Comparative Assessment of In Vitro Xanthine Oxidase and α-Glucosidase Inhibitory Activities of Cultured Cambial Meristematic Cells, Adventitious Roots, and Field-Cultivated Ginseng
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals, Reagents, and Different Ginseng Roots
2.2. Extraction and Measurement of Total Saponins
2.3. Measurement of Total Phenolic, Total Flavonoid, Total Proteins
2.4. Xanthine Oxidase Inhibitory Assay
2.5. α-Glucosidase Inhibitory Measurement
3. Results
3.1. Comparison of XO Inhibitory Activities among AGR, CMC, and CGR
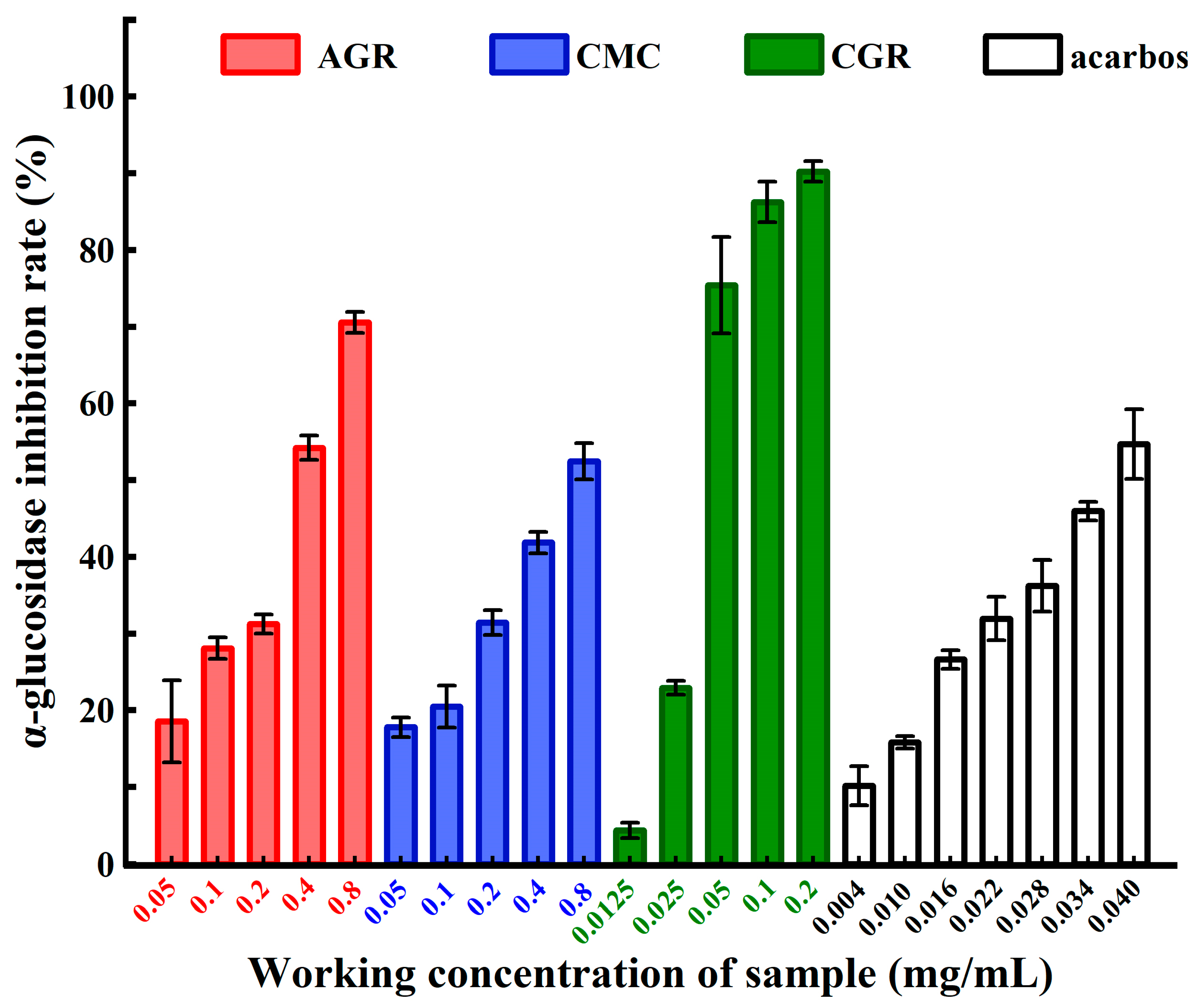
3.2. Comparison of α-Glucosidase Inhibitory Activities among AGR, CMC, and CGR
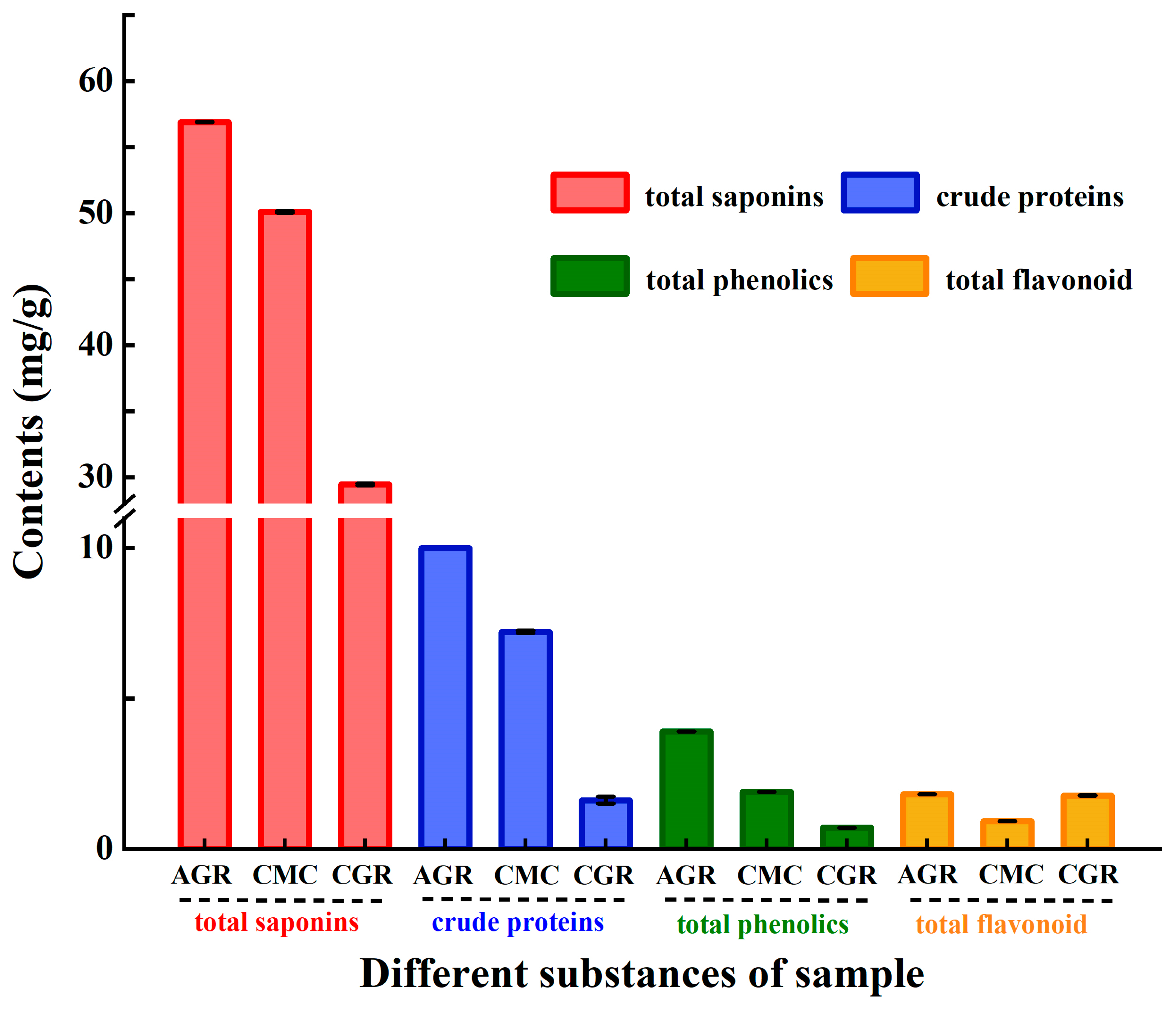
3.3. Comparison of Crude Proteins, Total Phenolics, and Flavonoids Contents among AGR, CMC, and CGR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, J.H.; Jang, M.J.; Park, Y.J. In Vitro alpha-Amylase, alpha-Glucosidase, Pancreatic Lipase, Xanthine Oxidase Inhibiting Activity of Agaricus bisporus Extracts. Mycobiology 2023, 51, 60–66. [Google Scholar] [CrossRef]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Nutmakul, T. A review on benefits of quercetin in hyperuricemia and gouty arthritis. Saudi Pharm. J 2022, 30, 918–926. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Pan, H.; Liu, Y.; Guan, M.; Li, X.; Chen, S.; Tong, X.; Luo, Y.; Wang, X.; Yang, X.; et al. Distinct hyperuricemia trajectories are associated with different risks of incident diabetes: A prospective cohort study. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.C.; Liu, J.; Solomon, D.H. Risk of incident diabetes in patients with gout: A cohort study. Arthritis Rheumatol. 2015, 67, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Tung, Y.C.; Lee, S.S.; Tsai, W.C.; Lin, G.T.; Chang, H.W.; Tu, H.P. Association Between Gout and Incident Type 2 Diabetes Mellitus: A Retrospective Cohort Study. Am. J. Med. 2016, 129, 1219.e17–1219.e25. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zhang, T.; Liu, Y.; Chang, Q.; Zhao, Y.; Guo, C.; Xia, Y. Prevalence of Diabetes in Patients with Hyperuricemia and Gout: A Systematic Review and Meta-analysis. Curr. Diabetes Rep. 2023, 23, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Guariguata, L.; Whiting, D.R.; Hambleton, I.; Beagley, J.; Linnenkamp, U.; Shaw, J.E. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res. Clin. Pract. 2014, 103, 137–149. [Google Scholar] [CrossRef]
- Xiong, Q.; Liu, J.; Xu, Y. Effects of Uric Acid on Diabetes Mellitus and Its Chronic Complications. Int. J. Endocrinol. 2019, 2019, 9691345. [Google Scholar] [CrossRef]
- Chen-Xu, M.; Yokose, C.; Rai, S.K.; Pillinger, M.H.; Choi, H.K. Contemporary Prevalence of Gout and Hyperuricemia in the United States and Decadal Trends: The National Health and Nutrition Examination Survey, 2007–2016. Arthritis Rheumatol. 2019, 71, 991–999. [Google Scholar] [CrossRef]
- Glantzounis, G.K.; Tsimoyiannis, E.C.; Kappas, A.M.; Galaris, D.A. Uric acid and oxidative stress. Curr. Pharm. Des. 2005, 11, 4145–4151. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yang, H.; Guo, Y.; Wei, F.; Yang, X.; Li, D.; Li, M.; Xu, W.; Li, W.; Sun, L.; et al. Comparative efficacy and safety of urate-lowering therapy for the treatment of hyperuricemia: A systematic review and network meta-analysis. Sci. Rep. 2016, 6, 33082. [Google Scholar] [CrossRef] [PubMed]
- Quy, T.N.; Xuan, T.D. Xanthine Oxidase Inhibitory Potential, Antioxidant and Antibacterial Activities of Cordyceps militaris (L.) Link Fruiting Body. Medicines 2019, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Lankatillake, C.; Huynh, T.; Dias, D.A. Understanding glycaemic control and current approaches for screening antidiabetic natural products from evidence-based medicinal plants. Plant Methods 2019, 15, 105. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, D.; Sharma, P.; Adhikari, A.; Mandal, K.A.; Verma, A. A Review on Nepalese Medicinal Plants Used Traditionally as Alpha-Amylase and Alpha-Glucosidase Inhibitors Against Diabetes Mellitus. Curr. Tradit. Med. 2021, 7, e020721194443. [Google Scholar] [CrossRef]
- Pokhrel, T.; Shrestha, D.; Dhakal, K.; Yadav, P.M.; Adhikari, A. Comparative Analysis of the Antioxidant and Antidiabetic Potential of Nelumbo nucifera Gaertn. and Nymphaea lotus L. var. pubescens (Willd.). J. Chem. 2022, 2022, 4258124. [Google Scholar] [CrossRef]
- Fatmawati, S.; Shimizu, K.; Kondo, R. Ganoderol B: A potent alpha-glucosidase inhibitor isolated from the fruiting body of Ganoderma lucidum. Phytomedicine 2011, 18, 1053–1055. [Google Scholar] [CrossRef] [PubMed]
- Satoh, T.; Igarashi, M.; Yamada, S.; Takahashi, N.; Watanabe, K. Inhibitory effect of black tea and its combination with acarbose on small intestinal alpha-glucosidase activity. J. Ethnopharmacol. 2015, 161, 147–155. [Google Scholar] [CrossRef]
- Xue, H.; Tan, J.; Zhu, X.; Li, Q.; Tang, J.; Cai, X. Counter-current fractionation-assisted and bioassay-guided separation of active compounds from cranberry and their interaction with α-glucosidase. LWT 2021, 145, 111374. [Google Scholar] [CrossRef]
- Joshi, S.R.; Standl, E.; Tong, N.; Shah, P.; Kalra, S.; Rathod, R. Therapeutic potential of alpha-glucosidase inhibitors in type 2 diabetes mellitus: An evidence-based review. Expert. Opin. Pharmacother. 2015, 16, 1959–1981. [Google Scholar] [CrossRef]
- Qin, N.; Hu, X.; Li, S.; Wang, J.; Li, Z.; Li, D.; Xu, F.; Gao, M.; Hua, H. Hypoglycemic effect of silychristin A from Silybum marianum fruit via protecting pancreatic islet b cells from oxidative damage and inhibiting a-glucosidase activity in vitro and in rats with type 1 diabetes. J. Funct. Foods 2017, 38, 168–179. [Google Scholar] [CrossRef]
- Reshma, M.V.; Namitha, L.K.; Sundaresan, A.; Kiran, C.R. Total Phenol Content, Antioxidant Activities and α-Glucosidase Inhibition of Sesame Cake Extracts. J. Food Biochem. 2012, 37, 723–731. [Google Scholar] [CrossRef]
- Choi, G.; Han, Y.; Sim, K.; Kim, M. Phenolic compounds, antioxidant capacity, and α-amylase and α-glucosidase inhibitory activity of ethanol extracts of perilla seed meal. Food Sci. Nutr. 2023, 11, 4596–4606. [Google Scholar] [CrossRef]
- Neale, C.; Camfield, D.; Reay, J.; Stough, C.; Scholey, A. Cognitive effects of two nutraceuticals Ginseng and Bacopa benchmarked against modafinil: A review and comparison of effect sizes. Br. J. Clin. Pharmacol. 2013, 75, 728–737. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Valappil, A.K.; Mathiyalagan, R.; Tran, T.N.A.; Ramadhania, Z.M.; Awais, M.; Yang, D.C. In Vitro Cultivation and Ginsenosides Accumulation in Panax ginseng: A Review. Plants 2023, 12, 3165. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Qi, X.; Lu, X.T.; Cui, C.B.; Gao, X.F. Study on hypoglycemic effects of irradiated ginseng adventitious roots. Food Chem. X 2022, 13, 100234. [Google Scholar] [CrossRef]
- Barzee, T.J.; Mashad, H.M.E.; Cao, L.; Chio, A.; Pan, Z.; Zhang, R. Cell-cultivated food production and processing: A review. Food Bioeng. 2022, 1, 4–25. [Google Scholar] [CrossRef]
- Li, J.; Yang, C.; Zhang, S.; Liu, S.; Zhao, L.; Luo, H.; Chen, Y.; Huang, W. Ginsenoside Rg1 inhibits inflammatory responses via modulation of the nuclear factor-kappaB pathway and inhibition of inflammasome activation in alcoholic hepatitis. Int. J. Mol. Med. 2018, 41, 899–907. [Google Scholar] [CrossRef]
- Ong, W.Y.; Farooqui, T.; Koh, H.L.; Farooqui, A.A.; Ling, E.A. Protective effects of ginseng on neurological disorders. Front. Aging Neurosci. 2015, 7, 129. [Google Scholar] [CrossRef]
- Chen, X.J.; Zhang, X.J.; Shui, Y.M.; Wan, J.B.; Gao, J.L. Anticancer Activities of Protopanaxadiol- and Protopanaxatriol-Type Ginsenosides and Their Metabolites. Evid. Based Complement. Alternat. Med. 2016, 2016, 5738694. [Google Scholar] [CrossRef]
- Murthy, H.N.; Dandin, V.S.; Lee, E.J.; Paek, K.Y. Efficacy of ginseng adventitious root extract on hyperglycemia in streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2014, 153, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Ju, C.; Jeon, S.M.; Jun, H.S.; Moon, C.K. Diol-ginsenosides from Korean Red Ginseng delay the development of type 1 diabetes in diabetes-prone biobreeding rats. J. Ginseng. Res. 2020, 44, 619–626. [Google Scholar] [CrossRef]
- Ratan, Z.A.; Haidere, M.F.; Hong, Y.H.; Park, S.H.; Lee, J.O.; Lee, J.; Cho, J.Y. Pharmacological potential of ginseng and its major component ginsenosides. J. Ginseng Res. 2021, 45, 199–210. [Google Scholar] [CrossRef]
- Karmazyn, M.; Gan, X.T. Ginseng for the treatment of diabetes and diabetes-related cardiovascular complications: A discussion of the evidence. Can. J. Physiol. Pharmacol. 2019, 97, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Balan, P.; Popovich, D.G. Review of Ginseng Anti-Diabetic Studies. Molecules 2019, 24, 4501. [Google Scholar] [CrossRef]
- Fu, C.; Yin, D.; Nie, H.; Sun, D. Notoginsenoside R1 Protects HUVEC Against Oxidized Low Density Lipoprotein (Ox-LDL)-Induced Atherogenic Response via Down-Regulating miR-132. Cell Physiol. Biochem. 2018, 51, 1739–1750. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Yi, Y.S.; Kim, D.; Kim, M.H.; Park, J.G.; Kim, E.; Lee, S.Y.; Yoon, K.; Kim, J.H.; Park, J.; et al. Nuclear factor kappa-B- and activator protein-1-mediated immunostimulatory activity of compound K in monocytes and macrophages. J. Ginseng Res. 2017, 41, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, J.J.; An, C.; Liu, C.; Zhang, Q.W.; Ding, H.; Ma, S.J.; Xue, W.J.; Fan, D.D. Ginsenoside Rg1 alleviates the postprandial blood glucose by inhibiting α-glucosidase. J. Funct. Foods 2023, 107, 105648. [Google Scholar] [CrossRef]
- Li, S.; Tang, Y.; Liu, C.; Li, J.; Guo, L.; Zhang, Y. Development of a method to screen and isolate potential xanthine oxidase inhibitors from Panax japlcus var via ultrafiltration liquid chromatography combined with counter-current chromatography. Talanta 2015, 134, 665–673. [Google Scholar] [CrossRef]
- Sung, Y.Y.; Yuk, H.J.; Kim, D.S. Saengmaeksan, a traditional herbal formulation consisting of Panax ginseng, ameliorates hyperuricemia by inhibiting xanthine oxidase activity and enhancing urate excretion in rats. J. Ginseng Res. 2021, 45, 565–574. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, X.l.; Xiang, T.; Sun, B.G.; Luo, H.X.; Liu, M.T.; Chen, Z.X.; Zhang, S.J.; Wang, C.J. Total saponins from dioscorea septemloba thunb reduce serum uric acid levels in rats with hyperuricemia through OATP1A1 up-regulation. J. Huazhong Univ. Sci. Technol. 2016, 36, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Pang, M.; Fang, Y.; Chen, S.; Zhu, X.; Shan, C.; Su, J.; Yu, J.; Li, B.; Yang, Y.; Chen, B.; et al. Gypenosides Inhibits Xanthine Oxidoreductase and Ameliorates Urate Excretion in Hyperuricemic Rats Induced by High Cholesterol and High Fat Food (Lipid Emulsion). Med. Sci. Monit. 2017, 23, 1129–1140. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.P.; Fan, C.L.; Lin, Z.Z.; Yin, Q.; Zhao, C.; Peng, P.; Zhang, R.; Wang, Z.J.; Du, J.; Wang, Z.B. Screening of Potential alpha-Glucosidase Inhibitors from the Roots and Rhizomes of Panax Ginseng by Affinity Ultrafiltration Screening Coupled with UPLC-ESI-Orbitrap-MS Method. Molecules 2023, 28, 2069. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cheng, Y.; Zhang, X.; Wang, Y.; Zhao, H. Comparative analysis of physicochemical properties, ginsenosides content and α-amylase inhibitory effects in white ginseng and red ginsen. Food Sci. Hum. Wellness 2023, 12, 14–27. [Google Scholar] [CrossRef]
- Deng, J.; Liu, Y.; Duan, Z.; Zhu, C.; Hui, J.; Mi, Y.; Ma, P.; Ma, X.; Fan, D.; Yang, H. Protopanaxadiol and Protopanaxatriol-Type Saponins Ameliorate Glucose and Lipid Metabolism in Type 2 Diabetes Mellitus in High-Fat Diet/Streptozocin-Induced Mice. Front. Pharmacol. 2017, 8, 506. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S. Investigation of Phenolic, Flavonoid, and Vitamin Contents in Different Parts of Korean Ginseng (Panax ginseng C.A. Meyer). Prev. Nutr. Food Sci. 2016, 21, 263–270. [Google Scholar] [CrossRef]
- Malathy, R.; Prabakaran, M.; Kalaiselvi, K.; Chung, I.-M.; Kim, S.-H. Comparative Polyphenol Composition, Antioxidant and Anticorrosion Properties in Various Parts of Panax ginseng Extracted in Different Solvents. Appl. Sci. 2021, 11, 93. [Google Scholar] [CrossRef]
- Thaha, A.; Wang, B.S.; Chang, Y.W.; Hsia, S.M.; Huang, T.C.; Shiau, C.Y.; Hwang, D.F.; Chen, T.Y. Food-Derived Bioactive Peptides with Antioxidative Capacity, Xanthine Oxidase and Tyrosinase Inhibitory Activity. Processes 2021, 9, 747. [Google Scholar] [CrossRef]
- Orhan, I.E.; Deniz, F.S.S. Natural Products and Extracts as Xantine Oxidase Inhibitors—A Hope for Gout Disease? Curr. Pharm. Des. 2021, 27, 143–158. [Google Scholar] [CrossRef]
- Bai, L.; Gao, J.; Wei, F.; Zhao, J.; Wang, D.; Wei, J. Therapeutic Potential of Ginsenosides as an Adjuvant Treatment for Diabetes. Front. Pharmacol. 2018, 9, 423. [Google Scholar] [CrossRef]
- Irondi, E.A.; Adegoke, B.M.; Effion, E.S.; Oyewo, S.O.; Alamu, E.O.; Boligon, A.A. Enzymes inhibitory property, antioxidant activity and phenolics profile of raw and roasted red sorghum grains in vitro. Food Sci. Hum. Wellness 2019, 8, 142–148. [Google Scholar] [CrossRef]
- Li, R.; Ru, Y.; Wang, Z.; He, X.; Kong, K.W.; Zheng, T.; Zhang, X. Phytochemical Composition, Antioxidant Activity, and Enzyme Inhibitory Activities (alpha-Glucosidase, Xanthine Oxidase, and Acetylcholinesterase) of Musella lasiocarpa. Molecules 2021, 26, 4472. [Google Scholar] [CrossRef] [PubMed]
- Benrahou, K.; Guourrami, O.E.; Mrabti, H.N.; Cherrah, Y.; Faouzi, M.E.A. Investigation of Antioxidant, Hypoglycemic and Anti-Obesity Effects of Euphorbia resinifera L. J. Pharmacopunct. 2022, 25, 242–249. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Liu, L.; Chen, Q.; Wang, Y.; Gao, X.; Ma, X.; Yan, P. Comparative Assessment of In Vitro Xanthine Oxidase and α-Glucosidase Inhibitory Activities of Cultured Cambial Meristematic Cells, Adventitious Roots, and Field-Cultivated Ginseng. Nutrients 2024, 16, 443. https://doi.org/10.3390/nu16030443
Zhang T, Liu L, Chen Q, Wang Y, Gao X, Ma X, Yan P. Comparative Assessment of In Vitro Xanthine Oxidase and α-Glucosidase Inhibitory Activities of Cultured Cambial Meristematic Cells, Adventitious Roots, and Field-Cultivated Ginseng. Nutrients. 2024; 16(3):443. https://doi.org/10.3390/nu16030443
Chicago/Turabian StyleZhang, Tianhe, Lijun Liu, Qiqi Chen, Yifei Wang, Xiujun Gao, Xingyi Ma, and Peisheng Yan. 2024. "Comparative Assessment of In Vitro Xanthine Oxidase and α-Glucosidase Inhibitory Activities of Cultured Cambial Meristematic Cells, Adventitious Roots, and Field-Cultivated Ginseng" Nutrients 16, no. 3: 443. https://doi.org/10.3390/nu16030443
APA StyleZhang, T., Liu, L., Chen, Q., Wang, Y., Gao, X., Ma, X., & Yan, P. (2024). Comparative Assessment of In Vitro Xanthine Oxidase and α-Glucosidase Inhibitory Activities of Cultured Cambial Meristematic Cells, Adventitious Roots, and Field-Cultivated Ginseng. Nutrients, 16(3), 443. https://doi.org/10.3390/nu16030443






