Micronutrient Status in Patients with Severe Obesity Before and After Laparoscopic Sleeve Gastrectomy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Anthropometric Indices
2.2. Laboratory Measurements
- iron deficiency: diagnosed based on iron levels below 50 μg/dL as well as on ferritin levels with threshold <28 ng/mL (male) and <5 ng/mL (female);
- anemia: defined according to the WHO criteria of Hb levels < 12 g/dL in women and <13 g/dL in men, respectively [39];
- 25(OH)D status: characterized as deficient if below <10 ng/mL, insufficient if between 10 and 29 ng/mL, and optimal if >30 ng/mL;
- secondary hyperparathyroidism: diagnosed based on excess PTH levels (>64 pg/mL) in the presence of low calcium (<8.6 mg/dL) or 25(OH)D (<30 ng/mL).
2.3. Nutritional Evaluation and Supplementation
2.4. Statistical Analysis
3. Results
3.1. Sample Characteristics
3.2. Longitudinal Change in Anthropometric Characteristics in All Obese Patients’ Samples
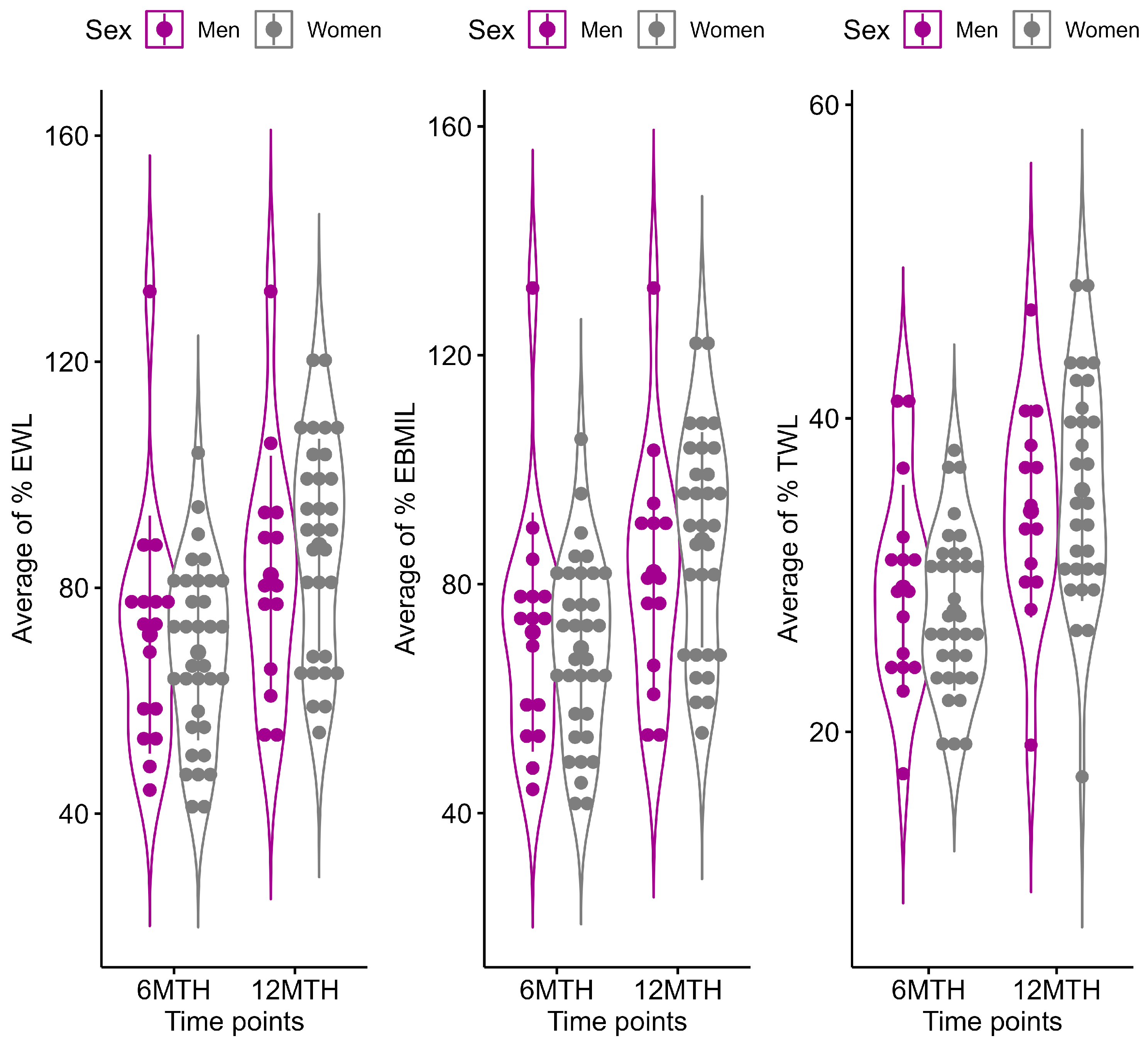
3.3. Longitudinal Change in Anthropometric Characteristics Stratified by Sex
3.4. Changes in Hematinic and Hematological Values over Time in All Patients’ Samples
3.5. Longitudinal Changes in Hematinic and Hematological Values by Sex Group and Baseline BMI
3.6. Longitudinal Changes in Vitamin D–Calcium Status and PTH Values in All Patients’ Samples
3.7. Longitudinal Changes in Vitamin D–Calcium Status and PTH Values by Sex and Baseline BMI
| Outcome | Subgroup | Baseline | 6 MTH | p-Value 1 | 12 MTH | p-Value 2 | p-Value 3 | Adjusted p-Values (Adjusted for Sex and BMI ≥ 40 kg/m2) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| LS-Means [95% CI] | LS-Means [95% CI] | LS-Means [95% CI] | Baseline | 6 MTH | 12 MTH | |||||
| 25(OH)D (ng/mL) a | Male | 8.8 [5.0, 15.3] # | 11.2 [7.9, 16.0] # | 0.76 | 20.6 [14.7, 28.7] # | <0.001 * | <0.001 * | 0.99 | 0.92 | 0.001 * |
| Female | 9.9 [6.5, 4.9] # | 9.9 [7.5, 13.0] # | 1.00 | 10.6 [8.1, 13.8] # | 0.98 | >0.99 | ||||
| BMI ≥ 40 kg/m2 | 8.8 [4.6, 17.0] # | 11.4 [9.0, 14.3] # | 0.77 | 14.3 [11.3, 18.1] # | 0.08 | 0.03 * | >0.99 | 0.90 | >0.99 | |
| BMI 35–40 kg/m2 | 9.8 [6.9, 13.8] # | 9.8 [6.5, 14.8] # | >0.99 | 15.3 [10.5, 22.1] # | 0.03 * | 0.14 | ||||
| PTH (pg/mL) b | Male | 29.8 [22.1, 37.5] | 34.5 [27.2, 42.5] | 0.84 | 41.2 [32.6, 49.7] | 0.76 | 0.15 | 0.95 | 1.00 | >0.99 |
| Female | 25.8 [20.3, 31.2] | 34.1 [28.8, 39.5] | 0.07 | 38.8 [33.3, 44.4] | 0.64 | <0.001 * | ||||
| BMI ≥ 40 kg/m2 | 30.3 [25.2, 35.4] | 34.1 [29.0, 39.2] | 0.75 | 40.1 [34.5, 45.7] | 0.38 | 0.02 * | 0.90 | 1.00 | 1.00 | |
| BMI 35–40 kg/m2 | 25.3 [17.0, 33.5] | 34.9 [26.8, 43.0] | 0.29 | 39.9 [31.4, 48.4] | 0.89 | 0.03 * | ||||
| Calcium (mg/dL) c | Male | 9.4 [9.2, 9.6] | 9.4 [9.2, 9.6] | 0.046 * | 9.4 [9.2, 9.6] | 1.00 | 0.09 | 0.99 | >0.99 | 0.86 |
| Female | 9.1 [8.9, 9.3] | 9.5 [9.3, 9.6] | 0.004 * | 9.3 [9.1, 9.4] | 0.22 | 0.65 | ||||
| BMI ≥ 40 kg/m2 | 9.0 [8.9, 9.1] | 9.4 [9.3, 9.5] | <0.001 * | 9.4 [9.3, 9.6] | >0.99 | <0.001 * | 0.79 | >0.99 | 0.59 | |
| BMI 35–40 kg/m2 | 9.2 [8.9, 9.4] | 9.5 [9.2, 9.7] | 0.23 | 9.2 [9.0, 9.5] | 0.49 | >0.99 | ||||
3.8. Differences in MNDs over Time
4. Discussion
- (i)
- Folate and vitamin B12 levels did not modify substantially between the baseline and 12 months after LSG; serum iron increased, and ferritin decreased, although in keeping with the normal range. The effect of time on the vitamin B12, folate, iron, and ferritin levels did not vary significantly across the different sexes or initial BMI categories. The significantly low levels of 25(OH)D documented prior to LSG did not suffer variation during the follow-up period. Although we did observe a significant increase in 25(OH)D from the baseline to the end of the follow-up period in men, its mean value remained low at 12 months following LSG. Throughout the course of this study, there was an increase in the mean calcium levels along with a significant increase in PTH, while remaining within the normal range.
- (ii)
- The frequency of several MNDs, folate (6.6 vs. 11.1%), vitamin B12 (16.6 vs. 12.5%), iron (2.2 vs. 4.5%), calcium (4.6 vs. 2.3%), and 25(OH)D (100 vs. 100%), respectively, did not modify significantly from the baseline to 12 months after LSG. Ferritin deficiency was not observed. PTH excess was identified in only one case (2.4%) both before and after 12 months following LSG.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Obesity: Health Consequences of Being Overweight. Available online: https://www.who.int/news-room/questions-and-answers/item/obesity-health-consequences-of-being-overweight (accessed on 15 September 2024).
- World Health Organization. WHO European Regional Obesity Report 2022; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Popa, S.; Moţa, M.; Popa, A.; Moţa, E.; Serafinceanu, C.; Guja, C.; Catrinoiu, D.; Hâncu, N.; Lichiardopol, R.; Bala, C.; et al. Prevalence of overweight/obesity, abdominal obesity and metabolic syndrome and atypical cardiometabolic phenotypes in the adult Romanian population: PREDATORR study. J. Endocrinol. Investig. 2016, 39, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, D.; Shikora, S.A.; Aarts, E.; Aminian, A.; Angrisani, L.; Cohen, R.V.; De Luca, M.; Faria, S.L.; Goodpaster, K.P.; Haddad, A.; et al. 2022 American Society for Metabolic and Bariatric Surgery (ASMBS) and International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO): Indications for Metabolic and Bariatric Surgery. Surg. Obes. Relat. Dis. 2022, 18, 1345–1356. [Google Scholar] [CrossRef]
- Shi, X.; Karmali, S.; Sharma, A.M.; Birch, D.W. A review of Laparoscopic sleeve gastrectomy for morbid obesity. Obes. Surg. 2010, 20, 1171–1177. [Google Scholar] [CrossRef] [PubMed]
- Salminen, P.; Helmiö, M.; Ovaska, J.; Juuti, A.; Leivonen, M.; Peromaa-Haavisto, P.; Hurme, S.; Soinio, M.; Nuutila, P.; Victorzon, M. Effect of Laparoscopic Sleeve Gastrectomy vs. Laparoscopic Roux-en-Y Gastric Bypass on Weight Loss at 5 Years Among Patients with Morbid Obesity. JAMA 2018, 319, 241–254. [Google Scholar] [CrossRef]
- Dang, J.T.; Karmali, S. Is RYGB more effective than sleeve gastrectomy? Nat. Rev. Endocrinol. 2019, 15, 134–135. [Google Scholar] [CrossRef] [PubMed]
- Angrisani, L.; Santonicola, A.; Iovino, P.; Ramos, A.; Shikora, S.; Kow, L. Bariatric Surgery Survey 2018: Similarities and Disparities Among the 5 IFSO Chapters. Obes. Surg. 2021, 31, 1937–1948. [Google Scholar] [CrossRef] [PubMed]
- Mureșan Ciobârcă, D.; Cătoi, A.F.; Copăescu, C.; Miere, D.; Crișan, G. Nutritional status prior to bariatric surgery for severe obesity: A review. Med. Pharm. Rep. 2021, 95, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Ciobârcă, D.; Cătoi, A.F.; Copăescu, C.; Miere, D.; Cris, G. Bariatric Surgery in Obesity: Effects on Gut. Nutrients 2020, 12, 235. [Google Scholar] [CrossRef] [PubMed]
- Jastrzębska-Mierzyńska, M.; Ostrowska, L.; Witczak-Sawczuk, K.; Razak Hady, H. Assessment of the Clinical Condition and Way of Patients’ Nutrition before and after Laparoscopic Sleeve Gastrectomy. Nutrients 2023, 15, 514. [Google Scholar] [CrossRef] [PubMed]
- van Rutte, P.W.J.; Aarts, E.O.; Smulders, J.F.; Nienhuijs, S.W. Nutrient Deficiencies Before and After Sleeve Gastrectomy. Obes. Surg. 2014, 24, 1639–1646. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.J.; Mundi, M.S.; Hurt, R.T.; Wolfe, B.; Martindale, R.G. Micronutrient Deficiencies after Bariatric Surgery: An Emphasis on Vitamins and Trace Minerals. Nutr. Clin. Pract. 2017, 32, 471–480. [Google Scholar] [CrossRef]
- Ben-Porat, T.; Weiss, R.; Sherf-Dagan, S.; Nabulsi, N.; Maayani, A.; Khalaileh, A.; Abed, S.; Brodie, R.; Harari, R.; Mintz, Y.; et al. Nutritional Deficiencies in Patients with Severe Obesity before Bariatric Surgery: What Should Be the Focus During the Preoperative Assessment? J. Acad. Nutr. Diet. 2020, 120, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Berardi, G.; Vitiello, A.; Abu-Abeid, A.; Schiavone, V.; Franzese, A.; Velotti, N.; Musella, M. Micronutrients Deficiencies in Candidates of Bariatric Surgery: Results from a Single Institution over a 1-Year Period. Obes. Surg. 2023, 33, 212–218. [Google Scholar] [CrossRef]
- Roust, L.R.; Dibaise, J.K. Nutrient deficiencies prior to bariatric surgery. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Frame-Peterson, L.A.; Megill, R.D.; Carobrese, S.; Schweitzer, M. Nutrient Deficiencies Are Common Prior to Bariatric Surgery. Nutr. Clin. Pract. 2017, 32, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Pellitero, S.; Martínez, E.; Puig, R.; Leis, A.; Zavala, R.; Granada, M.L.; Pastor, C.; Moreno, P.; Tarascó, J.; Puig-Domingo, M. Evaluation of Vitamin and Trace Element Requirements after Sleeve Gastrectomy at Long Term. Obes. Surg. 2017, 27, 1674–1682. [Google Scholar] [CrossRef] [PubMed]
- Lupoli, R.; Lembo, E.; Saldalamacchia, G.; Avola, C.K.; Angrisani, L.; Capaldo, B. Bariatric surgery and long-term nutritional issues. World J. Diabetes 2017, 8, 464–474. [Google Scholar] [CrossRef]
- Krzizek, E.C.; Brix, J.M.; Stöckl, A.; Parzer, V.; Ludvik, B. Prevalence of Micronutrient Deficiency after Bariatric Surgery. Obes. Facts 2021, 14, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Steenackers, N.; Van der Schueren, B.; Augustijns, P.; Vanuytsel, T.; Matthys, C. Development and complications of nutritional deficiencies after bariatric surgery. Nutr. Res. Rev. 2023, 36, 512–525. [Google Scholar] [CrossRef]
- Al-Mulhim, A.S. Laparoscopic Sleeve Gastrectomy and Nutrient Deficiencies: A Prospective Study. Surg. Laparosc. Endosc. Percutaneous Tech. 2016, 26, 208–211. [Google Scholar] [CrossRef]
- Aarts, E.O.; Janssen, I.M.C.; Berends, F.J. The gastric sleeve: Losing weight as fast as micronutrients? Obes. Surg. 2011, 21, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Gehrer, S.; Kern, B.; Peters, T.; Christofiel-Courtin, C.; Peterli, R. Fewer nutrient Deficiencies after laparoscopic sleeve gastrectomy (LSG) than after Laparoscopic Roux-Y-gastric bypass (LRYGB)—A prospective study. Obes. Surg. 2010, 20, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Tovar, J.; Llavero, C.; Zubiaga, L.; Boix, E.; OBELCHE Group. Maintenance of Multivitamin Supplements After Sleeve Gastrectomy. Obes. Surg. 2016, 26, 2324–2330. [Google Scholar] [CrossRef] [PubMed]
- Ben-Porat, T.; Elazary, R.; Goldenshluger, A.; Sherf Dagan, S.; Mintz, Y.; Weiss, R. Nutritional deficiencies four years after laparoscopic sleeve gastrectomy—Are supplements required for a lifetime? Surg. Obes. Relat. Dis. 2017, 13, 1138–1144. [Google Scholar] [CrossRef] [PubMed]
- Gillon, S.; Jeanes, Y.M.; Andersen, J.R.; Våge, V. Micronutrient Status in Morbidly Obese Patients Prior to Laparoscopic Sleeve Gastrectomy and Micronutrient Changes 5 years Post-surgery. Obes. Surg. 2017, 27, 606–612. [Google Scholar] [CrossRef]
- Gu, L.; Fu, R.; Chen, P.; Du, N.; Chen, S.; Mao, D.; Chen, B.; Mao, F.; Khadaroo, P.A.; Jin, Q. In Terms of Nutrition, the Most Suitable Method for Bariatric Surgery: Laparoscopic Sleeve Gastrectomy or Roux-en-Y Gastric Bypass? A Systematic Review and Meta-analysis. Obes. Surg. 2020, 30, 2003–2014. [Google Scholar] [CrossRef] [PubMed]
- Von Drygalski, A.; Andris, D.A. Invited Review: Anemia after bariatric surgery: More than just iron deficiency. Nutr. Clin. Pract. 2009, 24, 217–226. [Google Scholar] [CrossRef]
- Ben-Porat, T.; Elazary, R.; Yuval, J.B.; Wieder, A.; Khalaileh, A.; Weiss, R. Nutritional deficiencies after sleeve gastrectomy: Can they be predicted preoperatively? Surg. Obes. Relat. Dis. 2015, 11, 1029–1036. [Google Scholar] [CrossRef]
- Mechanick, J.I.; Apovian, C.; Brethauer, S.; Garvey, W.T.; Joffe, A.M.; Kim, J.; Kushner, R.F.; Lindquist, R.; Pessah-Pollack, R.; Seger, J.; et al. Clinical Practice Guidelines for the Perioperative Nutrition, Metabolic, and Nonsurgical Support of Patients Undergoing Bariatric Procedures—2019 Update: Cosponsored By American Association of Clinical Endocrinologists/American College of Endocrinology. Endocr. Pract. 2019, 25, 1346–1359. [Google Scholar] [CrossRef]
- O’Kane, M.; Parretti, H.M.; Pinkney, J.; Welbourn, R.; Hughes, C.A.; Mok, J.; Walker, N.; Thomas, D.; Devin, J.; Coulman, K.D.; et al. British Obesity and Metabolic Surgery Society Guidelines on perioperative and postoperative biochemical monitoring and micronutrient replacement for patients undergoing bariatric surgery—2020 update. Obes. Rev. 2020, 21, e13087. [Google Scholar] [CrossRef] [PubMed]
- Heusschen, L.; Berendsen, A.A.M.; Deden, L.N.; Hazebroek, E.J.; Aarts, E.O. Nutritional Deficiencies 3 Years After Sleeve Gastrectomy Can Be Limited by a Specialized Multivitamin Supplement. Obes. Surg. 2022, 32, 3561–3570. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.; Kwon, Y.; Kwon, J.W.; Kim, D.; Park, S.H.; Hwang, J.; Lee, C.M.; Park, S. Micronutrient status in bariatric surgery patients receiving postoperative supplementation per guidelines: Insights from a systematic review and meta-analysis of longitudinal studies. Obes. Rev. 2021, 22, e13249. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, N.; Antoniou, S.A.; Batterham, R.L.; Busetto, L.; Godoroja, D.; Iossa, A.; Carrano, F.M.; Agresta, F.; Alarçon, I.; Azran, C.; et al. Clinical practice guidelines of the European Association for Endoscopic Surgery (EAES) on bariatric surgery: Update 2020 endorsed by IFSO-EC, EASO and ESPCOP. Surg. Endosc. 2020, 34, 2332–2358. [Google Scholar] [CrossRef]
- Copăescu, C. Gastrectomia Longitudinală Pentru Tratamentul Obezității Morbide. In Enciclopedia de Chirurgie; Editura Celsius: Voluntari, Romania, 2010. [Google Scholar]
- Fried, M.; Hainer, V.; Basdevant, A.; Buchwald, H.; Deitel, M.; Finer, N.; Greve, J.W.M.; Horber, F.; Mathus–Vliegen, E.; Scopinaro, N.; et al. Interdisciplinary European Guidelines for Surgery for Severe (Morbid) Obesity. Obes. Surg. 2007, 17, 260–270. [Google Scholar] [CrossRef]
- Brethauer, S.A.; Kim, J.; Chaar, M.; Papasavas, P.; Eisenberg, D.; Rogers, A.; Ballem, N.; Kligman, M.; Kothari, S. Standardized outcomes reporting in metabolic and bariatric surgery. Obes. Surg. 2015, 25, 587–606. [Google Scholar] [CrossRef]
- World Health Organization. Nutritional Anemias: Report of a WHO Scientific Group; World Health Organization: Geneva, Switzerland, 1968. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.M.; Walker, S.C. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Russell, A.; Lenth, V.; Buerkner, P.; Herve, M.; Love, J.; Singmann, H. Package ‘emmeans’ R Topics Documented. Available online: https://rvlenth.github.io/emmeans/ (accessed on 15 September 2024).
- Damms-Machado, A.; Friedrich, A.; Kramer, K.M.; Stingel, K.; Meile, T.; Küper, M.A.; Königsrainer, A.; Bischoff, S.C. Pre- and postoperative nutritional deficiencies in obese patients undergoing laparoscopic sleeve gastrectomy. Obes. Surg. 2012, 22, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.A.; de Jersey, S.; Seymour, M.; Hopkins, G.; Hickman, I.; Osland, E. Iron, Vitamin B12, Folate and Copper Deficiency After Bariatric Surgery and the Impact on Anaemia: A Systematic Review. Obes. Surg. 2020, 30, 4542–4591. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Suh, H.; Karantanis, W.; Jia, S.; Yang, Y.; Loi, K.W.K. Evaluation of Micronutrient Status Post Laparoscopic Sleeve Gastrectomy: An Australian Perspective. Obes. Surg. 2021, 31, 1099–1104. [Google Scholar] [CrossRef]
- Allen, L.H. “Vitamin B-12” Advances in Nutrition. Am. Soc. Nutr. 2012, 3, 54–55. [Google Scholar]
- Landais, A. Neurological Complications of Bariatric Surgery. Obes. Surg. 2014, 24, 1800–1807. [Google Scholar] [CrossRef]
- Gasmi, A.; Bjørklund, G.; Mujawdiya, P.K.; Semenova, Y.; Peana, M.; Dosa, A.; Piscopo, S.; Benahmed, A.G.; Costea, D.O. Micronutrients deficiences in patients after bariatric surgery. Eur. J. Nutr. 2022, 61, 55–67. [Google Scholar] [CrossRef]
- Shipton, M.J.; Thachil, J. Vitamin B12 deficiency—A 21st century perspective. Clin. Med. 2015, 15, 145–150. [Google Scholar] [CrossRef]
- Kornerup, L.S.; Hvas, C.L.; Abild, C.B.; Richelsen, B.; Nexo, E. Early changes in vitamin B12 uptake and biomarker status following Roux-en-Y gastric bypass and sleeve gastrectomy. Clin. Nutr. 2018, 38, 906–911. [Google Scholar] [CrossRef]
- Hakeam, H.A.; O’Regan, P.J.; Salem, A.M.; Bamehriz, F.Y.; Eldali, A.M. Impact of laparoscopic sleeve gastrectomy on iron indices: 1 Year follow-up. Obes. Surg. 2009, 19, 1491–1496. [Google Scholar] [CrossRef]
- Kwon, Y.; Kim, H.J.; Lo Menzo, E.; Park, S.; Szomstein, S.; Rosenthal, R.J. Anemia, iron and vitamin B12 deficiencies after sleeve gastrectomy compared to Roux-en-Y gastric bypass: A meta-analysis. Surg. Obes. Relat. Dis. 2014, 10, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Selhub, J.; Morris, M.S.; Jacques, P.F.; Rosenberg, I.H. Folate-vitamin B-12 interaction in relation to cognitive impairment, anemia, and biochemical indicators of vitamin B-12 deficiency. Am. J. Clin. Nutr. 2009, 89, 1951. [Google Scholar] [CrossRef]
- Langan, R.C.; Goodbred, A.J. Vitamin B12 Deficiency: Recognition and Management. Am. Fam. Physician 2017, 96, 384–389. [Google Scholar] [PubMed]
- Friedrich, A.E.; Damms-Machado, A.; Meile, T.; Scheuing, N.; Stingel, K.; Basrai, M.; Küper, M.A.; Kramer, K.M.; Königsrainer, A.; Bischoff, S.C. Laparoscopic sleeve gastrectomy compared to a multidisciplinary weight loss program for obesity—Effects on body composition and protein status. Obes. Surg. 2013, 23, 1957–1965. [Google Scholar] [CrossRef]
- Nunes, R.; Santos-Sousa, H.; Vieira, S.; Nogueiro, J.; Bouça-Machado, R.; Pereira, A.; Carneiro, S.; Costa-Pinho, A.; Lima-Da-Costa, E.; Preto, J.; et al. Vitamin B Complex Deficiency After Roux-en-Y Gastric Bypass and Sleeve Gastrectomy—A Systematic Review and Meta-Analysis. Obes. Surg. 2022, 32, 873–891. [Google Scholar] [CrossRef] [PubMed]
- Visentin, M.; Diop-Bove, N.; Zhao, R.; Goldman, I.D. The Intestinal Absorption of Folates. Annu. Rev. Physiol. 2014, 76, 251–274. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.; Stier, C.; Raab, H.; Weiner, R. Review article: The nutritional and pharmacological consequences of obesity surgery. Aliment. Pharmacol. Ther. 2014, 40, 582–609. [Google Scholar] [CrossRef] [PubMed]
- Al-Mutawa, A.; Al-Sabah, S.; Anderson, A.K.; Al-Mutawa, M. Evaluation of Nutritional Status Post Laparoscopic Sleeve Gastrectomy—5-Year Outcomes. Obes Surg 2018, 28, 1473–1483. [Google Scholar] [CrossRef]
- Daru, J.; Colman, K.; Stanworth, S.J.; De La Salle, B.; Wood, E.M.; Pasricha, S.R. Serum ferritin as an indicator of iron status: What do we need to know? Am. J. Clin. Nutr. 2017, 106, 1634S–1639S60. [Google Scholar] [CrossRef] [PubMed]
- Shipton, M.J.; Johal, N.J.; Dutta, N.; Slater, C.; Iqbal, Z.; Ahmed, B.; Ammori, B.J.; Senapati, S.; Akhtar, K.; Summers, L.K.M.; et al. Haemoglobin and Hematinic Status Before and After Bariatric Surgery over 4 years of Follow-up. Obes. Surg. 2021, 31, 682–693. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.J.S.; Salazar, D.A.; Neves, J.S.; Pedro, J.M.P.; Guerreiro, V.A.; Viana, S.e.S.; Mendonça, F.; Silva, M.M.; Belo, S.P.; Sande, A.V. Which Factors Are Associated with a Higher Prevalence of Anemia Following Bariatric Surgery? Results from a Retrospective Study Involving 1999 Patients. Obes Surg. 2020, 30, 3496–3502. [Google Scholar] [CrossRef] [PubMed]
- Cătoi, A.F.; Iancu, M.; Pârvu, A.E.; Cecan, A.D.; Bidian, C.; Chera, E.I.; Pop, I.D.; Macri, A.M. Relationship between 25 Hydroxyvitamin D, Overweight/Obesity Status, Pro-Inflammatory and Oxidative Stress Markers in Patients with Type 2 Diabetes: A Simplified Empirical Path Model. Nutrients 2021, 13, 2889. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Deficiency | Excess |
|---|---|---|
| Iron | <50 μg/dL | - |
| Ferritin | <28 ng/mL (male) <5 ng/mL (female) | - |
| Folate | <2.7 ng/mL | - |
| Vitamin B12 | <193 pg/mL | - |
| Calcium | <8.6 mg/dL | - |
| 25(OH)D | <10 ng/mL | - |
| PTH | >64 pg/mL |
| Variables | All Sample (n = 50) | Males (n1 = 16) | Females (n2 = 34) | p-Value |
|---|---|---|---|---|
| Age | 46.5(10.9) | 47.0 (11.1) | 46.3(10.9) | 0.83 |
| BMI, kg/m2 | 43.1 (1.1) | 43.5 (1.1) | 42.9 (1.1) | 0.75 |
| WHR | 0.9 (0.1) | 1.1 (0.1) | 0.9 (0.1) | <0.001 * |
| Comorbidities | ||||
| T2D, n (%) | 12 (24) | 5 (42) | 7 (58) | 0.49 |
| Hypertension, n (%) | 25 (50) | 9 (36) | 16 (64) | 0.54 |
| Dyslipidemia, n (%) | 44 (88) | 16 (36) | 28 (64) | 0.16 |
| NAFLD, n (%) | 31 (62) | 10 (32) | 21 (68) | 0.96 |
| Outcome | Group | Baseline | 6 MTH | p-Value 1 | 12 MTH | p-Value 2 | p-Value 3 | Adjusted p-Value (Male vs. Female) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| LS-Means [95% CI] | LS-Means [95% CI] | LSMs [95% CI] | Baseline | 6 MTH | 12 MTH | |||||
| Weight (kg) a | Male | 139.8 [130.3, 149.9] # | 98.5 [91.8, 106.7] # | <0.001 * | 91.8 [85.6, 99.5] # | 0.03 * | <0.001 | <0.001 * | 0.004 * | <0.001 * |
| Female | 114.4 [108.9, 120.3] # | 83.1 [79.1, 87.4] # | <0.001 * | 73.7 [70.1, 78.3] # | <0.001 * | <0.001 * | ||||
| BMI, kg/m2 a | Male | 43.8 [41.4, 46.2] | 31.2 [29.6, 32.9] | <0.001 * | 28.9 [26.4, 31.4] | 0.13 | <0.001 * | >0.99 | 1.00 | 0.98 |
| Female | 43.3 [41.6, 44.9] | 31.0 [28.5, 33.4] | <0.001 * | 27.8 [26.1, 29.5] | <0.001 * | <0.001 * | ||||
| WHR b | Male | 1.06 [1.02, 1.10] | 1.03 [0.99, 1.07] | 0.58 | 1.01 [0.96, 1.06] | 0.94 | 0.19 | <0.001 * | <0.001 * | 0.002 * |
| Female | 0.93 [0.91, 0.96] | 0.91 [0.89, 0.94] | 0.75 | 0.90 [0.87, 0.93] | 0.94 | 0.22 | ||||
| Preoperative Deficiency n (%) | Postoperative Deficiency 1 n (%) | p-Value | |
|---|---|---|---|
| Vitamin B12 (pg/mL) a | 8 (16.7) | 6 (12.5) | 0.75 |
| Folate (ng/mL) b | 3 (6.7) | 5 (11.1) | 0.68 |
| Iron (μg/dL) c | 1 (2.3) | 2 (4.6) | 1.00 |
| Ferritin (ng/mL) | 0 (0.0) | 0 (0.0) | Na |
| 25(OH)D (ng/mL) d | 15 (100.0) | 15 (100.0) | Na |
| Calcium (mg/dL) e | 2 (4.7) | 1 (2.3) | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciobârcă, D.; Cătoi, A.F.; Copăescu, C.; Iancu, M.; Pop, I.D.; Vodnar, D.C.; Cecan, A.D.; Miere, D.; Filip, L.; Crișan, G. Micronutrient Status in Patients with Severe Obesity Before and After Laparoscopic Sleeve Gastrectomy. Nutrients 2024, 16, 4386. https://doi.org/10.3390/nu16244386
Ciobârcă D, Cătoi AF, Copăescu C, Iancu M, Pop ID, Vodnar DC, Cecan AD, Miere D, Filip L, Crișan G. Micronutrient Status in Patients with Severe Obesity Before and After Laparoscopic Sleeve Gastrectomy. Nutrients. 2024; 16(24):4386. https://doi.org/10.3390/nu16244386
Chicago/Turabian StyleCiobârcă, Daniela, Adriana Florinela Cătoi, Cătălin Copăescu, Mihaela Iancu, Ioana Delia Pop, Dan Cristian Vodnar, Andra Diana Cecan, Doina Miere, Lorena Filip, and Gianina Crișan. 2024. "Micronutrient Status in Patients with Severe Obesity Before and After Laparoscopic Sleeve Gastrectomy" Nutrients 16, no. 24: 4386. https://doi.org/10.3390/nu16244386
APA StyleCiobârcă, D., Cătoi, A. F., Copăescu, C., Iancu, M., Pop, I. D., Vodnar, D. C., Cecan, A. D., Miere, D., Filip, L., & Crișan, G. (2024). Micronutrient Status in Patients with Severe Obesity Before and After Laparoscopic Sleeve Gastrectomy. Nutrients, 16(24), 4386. https://doi.org/10.3390/nu16244386










