Lutein Maintains Bone Mass In Vitro and In Vivo Against Disuse-Induced Bone Loss in Hindlimb-Unloaded Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Reagents
2.2. Osteoclast Differentiation Induced by IL-1 in Cocultures of Mouse Primary Osteoblasts and Mouse Bone Marrow Cells
2.3. Osteoclast Differentiation Induced by Soluble RANKL in Cultures of Raw264.7 Cells
2.4. TRAP Staining
2.5. Organ Cultures of Calvariae from Neonatal Mice
2.6. Calcified Bone Nodule Formation in POB Cultures
2.7. mRNA Expression Analysis by Quantitative PCR
2.8. In Silico Molecular Docking Simulation
2.8.1. Ligand Preparation
2.8.2. Protein Preparation
2.8.3. Binding Site Definition
2.8.4. Molecular Docking
2.8.5. Post-Docking Analysis
2.8.6. Visualization and Interaction Analysis
2.9. In Vitro Assay of IKK Kinase Activity
2.10. Protein Expression Analysis by Western Blotting
2.11. Feeding 1% Lutein-Contained Diets to HLU Mice
2.12. Statistical Analysis
3. Results
3.1. Compared Effects of Lutein, Astaxanthin, and β-Cryptoxanthin on Osteoclastic Bone Resorption
3.2. Effects of Lutein, Astaxanthin, and β-Cryptoxanthin on Osteoblastic Bone Mineralization
3.3. Lutein Ameliorated the NF-κB Pathway in Osteoblasts
3.4. Feeding of a 1% Lutein-Contained Diet Ameliorated Bone Loss in HLU Mice
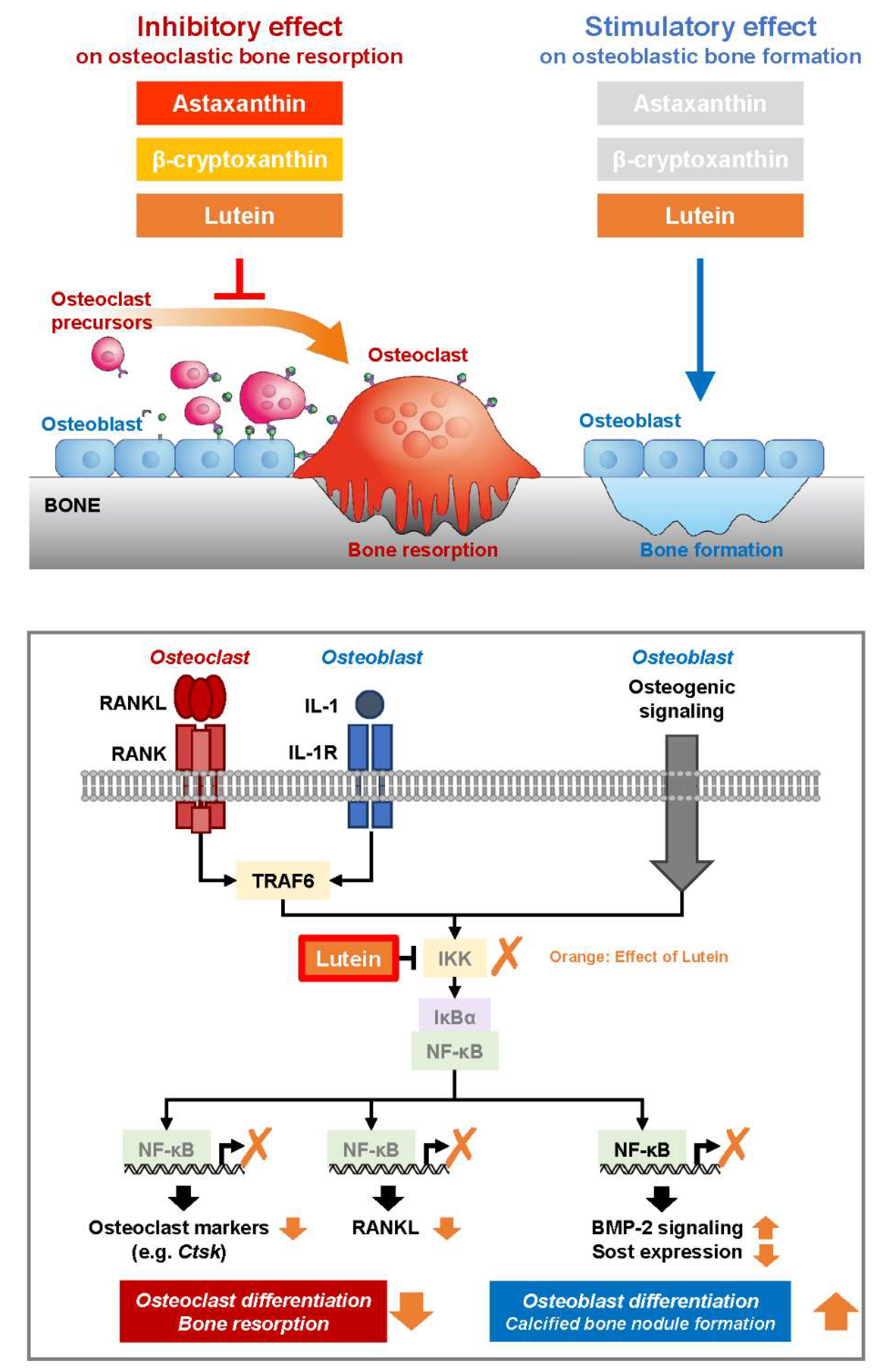
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bolamperti, S.; Villa, I.; Rubinacci, A. Bone Remodeling: An Operational Process Ensuring Survival and Bone Mechanical Competence. Bone Res. 2022, 10, 48. [Google Scholar] [CrossRef] [PubMed]
- Natesan, V.; Kim, S.-J. Metabolic Bone Diseases and New Drug Developments. Biomol. Ther. 2022, 30, 309–319. [Google Scholar] [CrossRef]
- Tanaka, S.; Tanaka, Y.; Ishiguro, N.; Yamanaka, H.; Takeuchi, T. RANKL: A Therapeutic Target for Bone Destruction in Rheumatoid Arthritis. Mod. Rheumatol. 2017, 28, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Inada, M.; Matsumoto, C.; Uematsu, S.; Akira, S.; Miyaura, C. Membrane-Bound Prostaglandin E Synthase-1-Mediated Prostaglandin E2 Production by Osteoblast Plays a Critical Role in Lipopolysaccharide-Induced Bone Loss Associated with Inflammation. J. Immunol. 2006, 177, 1879–1885. [Google Scholar] [CrossRef] [PubMed]
- Steeve, K.T.; Marc, P.; Sandrine, T.; Dominique, H.; Yannick, F. IL-6, RANKL, TNF-Alpha/IL-1: Interrelations in Bone Resorption Pathophysiology. Cytokine Growth Factor Rev. 2004, 15, 49–60. [Google Scholar] [CrossRef]
- Tominari, T.; Matsumoto, C.; Tanaka, Y.; Shimizu, K.; Takatoya, M.; Sugasaki, M.; Karouji, K.; Kasuga, U.; Miyaura, C.; Miyata, S.; et al. Roles of Toll-like Receptor Signaling in Inflammatory Bone Resorption. Biology 2024, 13, 692. [Google Scholar] [CrossRef]
- Rolvien, T.; Amling, M. Disuse Osteoporosis: Clinical and Mechanistic Insights. Calcif. Tissue Int. 2022, 110, 592–604. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, B.-J.; Choi, H.J.; Cho, S.W.; Shin, C.S.; Park, S.-Y.; Lee, Y.-S.; Lee, S.-Y.; Kim, H.-H.; Kim, G.S.; et al. (–)-Epigallocathechin-3-Gallate, an AMPK Activator, Decreases Ovariectomy-Induced Bone Loss by Suppression of Bone Resorption. Calcif. Tissue Int. 2012, 90, 404–410. [Google Scholar] [CrossRef]
- Ermakov, I.V.; Ermakova, M.R.; Rosenberg, T.D.; Gellermann, W. Optical Detection of Carotenoid Antioxidants in Human Bone and Surrounding Tissue. J. Biomed. Opt. 2013, 18, 117006. [Google Scholar] [CrossRef]
- Ozaki, K.; Okamoto, M.; Fukasawa, K.; Iezaki, T.; Onishi, Y.; Yoneda, Y.; Sugiura, M.; Hinoi, E. Daily Intake of β-Cryptoxanthin Prevents Bone Loss by Preferential Disturbance of Osteoclastic Activation in Ovariectomized Mice. J. Pharmacol. Sci. 2015, 129, 72–77. [Google Scholar] [CrossRef]
- Adhikary, S.; Choudhary, D.; Ahmad, N.; Karvande, A.; Kumar, A.; Banala, V.T.; Mishra, P.R.; Trivedi, R. Dietary Flavonoid Kaempferol Inhibits Glucocorticoid-Induced Bone Loss by Promoting Osteoblast Survival. Nutrition 2018, 53, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, V.; Rivoira, M.; Picotto, G.; de Barboza, G.D.; Collin, A.; de Talamoni, N.T. Analysis of the Molecular Mechanisms by Flavonoids with Potential Use for Osteoporosis Prevention or Therapy. Curr. Med. Chem. 2022, 29, 2913–2936. [Google Scholar] [CrossRef]
- Tominari, T.; Ichimaru, R.; Yoshinouchi, S.; Matsumoto, C.; Watanabe, K.; Hirata, M.; Grundler, F.M.W.; Inada, M.; Miyaura, C. Effects of O-methylated (−)-epigallocatechin Gallate (EGCG) on LPS-induced Osteoclastogenesis, Bone Resorption, and Alveolar Bone Loss in Mice. FEBS Open Bio 2017, 7, 1972–1981. [Google Scholar] [CrossRef]
- Hirata, M.; Tominari, T.; Ichimaru, R.; Takiguchi, N.; Tanaka, Y.; Takatoya, M.; Arai, D.; Yoshinouchi, S.; Miyaura, C.; Matsumoto, C.; et al. Effects of 4′-Demethylnobiletin and 4′-Demethyltangeretin on Osteoclast Differentiation In Vitro and in a Mouse Model of Estrogen-Deficient Bone Resorption. Nutrients 2023, 15, 1403. [Google Scholar] [CrossRef] [PubMed]
- Hirata, N.; Ichimaru, R.; Tominari, T.; Matsumoto, C.; Watanabe, K.; Taniguchi, K.; Hirata, M.; Ma, S.; Suzuki, K.; Grundler, F.M.W.; et al. Beta-Cryptoxanthin Inhibits Lipopolysaccharide-Induced Osteoclast Differentiation and Bone Resorption via the Suppression of Inhibitor of NF-ΚB Kinase Activity. Nutrients 2019, 11, 368. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, S.; Tominari, T.; Matsumoto, C.; Yoshinouchi, S.; Ichimaru, R.; Watanabe, K.; Hirata, M.; Grundler, F.M.W.; Miyaura, C.; Inada, M. Effects of Polymethoxyflavonoids on Bone Loss Induced by Estrogen Deficiency and by LPS-Dependent Inflammation in Mice. Pharmaceuticals 2018, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Tousen, Y.; Ichimaru, R.; Kondo, T.; Inada, M.; Miyaura, C.; Ishimi, Y. The Combination of Soy Isoflavones and Resveratrol Preserve Bone Mineral Density in Hindlimb-Unloaded Mice. Nutrients 2020, 12, 2043. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Igarashi, A.; Uchiyama, S.; Sugawara, K.; Sumida, T.; Morita, S.; Ogawa, H.; Nishitani, M.; Kajimoto, Y. Effect of β-Crytoxanthin on Circulating Bone Metabolic Markers: Intake of Juice (Citrus Unshiu) Supplemented with β-Cryptoxanthin Has an Effect in Menopausal Women. J. Health Sci. 2006, 52, 758–768. [Google Scholar] [CrossRef]
- Oliveira, G.R.; Vargas-Sanchez, P.K.; Fernandes, R.R.; Ricoldi, M.S.T.; Semeghini, M.S.; Pitol, D.L.; de Sousa, L.G.; Siessere, S.; Bombonato-Prado, K.F. Lycopene Influences Osteoblast Functional Activity and Prevents Femur Bone Loss in Female Rats Submitted to an Experimental Model of Osteoporosis. J. Bone Miner. Metab. 2019, 37, 658–667. [Google Scholar] [CrossRef]
- Kim, S.J.; Anh, N.H.; Diem, N.C.; Park, S.; Cho, Y.H.; Long, N.P.; Hwang, I.G.; Lim, J.; Kwon, S.W. Effects of β-Cryptoxanthin on Improvement in Osteoporosis Risk: A Systematic Review and Meta-Analysis of Observational Studies. Foods 2021, 10, 296. [Google Scholar] [CrossRef]
- Semeghini, M.S.; Scalize, P.H.; Coelho, M.C.; Fernandes, R.R.; Pitol, D.L.; Tavares, M.S.; de Sousa, L.G.; Coppi, A.A.; Siessere, S.; Bombonato-Prado, K.F. Lycopene Prevents Bone Loss in Ovariectomized Rats and Increases the Number of Osteocytes and Osteoblasts. J. Anat. 2022, 241, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, M.; Nakamura, M.; Ogawa, K.; Ikoma, Y.; Yano, M. High Serum Carotenoids Associated with Lower Risk for Bone Loss and Osteoporosis in Post-Menopausal Japanese Female Subjects: Prospective Cohort Study. PLoS ONE 2012, 7, e52643. [Google Scholar] [CrossRef]
- Zhang, Z.-Q.; Cao, W.-T.; Liu, J.; Cao, Y.; Su, Y.-X.; Chen, Y.-M. Greater Serum Carotenoid Concentration Associated with Higher Bone Mineral Density in Chinese Adults. Osteoporos. Int. 2016, 27, 1593–1601. [Google Scholar] [CrossRef]
- Kan, B.; Guo, D.; Yuan, B.; Vuong, A.M.; Jiang, D.; Zhang, M.; Cheng, H.; Zhao, Q.; Li, B.; Feng, L.; et al. Dietary Carotenoid Intake and Osteoporosis: The National Health and Nutrition Examination Survey, 2005–2018. Arch. Osteoporos. 2022, 17, 2. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Yuki, K.; Kurihara, T.; Miyake, S.; Noda, K.; Kobayashi, S.; Ishida, S.; Tsubota, K.; Ozawa, Y. Biological Role of Lutein in the Light-Induced Retinal Degeneration. J. Nutr. Biochem. 2012, 23, 423–429. [Google Scholar] [CrossRef]
- Widomska, J.; Subczynski, W.K.; Welc-Stanowska, R.; Luchowski, R. An Overview of Lutein in the Lipid Membrane. Int. J. Mol. Sci. 2023, 24, 12948. [Google Scholar] [CrossRef]
- Johra, F.T.; Bepari, A.K.; Bristy, A.T.; Reza, H.M. A Mechanistic Review of β-Carotene, Lutein, and Zeaxanthin in Eye Health and Disease. Antioxidants 2020, 9, 1046. [Google Scholar] [CrossRef] [PubMed]
- Tominari, T.; Matsumoto, C.; Watanabe, K.; Hirata, M.; Grundler, F.M.W.; Inada, M.; Miyaura, C. Lutein, a Carotenoid, Suppresses Osteoclastic Bone Resorption and Stimulates Bone Formation in Cultures. Biosci. Biotechnol. Biochem. 2017, 81, 302–306. [Google Scholar] [CrossRef]
- Takeda, H.; Tominari, T.; Hirata, M.; Watanabe, K.; Matsumoto, C.; Grundler, F.M.W.; Inada, M.; Miyaura, C. Lutein Enhances Bone Mass by Stimulating Bone Formation and Suppressing Bone Resorption in Growing Mice. Biol. Pharm. Bull. 2017, 40, 716–721. [Google Scholar] [CrossRef]
- Takeda, H.; Tominari, T.; Ichimaru, R.; Matsumoto, C.; Watanabe, K.; Hirata, M.; Inada, M.; Miyaura, C. Lutein, a Carotenoid, Inhibits Lipopolysaccharide-Induced Alveolar Bone Loss Associated with Inflammation in a Mouse Model of Periodontitis. Curr. Top. Biochem. Res. 2016, 17, 71–76. [Google Scholar]
- Srivastava, S.; Toraldo, G.; Weitzmann, M.N.; Cenci, S.; Ross, F.P.; Pacifici, R. Estrogen Decreases Osteoclast Formation by Down-Regulating Receptor Activator of NF-ΚB Ligand (RANKL)-Induced JNK Activation. J. Biol. Chem. 2001, 276, 8836–8840. [Google Scholar] [CrossRef] [PubMed]
- Weitzmann, M.N.; Pacifici, R. Estrogen Deficiency and Bone Loss: An Inflammatory Tale. J. Clin. Investig. 2006, 116, 1186–1194. [Google Scholar] [CrossRef]
- Mohamad, N.-V.; Ima-Nirwana, S.; Chin, K.-Y. Are Oxidative Stress and Inflammation Mediators of Bone Loss Due to Estrogen Deficiency? A Review of Current Evidence. Endocr. Metab. Immune Disord.-Drug Targets 2020, 20, 1478–1487. [Google Scholar] [CrossRef] [PubMed]
- Iantomasi, T.; Romagnoli, C.; Palmini, G.; Donati, S.; Falsetti, I.; Miglietta, F.; Aurilia, C.; Marini, F.; Giusti, F.; Brandi, M.L. Oxidative Stress and Inflammation in Osteoporosis: Molecular Mechanisms Involved and the Relationship with MicroRNAs. Int. J. Mol. Sci. 2023, 24, 3772. [Google Scholar] [CrossRef]
- Li, H.; Huang, C.; Zhu, J.; Gao, K.; Fang, J.; Li, H. Lutein Suppresses Oxidative Stress and Inflammation by Nrf2 Activation in an Osteoporosis Rat Model. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 5071–5075. [Google Scholar] [CrossRef] [PubMed]
- Bovier, E.R.; Hammond, B.R. The Macular Carotenoids Lutein and Zeaxanthin Are Related to Increased Bone Density in Young Healthy Adults. Foods 2017, 6, 78. [Google Scholar] [CrossRef]
- Regu, G.M.; Kim, H.; Kim, Y.J.; Paek, J.E.; Lee, G.; Chang, N.; Kwon, O. Association between Dietary Carotenoid Intake and Bone Mineral Density in Korean Adults Aged 30–75 Years Using Data from the Fourth and Fifth Korean National Health and Nutrition Examination Surveys (2008–2011). Nutrients 2017, 9, 1025. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Misquitta, Y.R.; Olland, A.; Johnson, M.A.; Kelleher, K.S.; Kriz, R.; Lin, L.L.; Stahl, M.; Mosyak, L. Crystal Structure of a Human IκB Kinase β Asymmetric Dimer. J. Biol. Chem. 2013, 288, 22758–22767. [Google Scholar] [CrossRef]
- Uchiyama, S.; Yamaguchi, M. Inhibitory Effect of β-Cryptoxanthin on Osteoclast-like Cell Formation in Mouse Marrow Cultures. Biochem. Pharmacol. 2004, 67, 1297–1305. [Google Scholar] [CrossRef]
- Mamun-Or-Rashid, A.N.M.; Lucy, T.T.; Yagi, M.; Yonei, Y. Inhibitory Effects of Astaxanthin on CML-HSA-Induced Inflammatory and RANKL-Induced Osteoclastogenic Gene Expression in RAW 264.7 Cells. Biomedicines 2021, 10, 54. [Google Scholar] [CrossRef]
- Uchiyama, S.; Yamaguchi, M. Beta-Cryptoxanthin Stimulates Cell Proliferation and Transcriptional Activity in Osteoblastic MC3T3-E1 Cells. Int. J. Mol. Med. 2005, 15, 675–681. [Google Scholar] [PubMed]
- Zhao, G.; Zhong, H.; Rao, T.; Pan, Z. Metabolomic Analysis Reveals That the Mechanism of Astaxanthin Improves the Osteogenic Differentiation Potential in Bone Marrow Mesenchymal Stem Cells. Oxidative Med. Cell. Longev. 2020, 2020, 3427430. [Google Scholar] [CrossRef] [PubMed]
- Sontakke, A.N.; Tare, R.S. A Duality in the Roles of Reactive Oxygen Species with Respect to Bone Metabolism. Clin. Chim. Acta 2002, 318, 145–148. [Google Scholar] [CrossRef]
- Agidigbi, T.S.; Kim, C. Reactive Oxygen Species in Osteoclast Differentiation and Possible Pharmaceutical Targets of ROS-Mediated Osteoclast Diseases. Int. J. Mol. Sci. 2019, 20, 3576. [Google Scholar] [CrossRef] [PubMed]
- Callaway, D.A.; Jiang, J.X. Reactive Oxygen Species and Oxidative Stress in Osteoclastogenesis, Skeletal Aging and Bone Diseases. J. Bone Miner. Metab. 2015, 33, 359–370. [Google Scholar] [CrossRef]
- Schröder, K. NADPH Oxidases in Bone Homeostasis and Osteoporosis. Free. Radic. Biol. Med. 2019, 132, 67–72. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Y.; Xu, M.; Zheng, J.; Xu, Y.; Chen, G.; Guo, Q.; Tian, W.; Guo, W. The Dual Effects of Reactive Oxygen Species on the Mandibular Alveolar Bone Formation in SOD1 Knockout Mice: Promotion or Inhibition. Oxidative Med. Cell. Longev. 2021, 2021, 8847140. [Google Scholar] [CrossRef]
- Rojasawasthien, T.; Usui, M.; Addison, W.N.; Matsubara, T.; Shirakawa, T.; Tsujisawa, T.; Nakashima, K.; Kokabu, S. Nobiletin, a NF-κB Signaling Antagonist, Promotes BMP-induced Bone Formation. FASEB BioAdvances 2023, 5, 62–70. [Google Scholar] [CrossRef]
- Baek, K.; Hwang, H.R.; Park, H.; Kwon, A.; Qadir, A.S.; Ko, S.; Woo, K.M.; Ryoo, H.; Kim, G.; Baek, J. TNF-α Upregulates Sclerostin Expression in Obese Mice Fed a High-Fat Diet. J. Cell. Physiol. 2014, 229, 640–650. [Google Scholar] [CrossRef]
- Alles, N.; Soysa, N.S.; Hayashi, J.; Khan, M.; Shimoda, A.; Shimokawa, H.; Ritzeler, O.; Akiyoshi, K.; Aoki, K.; Ohya, K. Suppression of NF-ΚB Increases Bone Formation and Ameliorates Osteopenia in Ovariectomized Mice. Endocrinology 2010, 151, 4626–4634. [Google Scholar] [CrossRef]
- Chang, J.; Wang, Z.; Tang, E.; Fan, Z.; McCauley, L.; Franceschi, R.; Guan, K.; Krebsbach, P.H.; Wang, C.-Y. Inhibition of Osteoblastic Bone Formation by Nuclear Factor-ΚB. Nat. Med. 2009, 15, 682–689. [Google Scholar] [CrossRef]
- Ahn, Y.J.; Kim, H. Lutein as a Modulator of Oxidative Stress-Mediated Inflammatory Diseases. Antioxidants 2021, 10, 1448. [Google Scholar] [CrossRef] [PubMed]
- Frede, K.; Ebert, F.; Kipp, A.P.; Schwerdtle, T.; Baldermann, S. Lutein Activates the Transcription Factor Nrf2 in Human Retinal Pigment Epithelial Cells. J. Agric. Food Chem. 2017, 65, 5944–5952. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Liu, W.; Zhao, J.; Meng, F.; Wang, H. Lutein Protects against β-Amyloid Peptide-Induced Oxidative Stress in Cerebrovascular Endothelial Cells through Modulation of Nrf-2 and NF-Κb. Cell Biol. Toxicol. 2017, 33, 57–67. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Zhang, H.; Wang, C.; Jiao, F.; Xu, H.; Wang, X.; Luan, W.; Ma, F.; Ni, L.; Tang, X.; et al. Activation of ROS/MAPKs/NF-κB/NLRP3 and Inhibition of Efferocytosis in Osteoclast-mediated Diabetic Osteoporosis. FASEB J. 2019, 33, 12515–12527. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Liu, H.; Wu, X.; Weng, W.; Wang, X.; Su, J. Reactive Oxygen Species (ROS)-Responsive Biomaterials for the Treatment of Bone-Related Diseases. Front. Bioeng. Biotechnol. 2022, 9, 820468. [Google Scholar] [CrossRef]
- Bartell, S.M.; Kim, H.-N.; Ambrogini, E.; Han, L.; Iyer, S.; Ucer, S.S.; Rabinovitch, P.; Jilka, R.L.; Weinstein, R.S.; Zhao, H.; et al. FoxO Proteins Restrain Osteoclastogenesis and Bone Resorption by Attenuating H2O2 Accumulation. Nat. Commun. 2014, 5, 3773. [Google Scholar] [CrossRef]
- Deng, S.; Dai, G.; Chen, S.; Nie, Z.; Zhou, J.; Fang, H.; Peng, H. Dexamethasone Induces Osteoblast Apoptosis through ROS-PI3K/AKT/GSK3β Signaling Pathway. Biomed. Pharmacother. 2019, 110, 602–608. [Google Scholar] [CrossRef]
- Khalid, S.; Yamazaki, H.; Socorro, M.; Monier, D.; Beniash, E.; Napierala, D. Reactive Oxygen Species (ROS) Generation as an Underlying Mechanism of Inorganic Phosphate (Pi)-Induced Mineralization of Osteogenic Cells. Free. Radic. Biol. Med. 2020, 153, 103–111. [Google Scholar] [CrossRef]
- Hu, G.; Yu, Y.; Sharma, D.; Pruett-Miller, S.M.; Ren, Y.; Zhang, G.-F.; Karner, C.M. Glutathione Limits RUNX2 Oxidation and Degradation to Regulate Bone Formation. JCI Insight 2023, 8, e166888. [Google Scholar] [CrossRef]
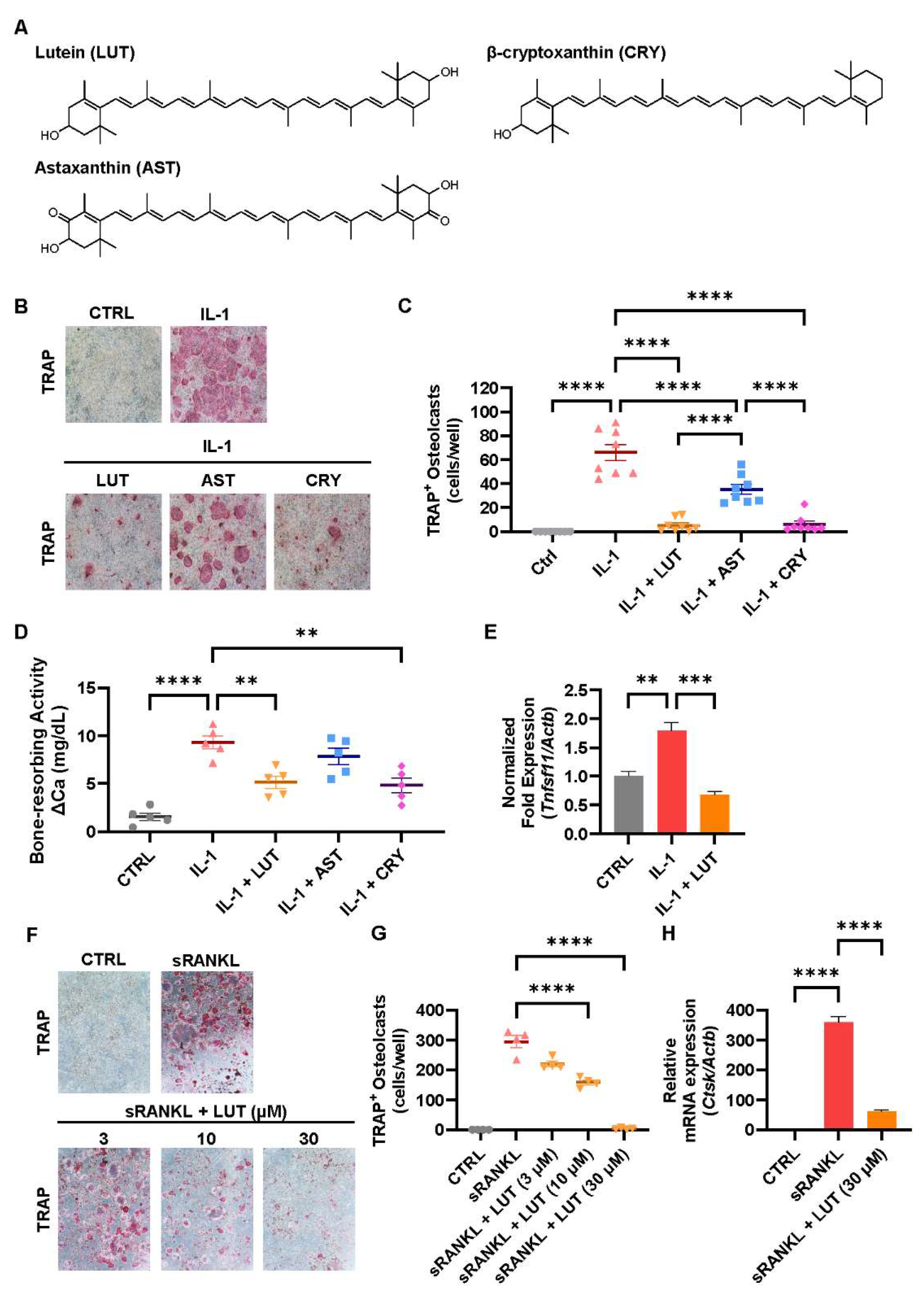
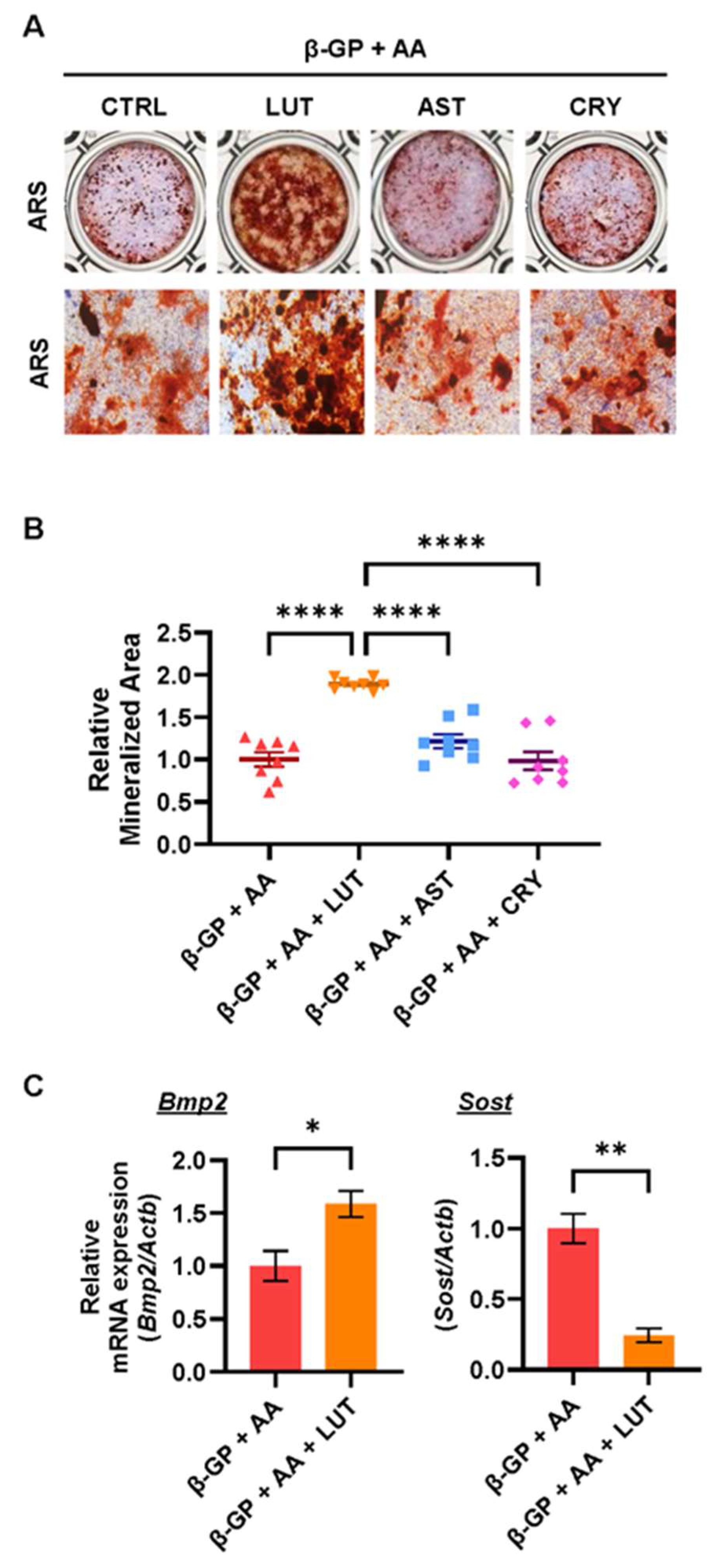

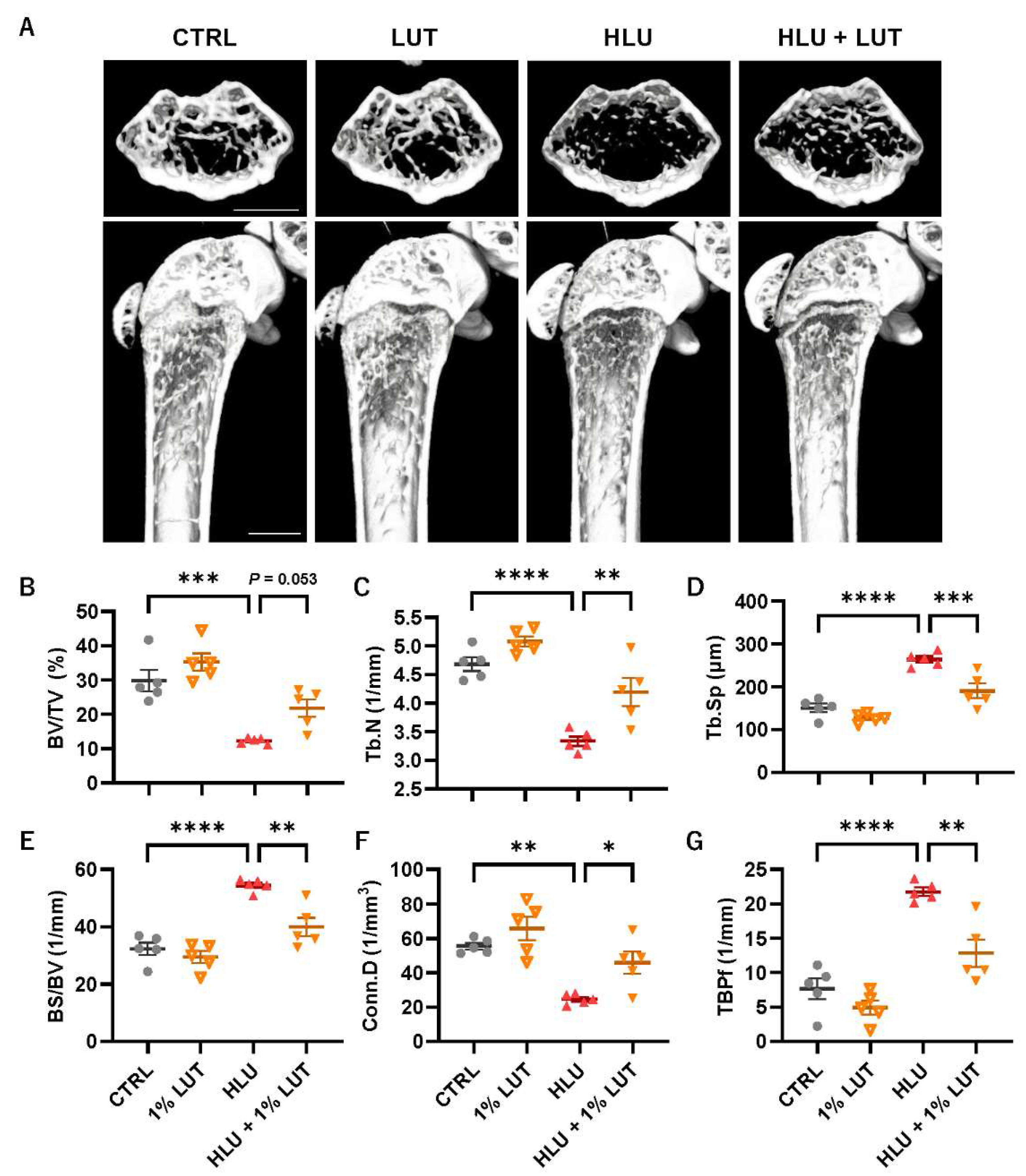
| Gene | Forward | Reverse |
|---|---|---|
| Actb | 5′-ccccattgaacatggcattg-3′ | 5′-acgaccagaggcatacagg-3′ |
| Tnfsf11 | 5′-aggctgggccaagatctcta-3′ | 5′-gtctgtaggtacgcttcccg-3′ |
| Ctsk | 5′-cattctcagacacacaatccac-3′ | 5′-gatactggacaccactggga-3′ |
| Bmp2 | 5′-gtcgaagctctcccactgac-3′ | 5′-caggaagctttgggaaacag-3′ |
| Sost | 5′-gtgtgatgttgggctacgtg-3′ | 5′-ccaccacaatctctccccta-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, Y.; Tominari, T.; Takatoya, M.; Arai, D.; Sugasaki, M.; Ichimaru, R.; Miyaura, C.; Matsumoto, C.; Ma, S.; Suzuki, K.; et al. Lutein Maintains Bone Mass In Vitro and In Vivo Against Disuse-Induced Bone Loss in Hindlimb-Unloaded Mice. Nutrients 2024, 16, 4271. https://doi.org/10.3390/nu16244271
Tanaka Y, Tominari T, Takatoya M, Arai D, Sugasaki M, Ichimaru R, Miyaura C, Matsumoto C, Ma S, Suzuki K, et al. Lutein Maintains Bone Mass In Vitro and In Vivo Against Disuse-Induced Bone Loss in Hindlimb-Unloaded Mice. Nutrients. 2024; 16(24):4271. https://doi.org/10.3390/nu16244271
Chicago/Turabian StyleTanaka, Yuki, Tsukasa Tominari, Masaru Takatoya, Daichi Arai, Moe Sugasaki, Ryota Ichimaru, Chisato Miyaura, Chiho Matsumoto, Sihui Ma, Katsuhiko Suzuki, and et al. 2024. "Lutein Maintains Bone Mass In Vitro and In Vivo Against Disuse-Induced Bone Loss in Hindlimb-Unloaded Mice" Nutrients 16, no. 24: 4271. https://doi.org/10.3390/nu16244271
APA StyleTanaka, Y., Tominari, T., Takatoya, M., Arai, D., Sugasaki, M., Ichimaru, R., Miyaura, C., Matsumoto, C., Ma, S., Suzuki, K., Hirata, M., Grundler, F. M. W., & Inada, M. (2024). Lutein Maintains Bone Mass In Vitro and In Vivo Against Disuse-Induced Bone Loss in Hindlimb-Unloaded Mice. Nutrients, 16(24), 4271. https://doi.org/10.3390/nu16244271










