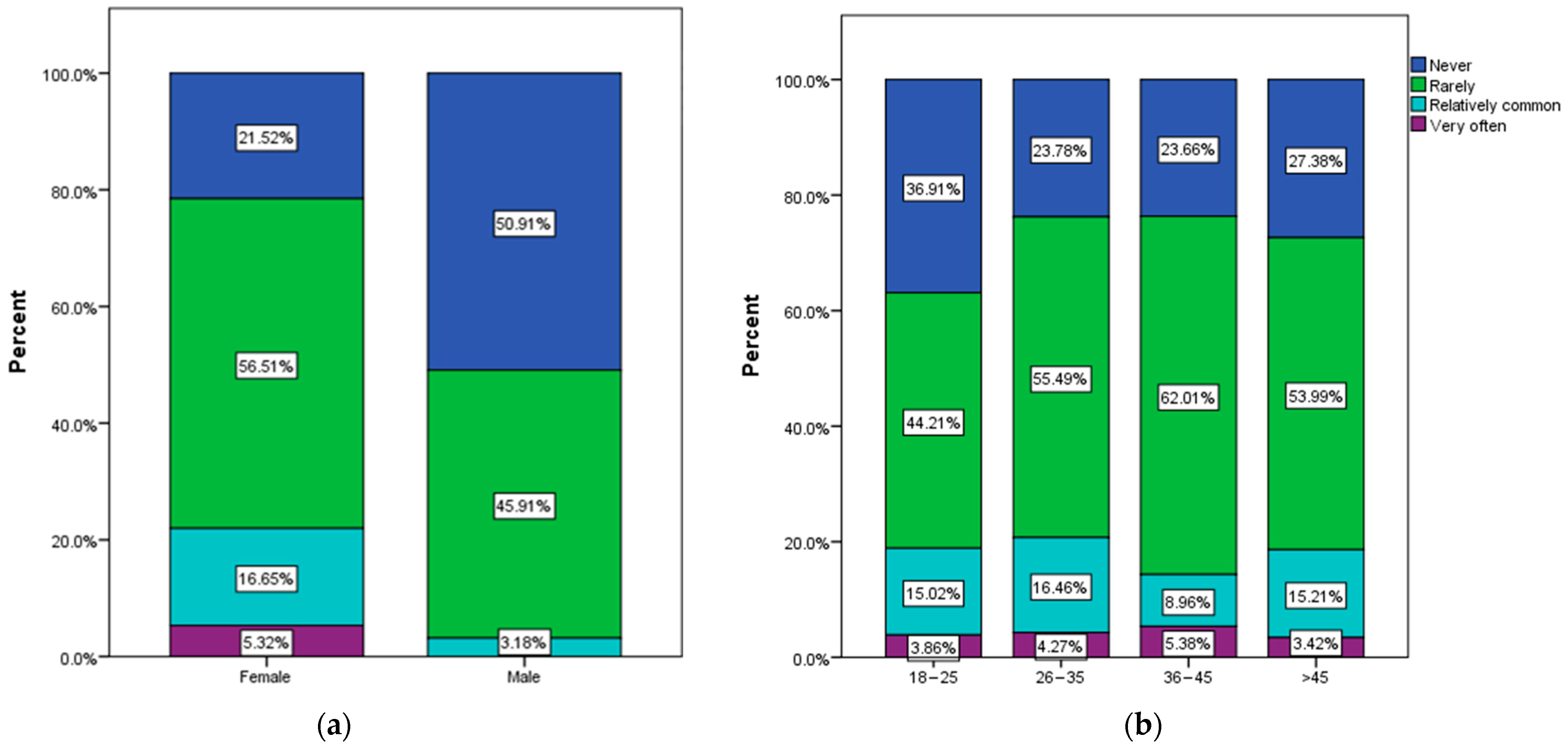
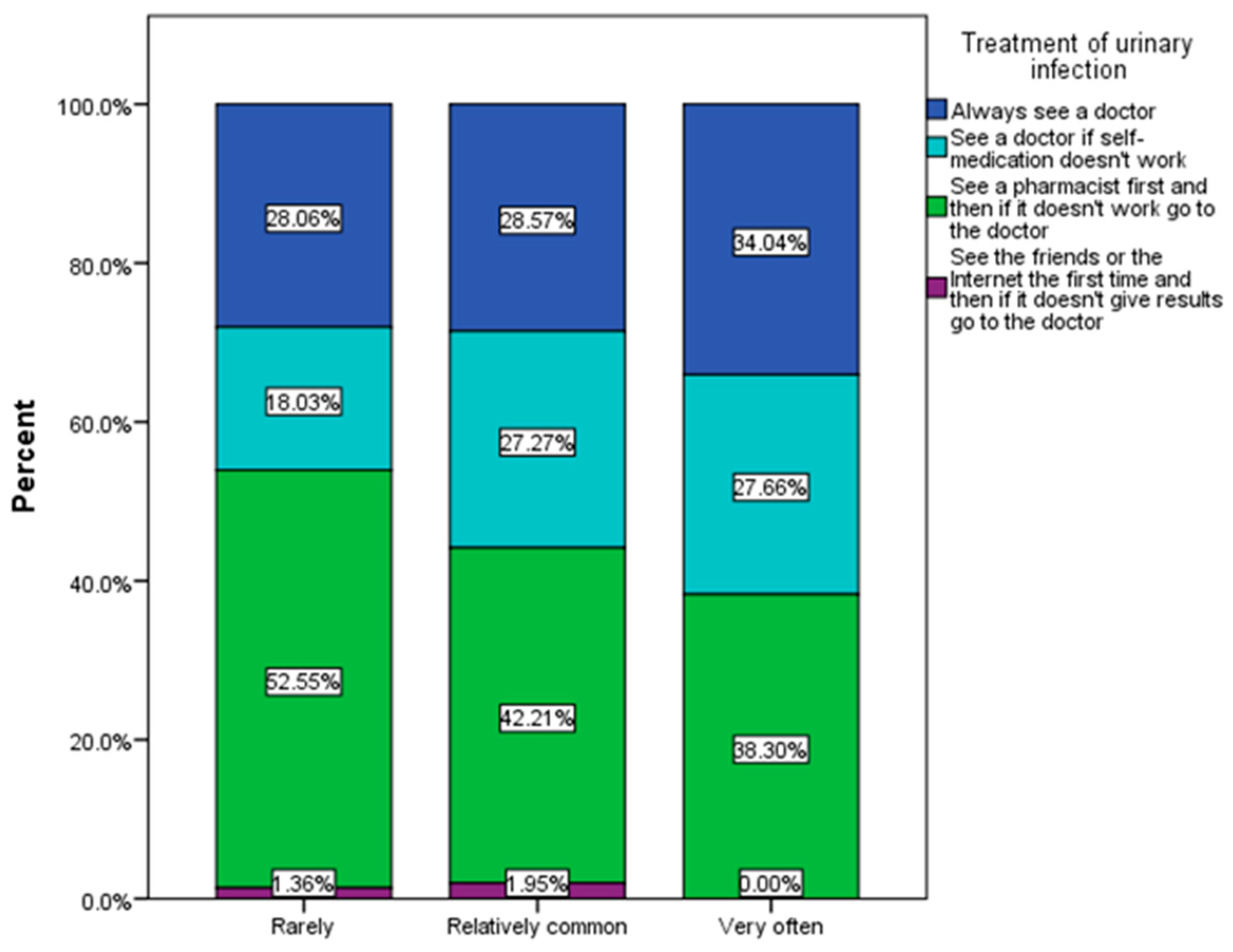
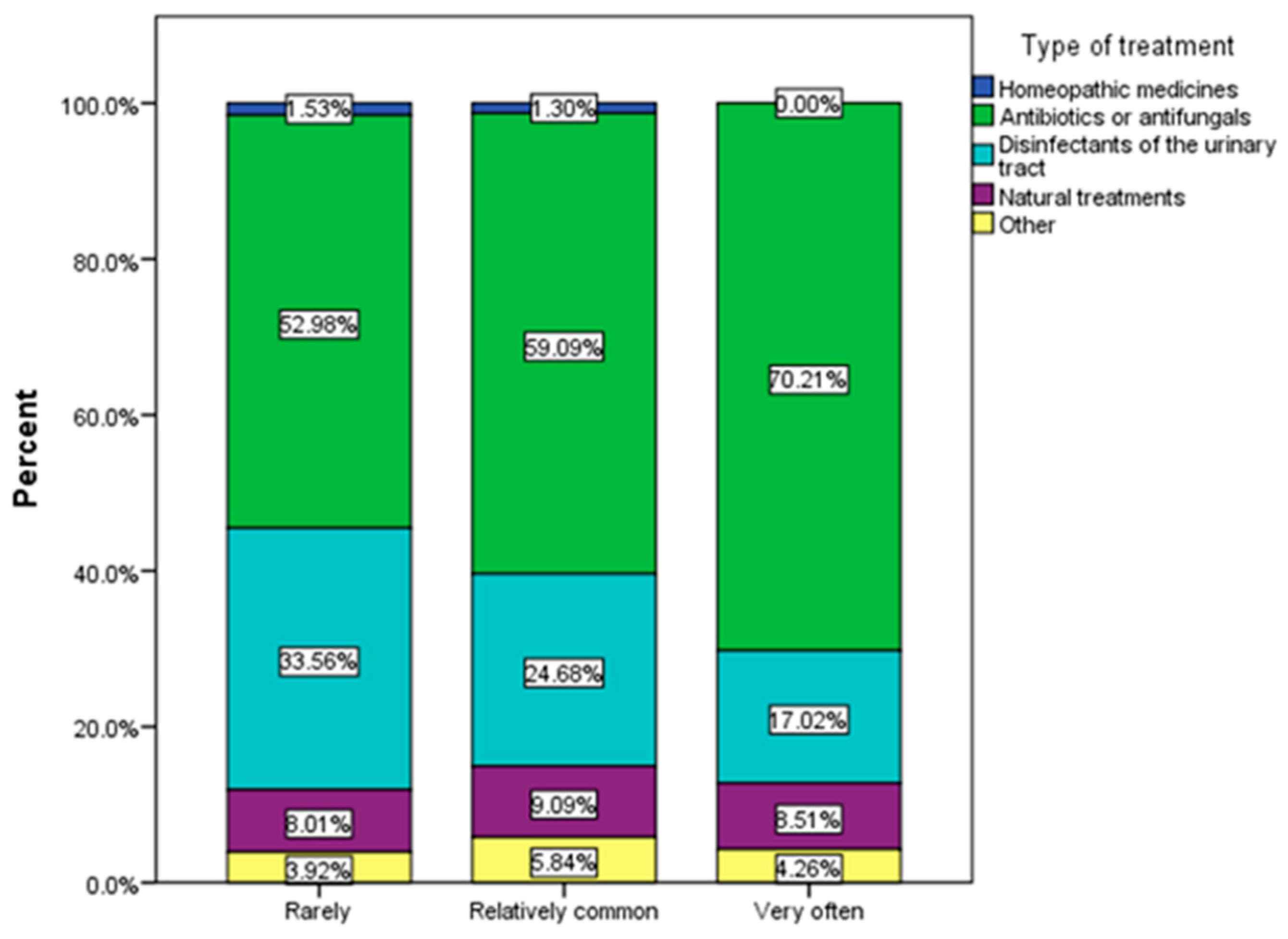
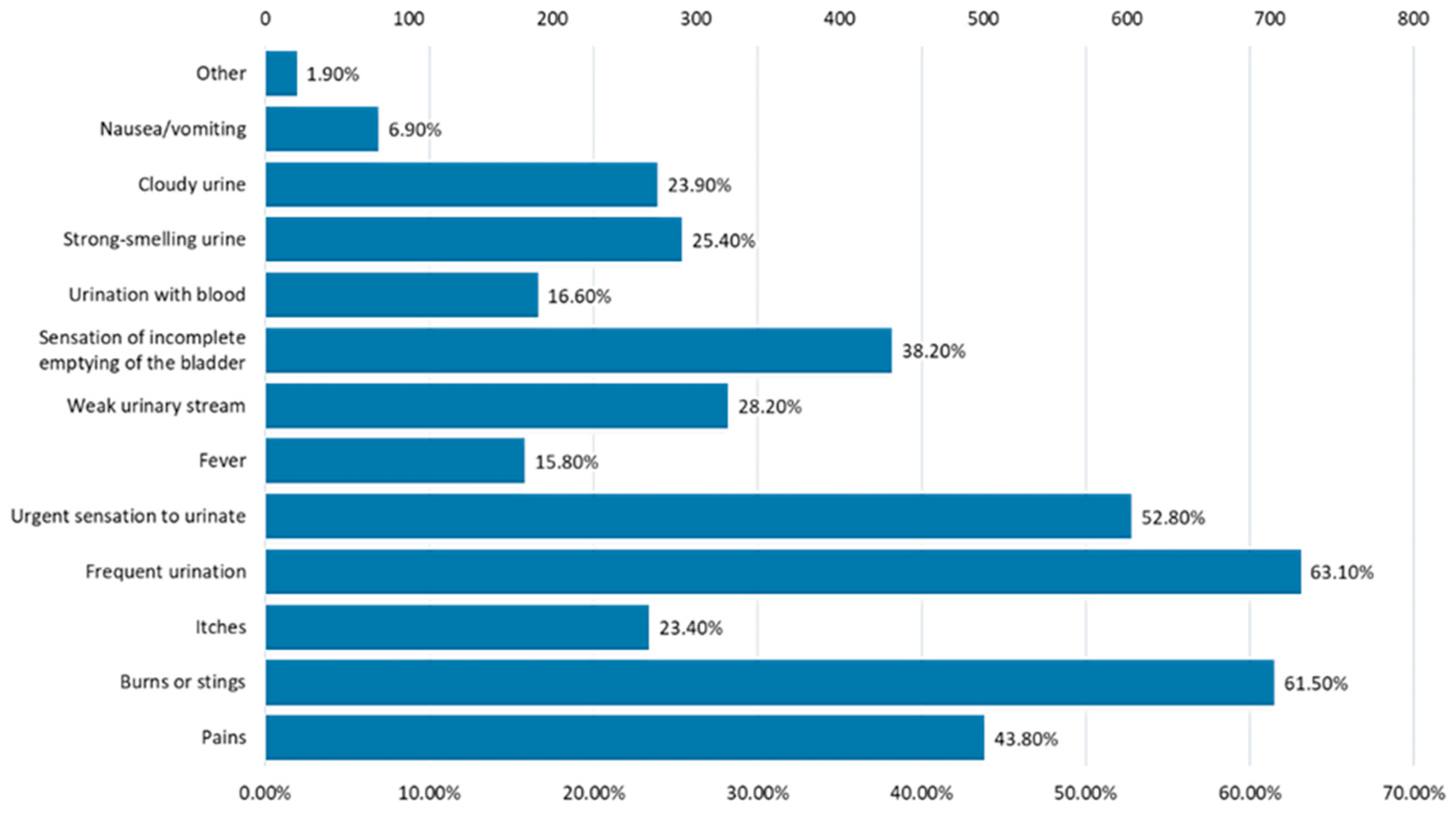
3.2. Frequency and Causes of Urinary Infections
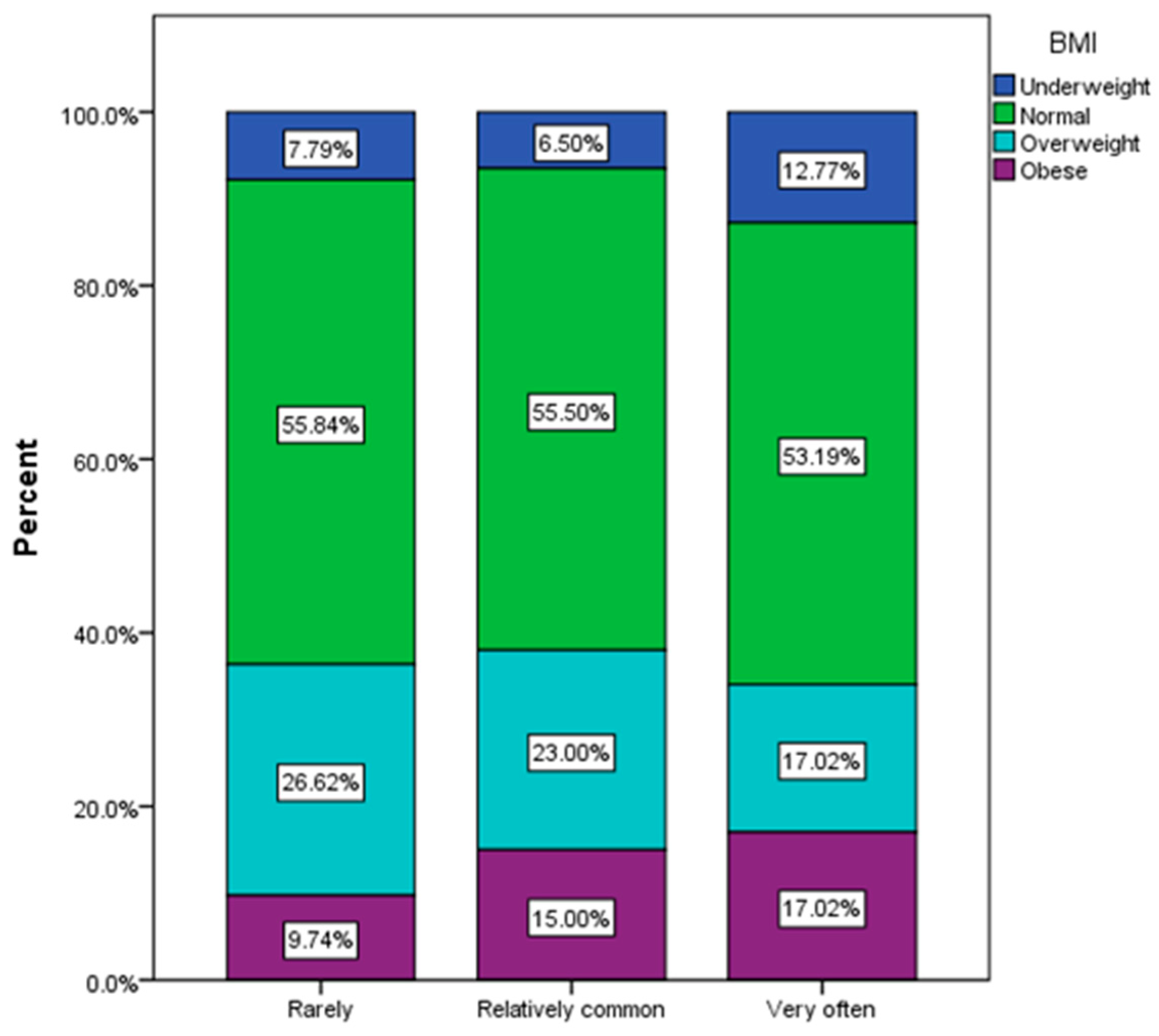
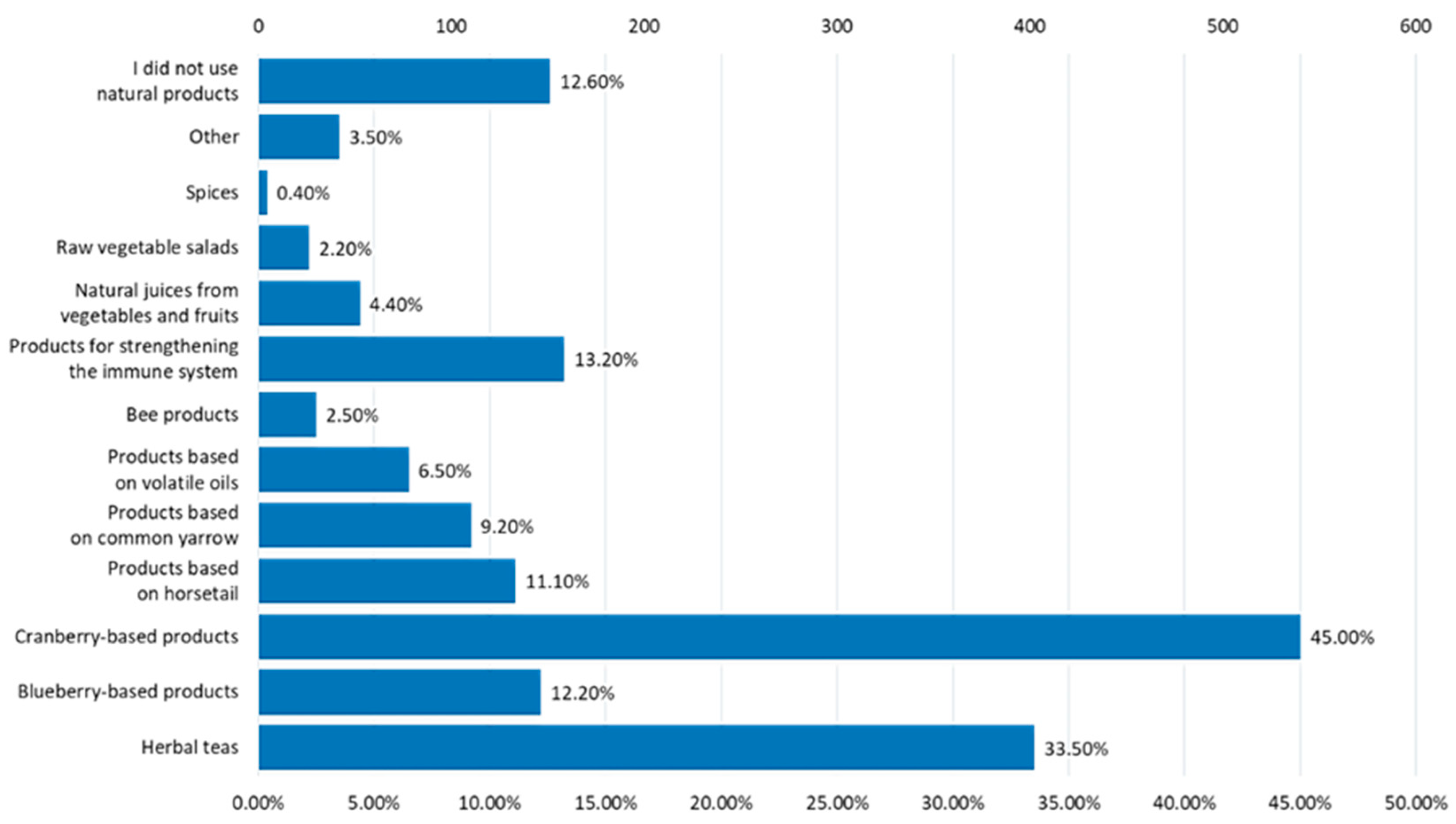
3.3. Assessment of Behavioral Risk Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zeng, Z.; Zhan, J.; Zhang, K.; Chen, H.; Cheng, S. Global, Regional, and National Burden of Urinary Tract Infections from 1990 to 2019: An Analysis of the Global Burden of Disease Study 2019. World J. Urol. 2022, 40, 755–763. [Google Scholar] [CrossRef]
- Yang, X.; Chen, H.; Zheng, Y.; Qu, S.; Wang, H.; Yi, F. Disease Burden and Long-Term Trends of Urinary Tract Infections: A Worldwide Report. Front. Public Health 2022, 10, 888205. [Google Scholar] [CrossRef] [PubMed]
- Urinary Tract Infection|Antibiotic Use|CDC. Available online: https://www.cdc.gov/antibiotic-use/uti.html (accessed on 7 August 2023).
- Behzadi, P.; Behzadi, E.; Yazdanbod, H.; Aghapour, R.; Akbari Cheshmeh, M.; Salehian Omran, D. A Survey on Urinary Tract Infections Associated with the Three Most Common Uropathogenic Bacteria. Maedica 2010, 5, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Petca, R.C.; Mareș, C.; Petca, A.; Negoiță, S.; Popescu, R.I.; Boț, M.; Barabás, E.; Chibelean, C.B. Spectrum and Antibiotic Resistance of Uropathogens in Romanian Females. Antibiotics 2020, 9, 472. [Google Scholar] [CrossRef] [PubMed]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary Tract Infections: Epidemiology, Mechanisms of Infection and Treatment Options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef]
- Storme, O.; Saucedo, J.T.; Garcia-Mora, A.; Dehesa-Dávila, M.; Naber, K.G. Risk Factors and Predisposing Conditions for Urinary Tract. Ther. Adv. Urol. 2019, 11, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Patangia, D.V.; Anthony Ryan, C.; Dempsey, E.; Paul Ross, R.; Stanton, C. Impact of Antibiotics on the Human Microbiome and Consequences for Host Health. Microbiologyopen 2022, 11, e1260. [Google Scholar] [CrossRef]
- Nicolle, L.E.; Evans, G.; Laverdieve, M.; Phillips, P.; Quan, C.; Rotstein, C. Complicated Urinary Tract Infection in Adults. Can. J. Infect. Dis. Med. Microbiol. 2005, 16, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Bono, M.J.; Leslie, S.W.; Reygaert, W.C. Uncomplicated Urinary Tract Infections; Updated 28 November 2022; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470195/ (accessed on 10 October 2023).
- Al-Badr, A.; Al-Shaikh, G. Recurrent Urinary Tract Infections Management in Women: A Review. Sultan Qaboos Univ. Med. J. 2013, 13, 359. [Google Scholar] [CrossRef]
- Valdevenito, S.J.P. Infección urinaria recurrente en la mujer [Recurrent urinary tract infection in women]. Rev. Chil. Infectol. 2008, 25, 268–276. [Google Scholar] [CrossRef]
- Godaly, G.; Ambite, I.; Svanborg, C. Innate immunity and genetic determinants of urinary tract infection susceptibility. Curr. Opin. Infect. Dis. 2015, 28, 88–96. [Google Scholar] [CrossRef]
- Chuang, F.C.; Kuo, H.C. Increased urothelial cell apoptosis and chronic inflammation are associated with recurrent urinary tract infection in women. PLoS ONE 2013, 8, e63760. [Google Scholar] [CrossRef] [PubMed]
- Semins, M.J.; Shore, A.D.; Makary, M.A.; Weiner, J.; Matlaga, B.R. The impact of obesity on urinary tract infection risk. Urology 2012, 79, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Bergamin, P.A.; Kiosoglous, A.J. Non-surgical management of recurrent urinary tract infections in women. Transl. Androl. Urol. 2017, 6 (Suppl. S2), S142–S152. [Google Scholar] [CrossRef]
- Singh, N.P.; Ingle, G.K.; Saini, V.K.; Jami, A.; Beniwal, P.; Lal, M.; Meena, G.S. Prevalence of low glomerular filtration rate, proteinuria and associated risk factors in North India using Cockcroft-Gault and Modification of Diet in Renal Disease equation: An observational, cross-sectional study. BMC Nephrol. 2009, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Tavakol, Z.; Ghannadi, S.; Tabesh, M.R.; Halabchi, F.; Noormohammadpour, P.; Akbarpour, S.; Alizadeh, Z.; Nezhad, M.H.; Reyhan, S.K. Relationship between physical activity, healthy lifestyle and COVID-19 disease severity; a cross-sectional study. Z. Gesundh. Wiss. 2023, 31, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, F.; Wang, L.; Wang, W.; Liu, B.; Liu, J.; Chen, M.; He, Q.; Liao, Y.; Yu, X.; et al. Prevalence of chronic kidney disease in China: A cross-sectional survey. Lancet 2012, 379, 815–822, Erratum in Lancet 2012, 380, 650. [Google Scholar] [CrossRef]
- Wallace, T.C.; Blumberg, J.B.; Johnson, E.J.; Shao, A. Dietary bioactives: Establishing a scientific framework for recommended intakes. Adv. Nutr. 2015, 6, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.C.; Holmes, B.A.; Cotillard, A.; Habi-Rachedi, F.; Brazeilles, R.; Gougis, S.; Gausserès, N.; Cani, P.D.; Fellahi, S.; Bastard, J.P.; et al. Dietary patterns differently associate with inflammation and gut microbiota in overweight and obese subjects. PLoS ONE 2014, 9, e109434. [Google Scholar] [CrossRef] [PubMed]
- Klurfeld, D.M.; Davis, C.D.; Karp, R.W.; Allen-Vercoe, E.; Chang, E.B.; Chassaing, B.; Fahey, G.C., Jr.; Hamaker, B.R.; Holscher, H.D.; Lampe, J.W.; et al. Considerations for best practices in studies of fiber or other dietary components and the intestinal microbiome. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E1087–E1097. [Google Scholar] [CrossRef]
- Holscher, H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef]
- Samtiya, M.; Aluko, R.E.; Dhewa, T.; Moreno-Rojas, J.M. Potential Health Benefits of Plant Food-Derived Bioactive Components: An Overview. Foods 2021, 10, 839. [Google Scholar] [CrossRef]
- Erlund, I. Review of the flavonoids quercetin, hesperetin, and naringenin. Dietary sources, bioactivities, bioavailability, and epidemiology. Nutr. Res. 2004, 24, 851–874. [Google Scholar] [CrossRef]
- D’Andrea, G. Quercetin: A flavonol with multifaceted therapeutic applications? Fitoterapia 2015, 106, 256–271. [Google Scholar] [CrossRef] [PubMed]
- Mojikon, F.D.; Kasimin, M.E.; Molujin, A.M.; Gansau, J.A.; Jawan, R. Probiotication of Nutritious Fruit and Vegetable Juices: An Alternative to Dairy-Based Probiotic Functional Products. Nutrients 2022, 14, 3457. [Google Scholar] [CrossRef]
- Martins, E.M.F.; Ramos, A.M.; Martins, M.L.; Leite Junior, B.R.C. Fruit salad as a new vehicle for probiotic bacteria. Food Sci. Technol. 2016, 36, 540–548. [Google Scholar] [CrossRef]
- Mititelu, M.; Nicolescu, T.O.; Ioniţă, C.A.; Nicolescu, F. Heavy Metals Analisys in Some Wild Edible Mushrooms. J. Environ. Prot. Ecol. 2012, 13, 875–879. [Google Scholar]
- Ioniţă, A.C.; Ghica, M.; Moroşan, E.; Nicolescu, F.; Mititelu, M. In vitro effects of some synthesized aminoacetanilide n’-substituted on human leukocytes separated from peripheral blood. Farmacia 2019, 67, 684–690. [Google Scholar] [CrossRef]
- Mititelu, M.; Ioniţă, A.C.; Moroşan, E. Research regarding integral processing of mussels from Black Sea. Farmacia 2014, 62, 625–632. [Google Scholar]
- Mititelu, M.; Neacsu, S.M.; Oprea, E.; Dumitrescu, D.-E.; Nedelescu, M.; Drăgănescu, D.; Nicolescu, T.O.; Rosca, A.C.; Ghica, M. Black Sea Mussels Qualitative and Quantitative Chemical Analysis: Nutritional Benefits and Possible Risks through Consumption. Nutrients 2022, 14, 964. [Google Scholar] [CrossRef]
- Jequier, E.; Constant, F. Water as an essential nutrient: The physiological basis of hydration. Eur. J. Nutr. 2010, 64, 115–123. [Google Scholar] [CrossRef]
- Lorenzo, I.; Serra-Prat, M.; Yébenes, J.C. The Role of Water Homeostasis in Muscle Function and Frailty: A Review. Nutrients 2019, 11, 1857. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Watanabe, H.; Tanaka, A.; Yasui, M.; Nishihira, J.; Murayama, N. Effect of Increased Daily Water Intake and Hydration on Health in Japanese Adults. Nutrients 2020, 12, 1191. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, L.E.; Ganio, M.S.; Casa, D.J.; Lee, E.C.; MacDermott, B.P.; Klau, J.F.; Jimenez, L.; Lieberman, H.R. Mild dehydration affects mood in healthy young women. J. Nutr. 2012, 142, 328–388. [Google Scholar] [CrossRef] [PubMed]
- Kontiokari, T.; Laitinen, J.; Järvi, L.; Pokka, T.; Sundqvist, K.; Uhari, M. Dietary factors protecting women from urinary tract infection. Am. J. Clin. Nutr. 2003, 77, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Wang, S.; Zhu, Y.; Wang, Z.; Zhao, M.; Chen, D.; Zhou, C. Behavioral and dietary risk factors of recurrent urinary tract infection in Chinese postmenopausal women: A case-control study. J. Int. Med. Res. 2020, 48, 300060519889448. [Google Scholar] [CrossRef] [PubMed]
- Jelly, P.; Verma, R.; Kumawat, R.; Choudhary, S.; Chadha, L.; Sharma, R. Occurrence of urinary tract infection and preventive strategies practiced by female students at a tertiary care teaching institution. J. Educ. Health Promot. 2022, 11, 122. [Google Scholar] [CrossRef] [PubMed]
- Ng, R.; Sutradhar, R.; Yao, Z.; Wodchis, W.P.; Rosella, L.C. Smoking, drinking, diet and physical activity-modifiable lifestyle risk factors and their associations with age to first chronic disease. Int. J. Epidemiol. 2020, 49, 113–130. [Google Scholar] [CrossRef] [PubMed]
- Ioniță-Mîndrican, C.-B.; Mititelu, M.; Musuc, A.M.; Oprea, E.; Ziani, K.; Neacșu, S.M.; Grigore, N.D.; Negrei, C.; Dumitrescu, D.-E.; Mireșan, H.; et al. Honey and Other Beekeeping Products Intake among the Romanian Population and Their Therapeutic Use. Appl. Sci. 2022, 12, 9649. [Google Scholar] [CrossRef]
- Năstăsescu, V.; Mititelu, M.; Stanciu, T.I.; Drăgănescu, D.; Grigore, N.D.; Udeanu, D.I.; Stanciu, G.; Neacșu, S.M.; Dinu-Pîrvu, C.E.; Oprea, E.; et al. Food Habits and Lifestyle of Romanians in the Context of the COVID-19 Pandemic. Nutrients 2022, 14, 504. [Google Scholar] [CrossRef]
- Cochran, W.G. Sampling Techniques, 3rd ed.; John Wiley & Sons: New York, NY, USA, 1977. [Google Scholar]
- Available online: https://www.recensamantromania.ro/rezultate-rpl-2021/rezultate-definitive-caracteristici-demografice/ (accessed on 5 May 2023).
- Whitehead, A.L.; Julious, S.A.; Cooper, C.L.; Campbell, M.J. Estimating the sample size for a pilot randomised trial to minimise the overall trial sample size for the external pilot and main trial for a continuous outcome variable. Stat. Methods Med. Res. 2016, 25, 1057–1073. [Google Scholar] [CrossRef]
- Julious, S.A. Sample size of 12 per group rule of thumb for a pilot study. Pharm. Stat. 2005, 4, 287–291. [Google Scholar] [CrossRef]
- Connelly, L.M. Pilot studies. Medsurg Nurs. 2008, 17, 411–412. [Google Scholar]
- Viechtbauer, W.; Smits, L.; Kotz, D.; Budé, L.; Spigt, M.; Serroyen, J.; Crutzen, R. A simpleformula for the calculation of sample size in pilot studies. J. Clin. Epidemiol. 2015, 68, 1375–1379. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, H.F. An index of factorial simplicity. Psychometrika 1974, 39, 31–36. [Google Scholar] [CrossRef]
- Bartlett, M.S. A note on the multiplying factors for various χ2 approximations. J. R. Stat. Soc. 1954, 16, 296–298. [Google Scholar] [CrossRef]
- Bonkat, G.; Bartoletti, R.; Bruyére, F.; Cai, T.; Geerlings, S.E.; Köves, B.; Schubert, S.; Wagenlehner, F. EAU Guidelines on Urological Infections 2020. In European Association of Urology Guidelines; Vol. presented at the EAU Annual Congress Amsterdam 2020; European Association of Urology Guidelines Office; Available online: http://uroweb.org/guideline/urological-infections/ (accessed on 5 May 2023).
- Branca, F.; Nikogosian, H.; Lobstein, T. The Challenge of Obesity in the WHO European Region and the Strategies for Response; WHO Regional Office for Europe: Copenhagen, Denmark, 2007; ISBN 9789289014083. [Google Scholar]
- Ashwell, M.; Gibson, S. Waist-to-height ratio as an indicator of early health risk: Simpler and more predictive than using a matrix based on BMI and waist circumference. BMJ Open 2016, 6, e010159. [Google Scholar] [CrossRef] [PubMed]
- Aydin, A.; Ahmed, K.; Zaman, I.; Khan, M.S.; Dasgupta, P. Recurrent urinary tract infections in women. Int. Urogynecol. J. 2015, 26, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Scholes, D.; Hooton, T.M.; Roberts, P.L.; Stapleton, A.E.; Gupta, K.; Stamm, W.E. Risk factors for recurrent urinary tract infection in young women. J. Infect. Dis. 2000, 182, 1177–1182. [Google Scholar] [CrossRef] [PubMed]
- Foxman, B. Epidemiology of urinary tract infections: Incidence, morbidity, and economic costs. Am. J. Med. 2002, 113 (Suppl. S1A), 5S–13S. [Google Scholar] [CrossRef] [PubMed]
- Scholes, D.; Hawn, T.R.; Roberts, P.L.; Li, S.S.; Stapleton, A.E.; Zhao, L.P.; Stamm, W.E.; Hooton, T.M. Family history and risk of recurrent cystitis and pyelonephritis in women. J. Urol. 2010, 184, 564–569. [Google Scholar] [CrossRef]
- Bustamante, M.; Oomah, B.D.; Oliveira, W.P.; Burgos-Díaz, C.; Rubilar, M.; Shene, C. Probiotics and prebiotics potential for the care of skin, female urogenital tract, and respiratory tract. Folia Microbiol. 2020, 65, 245–264. [Google Scholar] [CrossRef] [PubMed]
- De Souza, V.M.C.; dos Santos, E.F.; Sgarbieri, V.C. The importance of prebiotics in functional foods and clinical practice. Food Nutr. Sci. 2011, 2, 133–144. [Google Scholar]
- Syngai, G.G.; Gopi, R.; Bharali, R.; Dey, S.; Lakshmanan, G.M.; Ahmed, G. Probiotics—The versatile functional food ingredients. J. Food Sci. Technol. 2016, 53, 921–933. [Google Scholar] [CrossRef]
- Swedish National Institute of Public Health. Physical Activity in the Prevention and Treatment of Disease, SE-120 88 Stockholm, 2010, ISBN 978-91-7257-715-2. Available online: https://www.fyss.se/wp-content/uploads/2018/01/fyss_2010_english.pdf (accessed on 15 November 2023).
- Platt, F.W.; Keating, K.N. Differences in physician and patient perceptions of uncomplicated UTI symptom severity: Understanding the communication gap. Int. J. Clin. Pract. 2007, 61, 303–308. [Google Scholar] [CrossRef]
- Pasay, D.K.; Guirguis, M.S.; Shkrobot, R.C.; Slobodan, J.P.; Wagg, A.S.; Sadowski, C.A.; Conly, J.M.; Saxinger, L.M.; Bresee, L.C. Antimicrobial stewardship in rural nursing homes: Impact of interprofessional education and clinical decision tool implementation on urinary tract infection treatment in a cluster randomized trial. Infect. Control Hosp. Epidemiol. 2019, 40, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Medina, M.; Castillo-Pino, E. An introduction to the epidemiology and burden of urinary tract infections. Ther. Adv. Urol. 2019, 11, 1756287219832172. [Google Scholar] [CrossRef] [PubMed]

| Total Population | Female | Male | ||||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| 1103 | 100 | 883 | 80.05 | 220 | 19.95 | |
| Age (years) (χ2 = 15.45, p < 0.001) | ||||||
| 18–25 | 233 | 21.12 | 196 | 22.20 | 37 | 16.82 |
| 26–35 | 328 | 29.74 | 275 | 31.14 | 53 | 24.09 |
| 36–45 | 279 | 25.29 | 222 | 25.14 | 57 | 25.91 |
| >45 | 263 | 23.84 | 190 | 21.52 | 73 | 33.18 |
| Residence area (χ2 = 10.089, p = 0.001) | ||||||
| Urban area | 873 | 79.15 | 716 | 81.09 | 157 | 71.36 |
| Rural area | 230 | 20.85 | 167 | 18.91 | 63 | 28.64 |
| Level of education (χ2 = 15.529, p = 0.004) | ||||||
| General/primary school | 29 | 2.63 | 20 | 2.27 | 9 | 4.09 |
| Secondary education (baccalaureate) | 181 | 16.41 | 128 | 14.50 | 53 | 24.09 |
| Postsecondary school | 124 | 11.24 | 105 | 11.89 | 19 | 8.64 |
| Higher education (bachelor’s degree) | 412 | 37.35 | 339 | 38.39 | 73 | 33.18 |
| Postgraduate studies (master’s degree, residency, doctorate, other specializations) | 347 | 32.37 | 291 | 32.96 | 66 | 30.00 |
| Employment status (χ2 = 17.87, p = 0.013) | ||||||
| Unemployed | 5 | 0.45 | 4 | 0.45 | 1 | 0.45 |
| Socially assisted | 3 | 0.27 | 1 | 0.45 | 2 | 0.23 |
| Homemaker | 37 | 3.35 | 29 | 3.28 | 8 | 3.64 |
| Retired | 30 | 2.72 | 18 | 2.04 | 12 | 5.45 |
| Student | 228 | 20.67 | 200 | 22.65 | 28 | 12.73 |
| Teleworking | 31 | 2.81 | 76 | 8.61 | 19 | 8.64 |
| I go to work every day | 674 | 61.11 | 528 | 59.80 | 146 | 66.36 |
| I work in a hybrid regime (telework and commuting) | 95 | 8.61 | 49 | 7.90 | 27 | 9.89 |
| Body mass index (BMI) (χ2 = 32.46, p < 0.001) | ||||||
| Underweight | 80 | 11.43% | 80 | 9.06 | 0 | 0.00 |
| Normal weight | 598 | 46.67% | 547 | 61.95 | 51 | 23.18 |
| Overweight | 265 | 24.76% | 158 | 17.89 | 107 | 48.64 |
| Obese | 160 | 17.14% | 98 | 11.10 | 62 | 28.18 |
| Variable | Adherence to a Healthy Diet | |||||
|---|---|---|---|---|---|---|
| Mean = 49.82, SD = 6.06, Min = 22, Max = 66 | ||||||
| Unhealthy Diet (A) | Moderately Healthy Diet (B) | Healthy Diet (C) | ||||
| n | % | n | % | n | % | |
| Total | 210 | 19.04 | 620 | 56.21 | 273 | 24.75 |
| Gender (χ2 = 2.55, p = 0.279) | ||||||
| Female | 160 | 76.19 | 500 | 80.65 | 223 | 81.68 |
| Male | 50 | 23.81 | 120 | 19.35 | 50 | 18.32 |
| Age (χ2 = 36.941, p < 0.001) | ||||||
| 18–25 | 63 B,C | 30.00 | 127 | 20.48 | 43 | 15.75 |
| 26–35 | 61 | 29.05 | 201 C | 32.42 | 66 | 24.18 |
| 36–45 | 55 | 26.19 | 154 | 24.84 | 70 | 25.64 |
| >45 | 31 | 14.76 | 138 | 22.26 | 94 A,B | 34.43 |
| Residence area (χ2 = 26.39, p = 0.471) | ||||||
| Urban area | 139 | 66.19 | 510 A | 82.26 | 224 A | 82.05 |
| Rural area | 71 B,C | 33.81 | 110 | 17.74 | 49 | 17.95 |
| Level of education (χ2 = 26.08, p < 0.001) | ||||||
| General/primary school | 13 B,C | 6.19 | 12 | 1.94 | 4 | 1.47 |
| Secondary education (baccalaureate) | 50 C | 23.81 | 104 C | 16.77 | 27 | 9.89 |
| Postsecondary school | 22 | 10.48 | 64 | 10.32 | 38 | 13.92 |
| Higher education (bachelor’s degree) | 88 | 41.90 | 223 | 35.97 | 101 | 37.00 |
| Postgraduate studies (master’s degree, residency, doctorate, other specializations) | 37 | 17.62 | 217 A | 35.00 | 103 A | 37.73 |
| Employment status (χ2 = 13.06, p =0.220) | ||||||
| Unemployed | 3 | 1.43 | 1 | 0.16% | 1 | 0.37 |
| Socially assisted | 0 | 0.00 | 2 | 0.32 | 1 | 0.37 |
| Homemaker | 6 | 2.86 | 24 | 3.87 | 7 | 2.56 |
| Retired | 3 | 1.43 | 17 | 2.74 | 10 | 3.66 |
| Student | 57 C | 27.14 | 122 | 19.68 | 49 | 17.95 |
| Teleworking | 3 | 1.43 | 22 | 3.55 | 6 | 2.20 |
| I go to work every day | 119 | 56.67 | 383 | 61.77 | 172 | 63.00 |
| I work in a hybrid regime (telework and commuting) | 19 | 9.05 | 49 | 7.90 | 27 | 9.89 |
| Body mass index (BMI) (χ2 = 12.515, p = 0.051) | ||||||
| Underweight | 24 C | 11.43% | 44 | 7.10% | 12 | 4.40% |
| Normal weight | 98 | 46.67% | 346 | 55.81% | 154 | 56.41% |
| Overweight | 52 | 24.76% | 144 | 23.23% | 69 | 25.27% |
| Obese | 36 | 17.14% | 86 | 13.87% | 38 | 13.92% |
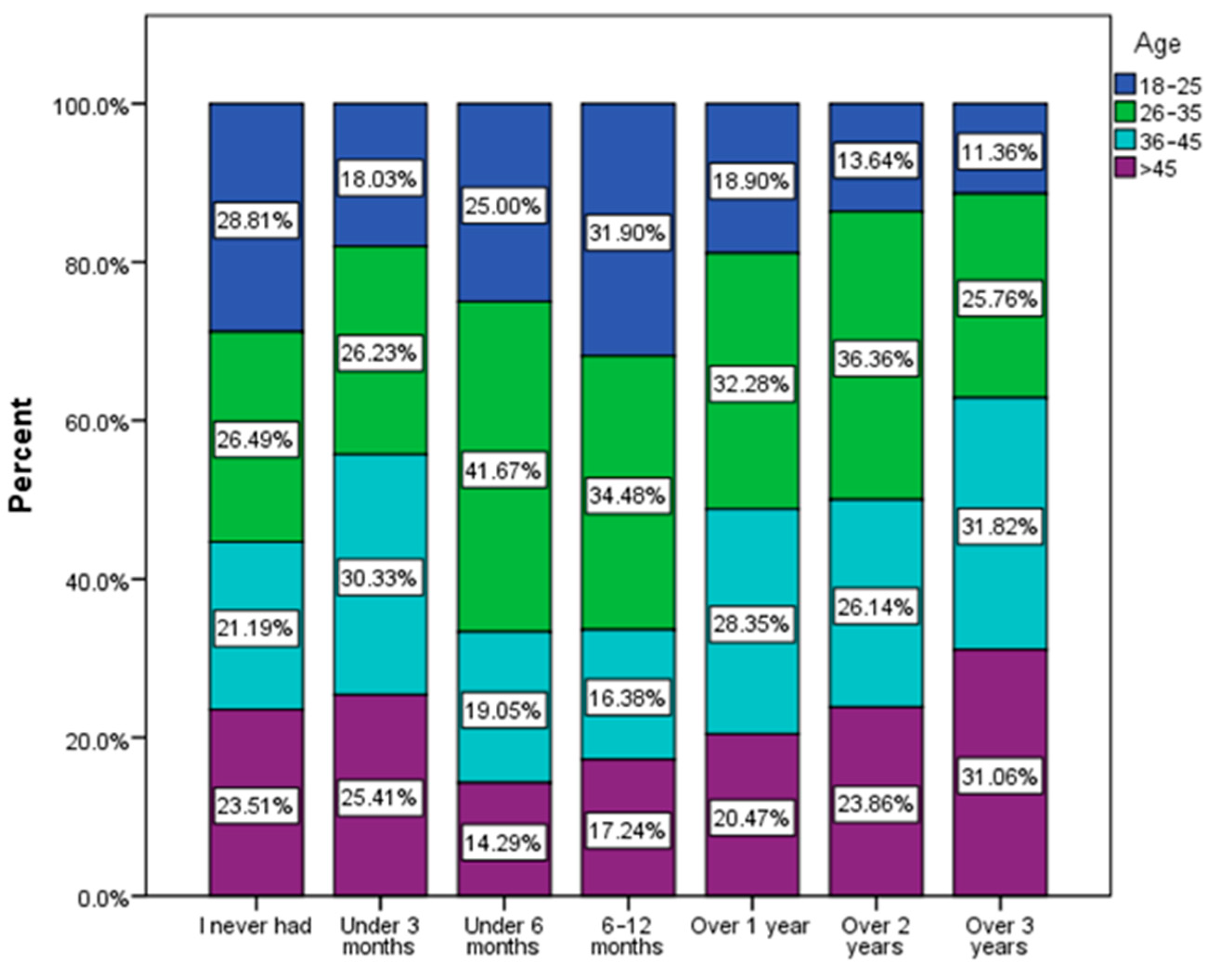
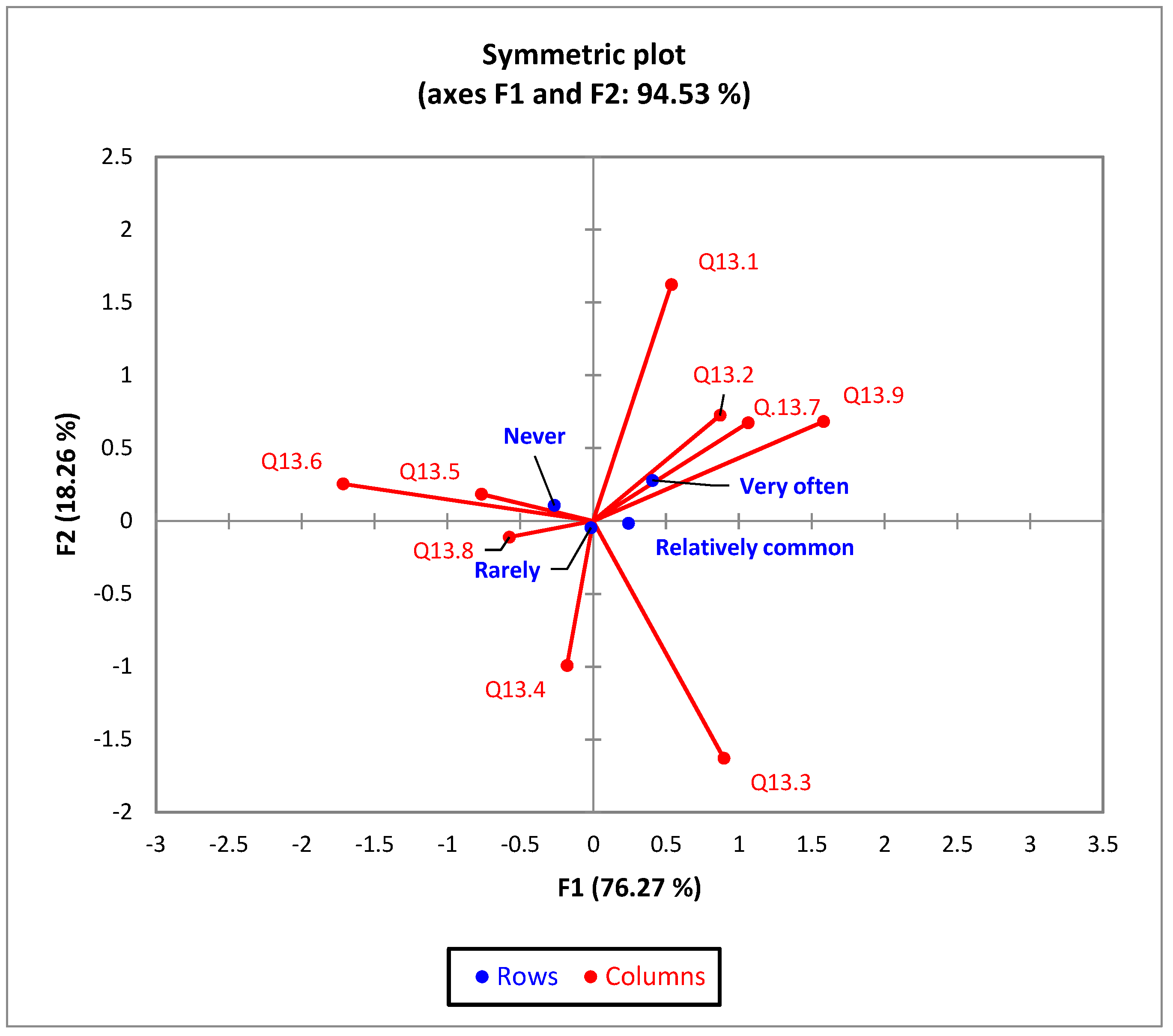
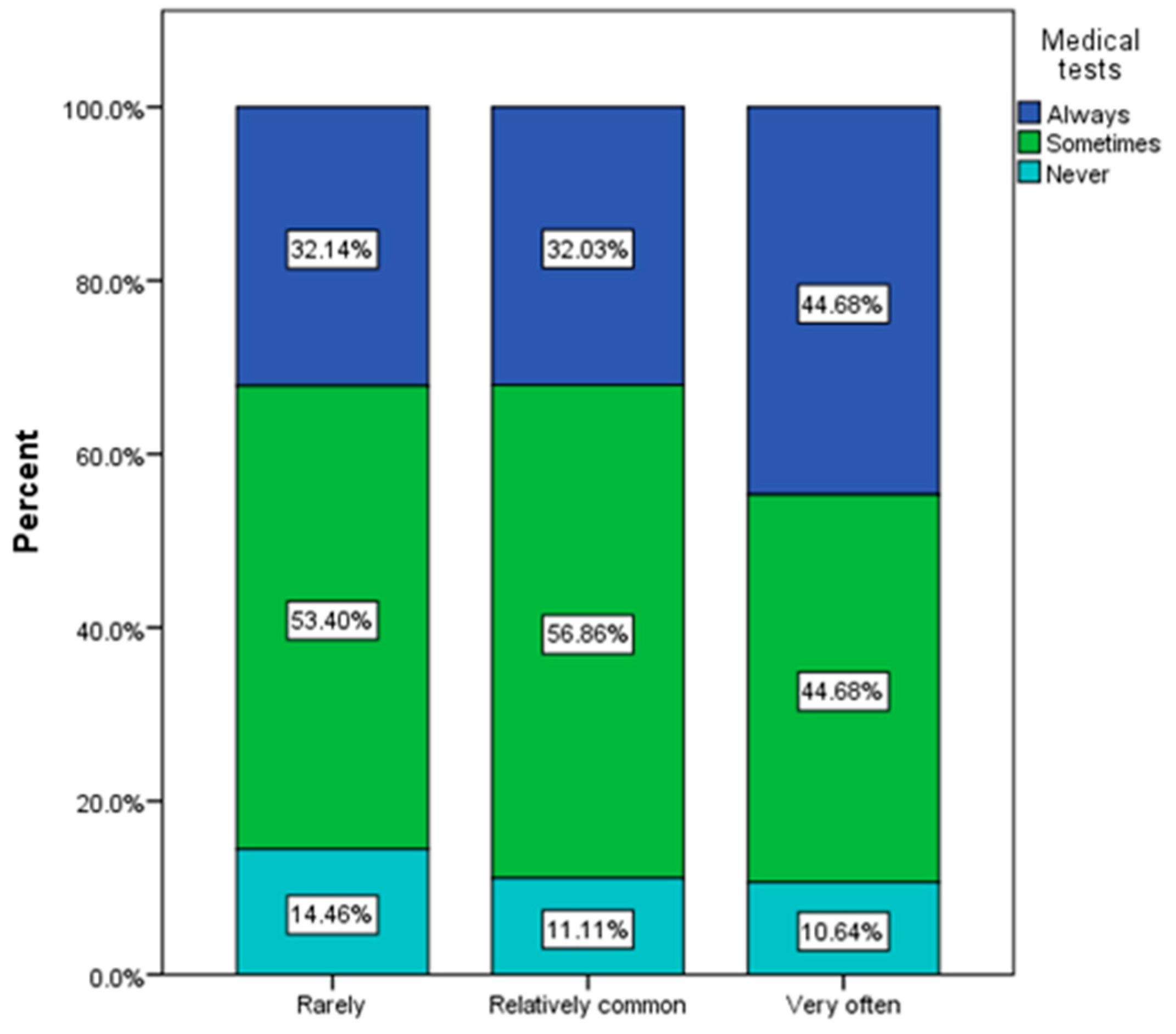
| Frequency of urinary infection (χ2 = 13.46, p = 0.036) | ||||||
| Very often | 15 | 7.14% | 22 | 3.55% | 10 | 3.66% |
| Relatively common | 24 | 11.43% | 103C | 16.61% | 27 | 9.89% |
| Rarely | 115 | 54.76% | 329 | 53.06% | 156 | 57.14% |
| Never | 56 | 26.67% | 166 | 26.77% | 80 | 29.30% |
| Independent Variables | Unhealthy Diet | Moderately Healthy Diet | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| Gender | ||||||
| Male | 1 | 1 | ||||
| Female | 0.619 | (0.363–1.055) | 0.078 | 0.822 | (0.538–1.256) | 0.365 |
| Age (years) | ||||||
| 18–23 | 3.789 | (2.004–7.167) | <0.001 | 2.321 | (1.204–4.476) | 0.012 |
| 24–35 | 3.615 | (1.994–6.535) | <0.001 | 2.220 | (1.464–3.367) | <0.001 |
| 35–45 | 2.674 | (1.491–4.795) | 0.001 | 1.605 | (1.067–2.414) | 0.023 |
| >45 | 1 | 1 | ||||
| Residence area | ||||||
| Urban area | 1 | 1 | ||||
| Rural area | 1.849 | (1.037–2.922) | 0.008 | 0.889 | (0.601–1.314) | 0.554 |
| Level of education | ||||||
| General/primary school | 3.635 | (1.037–7.743) | 0.044 | 1.558 | (0.465–5.220) | 0.473 |
| Secondary education (baccalaureate) | 1.949 | (1.054–3.606) | 0.033 | 1.887 | (1.111–3.206) | 0.019 |
| Postsecondary school | 0.780 | (0.415–1.466) | 0.440 | 0.832 | (0.514–1.348) | 0.455 |
| Higher education (bachelor’s degree) | 1 | 1 | ||||
| Postgraduate studies (master’s degree, residency, doctorate, other specializations) | 0.395 | (0.239–0.653) | <0.001 | 0.879 | (0.618–1.251) | 0.585 |
| Body mass index (BMI) | ||||||
| Underweight (<18.5) | 2.944 | (1.372–6.318) | 0.006 | 1.494 | (0.759–2.941) | 0.245 |
| Normal (18.5–24.9) | 1 | 1 | ||||
| Overweight (25–29.9) | 1.275 | (0.773–2.104) | 0.091 | 0.983 | (0.672–1.438) | 0.929 |
| Obese (≥30) | 1.351 | (0.753–2.423) | 0.313 | 1.081 | (0.684–1.707) | 0.739 |
| Frequency of urinary infection | ||||||
| Very often | 2.925 | (1.143–6.488) | 0.025 | 1.165 | (0.514–2.639) | 0.715 |
| Relatively common | 1.719 | (0.856–3.455) | 0.128 | 2.043 | (1.208–3.454) | 0.008 |
| Rarely | 1.417 | (0.898–2.235) | 0.134 | 1.071 | (0.758–1.514) | 0.698 |
| Never | 1 | 1 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mititelu, M.; Olteanu, G.; Neacșu, S.M.; Stoicescu, I.; Dumitrescu, D.-E.; Gheorghe, E.; Tarcea, M.; Busnatu, Ș.S.; Ioniță-Mîndrican, C.-B.; Tafuni, O.; et al. Incidence of Urinary Infections and Behavioral Risk Factors. Nutrients 2024, 16, 446. https://doi.org/10.3390/nu16030446
Mititelu M, Olteanu G, Neacșu SM, Stoicescu I, Dumitrescu D-E, Gheorghe E, Tarcea M, Busnatu ȘS, Ioniță-Mîndrican C-B, Tafuni O, et al. Incidence of Urinary Infections and Behavioral Risk Factors. Nutrients. 2024; 16(3):446. https://doi.org/10.3390/nu16030446
Chicago/Turabian StyleMititelu, Magdalena, Gabriel Olteanu, Sorinel Marius Neacșu, Iuliana Stoicescu, Denisa-Elena Dumitrescu, Emma Gheorghe, Monica Tarcea, Ștefan Sebastian Busnatu, Corina-Bianca Ioniță-Mîndrican, Ovidiu Tafuni, and et al. 2024. "Incidence of Urinary Infections and Behavioral Risk Factors" Nutrients 16, no. 3: 446. https://doi.org/10.3390/nu16030446
APA StyleMititelu, M., Olteanu, G., Neacșu, S. M., Stoicescu, I., Dumitrescu, D.-E., Gheorghe, E., Tarcea, M., Busnatu, Ș. S., Ioniță-Mîndrican, C.-B., Tafuni, O., Belu, I., Popescu, A., Lupu, S., & Lupu, C. E. (2024). Incidence of Urinary Infections and Behavioral Risk Factors. Nutrients, 16(3), 446. https://doi.org/10.3390/nu16030446









