Efficacy of ETB-F01, Heat-Killed Akkermansia muciniphila Strain EB-AMDK19, in Patients with Respiratory Symptoms: A Multicenter Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
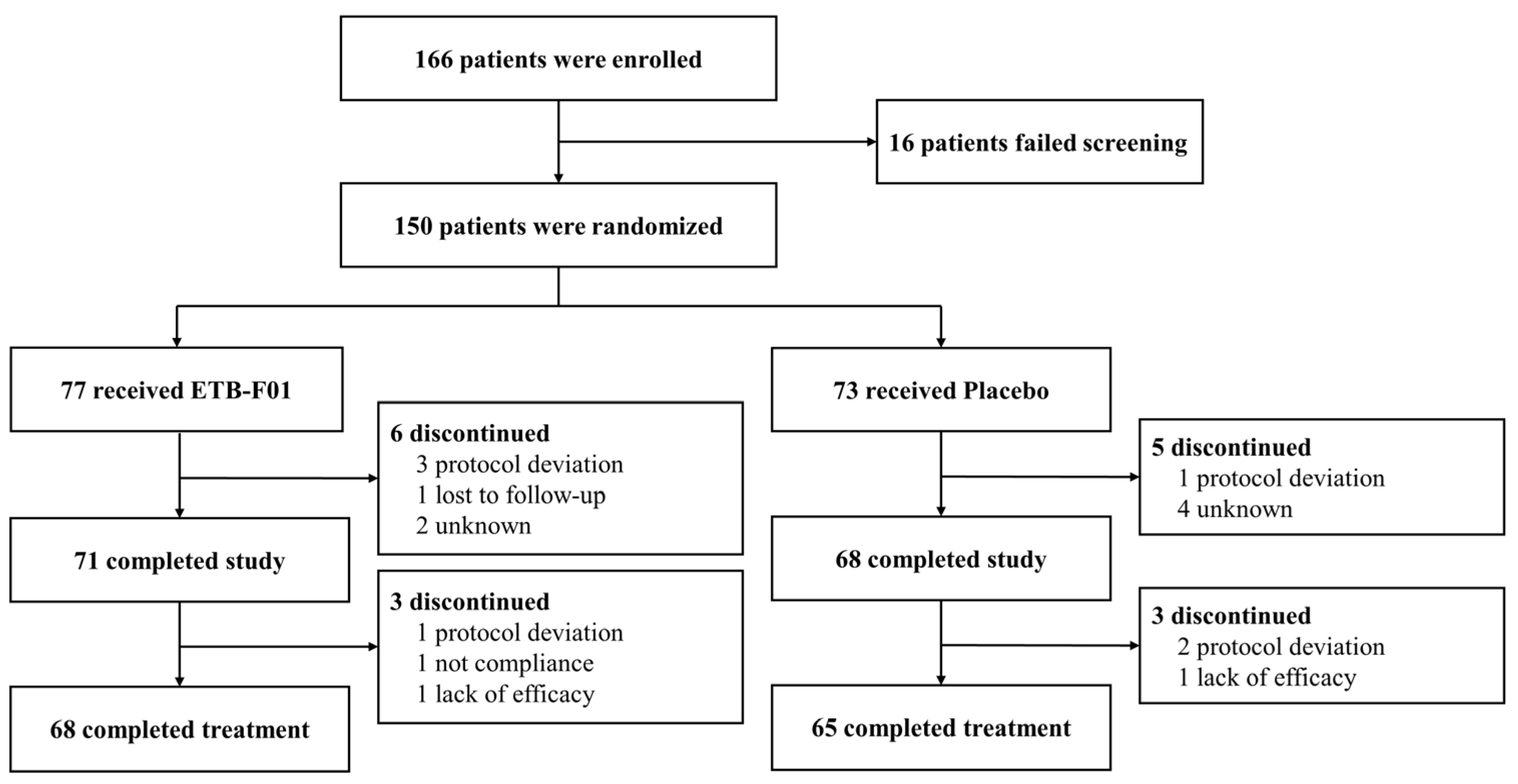
2.2. Patients
2.3. Procedures
2.4. Ethics
2.5. Outcomes
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. BCSS
3.3. Lung Function and FeNO
3.4. Analyses of Other Outcomes
3.5. Safety Profiles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Miravitlles, M.; Soler-Cataluña, J.J.; Soriano, J.B.; García-Río, F.; de Lucas, P.; Alfageme, I.; Casanova, C.; Rodríguez González-Moro, J.M.; Sánchez, G.; Ancochea, J.; et al. Respiratory symptoms and their determinants in the general Spanish population: Changes over 20 years. ERJ Open Res. 2022, 8, 00067–2022. [Google Scholar] [CrossRef] [PubMed]
- Harju, T.; Mäkinen, T.; Näyhä, S.; Laatikainen, T.; Jousilahti, P.; Hassi, J. Cold-related respiratory symptoms in the general population. Clin. Respir. J. 2010, 4, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Çolak, Y.; Nordestgaard, B.G.; Vestbo, J.; Lange, P.; Afzal, S. Prognostic significance of chronic respiratory symptoms in individuals with normal spirometry. Eur. Respir. J. 2019, 54, 1900734. [Google Scholar] [CrossRef] [PubMed]
- Jakeways, N.; McKeever, T.; Lewis, S.A.; Weiss, S.T.; Britton, J. Relationship between FEV1 reduction and respiratory symptoms in the general population. Eur. Respir. J. 2003, 21, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Krzyzanowski, M.; Camilli, A.E.; Lebowitz, M.D. Relationships between pulmonary function and changes in chronic respiratory symptoms. Comparison of Tucson and Cracow longitudinal studies. Chest 1990, 98, 62–70. [Google Scholar] [CrossRef]
- Stavem, K.; Johannessen, A.; Nielsen, R.; Gulsvik, A. Respiratory symptoms and respiratory deaths: A multi-cohort study with 45 years observation time. PLoS ONE 2021, 16, e0260416. [Google Scholar] [CrossRef]
- Gulsvik, A.; Bakke, P.S.; Brøgger, J.; Nielsen, R.; Stavem, K. Respiratory symptoms and mortality in four general population cohorts over 45 years. Respir. Med. 2020, 170, 106060. [Google Scholar] [CrossRef]
- Mousa, W.K.; Mousa, S.; Ghemrawi, R.; Obaid, D.; Sarfraz, M.; Chehadeh, F.; Husband, S. Probiotics Modulate Host Immune Response and Interact with the Gut Microbiota: Shaping Their Composition and Mediating Antibiotic Resistance. Int. J. Mol. Sci. 2023, 24, 13783. [Google Scholar] [CrossRef]
- Ghosh, S.; Whitley, C.S.; Haribabu, B.; Jala, V.R. Regulation of intestinal barrier function by microbial metabolites. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 1463–1482. [Google Scholar] [CrossRef]
- Derrien, M.; Collado, M.C.; Ben-Amor, K.; Salminen, S.; de Vos, W.M. The Mucin degrader Akkermansia muciniphila is an abundant resident of the human intestinal tract. Appl. Environ. Microbiol. 2008, 74, 1646–1648. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Depommier, C.; Derrien, M.; Everard, A.; de Vos, W.M. Akkermansia muciniphila: Paradigm for next-generation beneficial microorganisms. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 625–637. [Google Scholar] [CrossRef]
- Jakobsson, H.E.; Rodríguez-Piñeiro, A.M.; Schütte, A.; Ermund, A.; Boysen, P.; Bemark, M.; Sommer, F.; Bäckhed, F.; Hansson, G.C.; Johansson, M.E. The composition of the gut microbiota shapes the colon mucus barrier. EMBO Rep. 2015, 16, 164–177. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, P.W.; Marchesi, J.R.; Hill, C. Next-generation probiotics: The spectrum from probiotics to live biotherapeutics. Nat. Microbiol. 2017, 2, 17057. [Google Scholar] [CrossRef] [PubMed]
- Derrien, M.; Belzer, C.; de Vos, W.M. Akkermansia muciniphila and its role in regulating host functions. Microb. Pathog. 2017, 106, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Demirci, M.; Tokman, H.B.; Uysal, H.K.; Demiryas, S.; Karakullukcu, A.; Saribas, S.; Cokugras, H.; Kocazeybek, B.S. Reduced Akkermansia muciniphila and Faecalibacterium prausnitzii levels in the gut microbiota of children with allergic asthma. Allergol. Immunopathol. 2019, 47, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Michalovich, D.; Rodriguez-Perez, N.; Smolinska, S.; Pirozynski, M.; Mayhew, D.; Uddin, S.; Van Horn, S.; Sokolowska, M.; Altunbulakli, C.; Eljaszewicz, A.; et al. Obesity and disease severity magnify disturbed microbiome-immune interactions in asthma patients. Nat. Commun. 2019, 10, 5711. [Google Scholar] [CrossRef]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tang, L.; Feng, Y.; Zhao, S.; Han, M.; Zhang, C.; Yuan, G.; Zhu, J.; Cao, S.; Wu, Q.; et al. A purified membrane protein from Akkermansia muciniphila or the pasteurised bacterium blunts colitis associated tumourigenesis by modulation of CD8(+) T cells in mice. Gut 2020, 69, 1988–1997. [Google Scholar] [CrossRef]
- Plovier, H.; Everard, A.; Druart, C.; Depommier, C.; Van Hul, M.; Geurts, L.; Chilloux, J.; Ottman, N.; Duparc, T.; Lichtenstein, L.; et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 2017, 23, 107–113. [Google Scholar] [CrossRef]
- Segers, A.; de Vos, W.M. Mode of action of Akkermansia muciniphila in the intestinal dialogue: Role of extracellular proteins, metabolites and cell envelope components. Microbiome Res. Rep. 2023, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.A.; Lim, Y.; Byeon, H.R.; Jung, J.; Ma, S.; Hong, M.-G.; Kim, D.; Song, E.-J.; Nam, Y.-D.; Seo, J.-G.; et al. Heat-killed Akkermansia muciniphila ameliorates allergic airway inflammation in mice. Front. Microbiol. 2024, 15, 1386428. [Google Scholar] [CrossRef] [PubMed]
- Kirichenko, T.V.; Sobenin, I.A.; Markina, Y.V.; Gerasimova, E.V.; Grechko, A.V.; Kashirskikh, D.A.; Romanenko, E.B.; Wu, W.K.; Orekhov, A.N. Clinical Effectiveness of a Combination of Black Elder Berries, Violet Herb, and Calendula Flowers in Chronic Obstructive Pulmonary Disease: The Results of a Double-Blinded Placebo-Controlled Study. Biology 2020, 9, 83. [Google Scholar] [CrossRef] [PubMed]
- Marsland, B.J.; Trompette, A.; Gollwitzer, E.S. The Gut-Lung Axis in Respiratory Disease. Ann. Am. Thorac. Soc. 2015, 12 (Suppl. S2), S150–S156. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, K.E.; Sitarik, A.R.; Havstad, S.; Lin, D.L.; Levan, S.; Fadrosh, D.; Panzer, A.R.; LaMere, B.; Rackaityte, E.; Lukacs, N.W.; et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat. Med. 2016, 22, 1187–1191. [Google Scholar] [CrossRef]
- Leidy, N.K.; Rennard, S.I.; Schmier, J.; Jones, M.K.; Goldman, M. The breathlessness, cough, and sputum scale: The development of empirically based guidelines for interpretation. Chest 2003, 124, 2182–2191. [Google Scholar] [CrossRef]
- Kwon, N.H.; Oh, M.J.; Min, T.H.; Lee, B.J.; Choi, D.C. Causes and clinical features of subacute cough. Chest 2006, 129, 1142–1147. [Google Scholar] [CrossRef]
- Braman, S.S. Postinfectious cough: ACCP evidence-based clinical practice guidelines. Chest 2006, 129, 138S–146S. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.; Faber, K.N.; Hermoso, M.A. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Vinolo, M.A.; Rodrigues, H.G.; Nachbar, R.T.; Curi, R. Regulation of inflammation by short chain fatty acids. Nutrients 2011, 3, 858–876. [Google Scholar] [CrossRef]
- Bian, X.; Wu, W.; Yang, L.; Lv, L.; Wang, Q.; Li, Y.; Ye, J.; Fang, D.; Wu, J.; Jiang, X.; et al. Administration of Akkermansia muciniphila Ameliorates Dextran Sulfate Sodium-Induced Ulcerative Colitis in Mice. Front. Microbiol. 2019, 10, 2259. [Google Scholar] [CrossRef]
- Shin, N.R.; Lee, J.C.; Lee, H.Y.; Kim, M.S.; Whon, T.W.; Lee, M.S.; Bae, J.W. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut 2014, 63, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Bermudez-Brito, M.; Borghuis, T.; Daniel, C.; Pot, B.; de Haan, B.J.; Faas, M.M.; de Vos, P. L. plantarum WCFS1 enhances Treg frequencies by activating DCs even in absence of sampling of bacteria in the Peyer Patches. Sci. Rep. 2018, 8, 1785. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, S.; García-Vallejo, J.J.; Unger, W.W.; Fernandes, R.J.; Bruijns, S.; Laban, S.; Roep, B.O.; Hart, B.A.; van Kooyk, Y. TLR triggering on tolerogenic dendritic cells results in TLR2 up-regulation and a reduced proinflammatory immune program. J. Immunol. 2009, 183, 2984–2994. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Chao, K.; Wang, D.; Sun, Y.; Ding, X.; Zhang, X.; Liu, S.; Du, J.; Luo, Y.; Wang, H. A purified membrane protein from Akkermansia muciniphila blunted the sepsis-induced acute lung injury by modulation of gut microbiota in rats. Int. Immunopharmacol. 2023, 121, 110432. [Google Scholar] [CrossRef] [PubMed]
- Leidy, N.K.; Schmier, J.K.; Jones, M.K.; Lloyd, J.; Rocchiccioli, K. Evaluating symptoms in chronic obstructive pulmonary disease: Validation of the Breathlessness, Cough and Sputum Scale. Respir. Med. 2003, 97 (Suppl. S1), S59–S70. [Google Scholar] [CrossRef]
- Celli, B.; Halpin, D.; Hepburn, R.; Byrne, N.; Keating, E.T.; Goldman, M. Symptoms are an important outcome in chronic obstructive pulmonary disease clinical trials: Results of a 3-month comparative study using the Breathlessness, Cough and Sputum Scale (BCSS). Respir. Med. 2003, 97 (Suppl. A), S35–S43. [Google Scholar] [CrossRef] [PubMed]


| ETB-F01 (n = 68) | Placebo (n = 65) | p-Value | |
|---|---|---|---|
| Age, y, mean ± SD | 41.1 ± 12.6 | 41.3 ± 10.6 | 0.864 |
| Female, n (%) | 55 (80.9) | 46 (70.8) | 0.173 |
| Body weight, kg | 60.4 ± 12.2 | 64.3 ± 14.1 | 0.073 |
| Height, cm | 162 ± 8 | 164 ± 9 | 0.111 |
| Smoking history | 0.026 | ||
| Never smoker, n (%) | 68 (100) | 60 (92.3) | |
| Ex-smoker, n (%) | 0 | 5 (7.7) | |
| Patient-reported symptoms | |||
| Breathlessness, n (%) | 50 (73.5) | 42 (64.6) | 0.266 |
| Cough, n (%) | 67 (98.5) | 65 (100) | 1.000 |
| Sputum, n (%) | 65 (95.6) | 63 (96.9) | 1.000 |
| BCSS score, total, mean ± SD | 4.4 ± 1.5 | 4.3 ± 1.3 | 0.711 |
| Breathlessness score, mean ± SD | 1.1 ± 0.7 | 1.0 ± 0.6 | 0.248 |
| Cough score, mean ± SD | 1.9 ± 0.7 | 1.8 ± 0.7 | 0.517 |
| Sputum score, mean ± SD | 1.5 ± 0.7 | 1.5 ± 0.8 | 0.439 |
| SGRQ score, total, mean ± SD | 28.3 ± 18.4 | 31.7 ± 17.1 | 0.166 |
| mMRC score, mean ± SD | 1.1 ± 0.6 | 1.2 ± 0.7 | 0.971 |
| VAS, mm | |||
| Breathlessness, mean ± SD | 24.7 ± 22.0 | 24.7 ± 21.1 | 0.845 |
| Cough, mean ± SD | 32.1 ± 20.6 | 33.3 ± 20.0 | 0.690 |
| Sputum, mean ± SD | 34.6 ± 21.9 | 38.2 ± 23.9 | 0.415 |
| Lung function | |||
| Pre-BDR FEV1, L, mean ± SD | 2.9 ± 0.7 | 3.2 ± 0.8 | 0.066 |
| Pre-BDR FVC, L, mean ± SD | 3.6 ± 0.8 | 3.9 ± 1.0 | 0.147 |
| Pre-BDR FEV1/FVC, %, mean ± SD | 81.4 ± 6.2 | 82.6 ± 5.1 | 0.200 |
| FeNO, ppb | 32.8 ± 22.9 | 34.9 ± 26.1 | 0.753 |
| Comorbidities | |||
| Hypertension, n (%) | 1 (1.5) | 3 (4.6) | 0.358 |
| Diabetes mellitus, n (%) | 1 (1.5) | 0 (0) | 1.000 |
| Dyslipidemia, n (%) | 1 (1.5) | 2 (3.1) | 0.483 |
| Angina pectoris, n (%) | 0 (0) | 1 (1.5) | 0.489 |
| Cerebrovascular disease, n (%) | 1 (1.5) | 0 (0) | 1.000 |
| Chronic rhinitis, n (%) | 4 (5.9) | 2 (3.1) | 0.885 |
| Gastroesophageal reflux, n (%) | 0 (0) | 1 (1.5) | 0.489 |
| Outcome | ETB-F01 (n = 68) | Placebo (n = 65) |
|---|---|---|
| BCSS total score | ||
| At week 12, mean ± SD | 1.6 ± 1.4 | 2.3 ± 1.6 |
| Change from baseline to week 12, mean ± SD | −2.9 ± 1.6 | −2.0 ± 1.6 |
| p-value within groups | <0.001 | <0.001 |
| p-value between groups | 0.004 | |
| Change from baseline to week 12, LS mean ± SE | −2.4 ± 0.4 | −1.5 ± 0.4 |
| Difference in change from baseline to week 12, LS mean, (95% CI) | −0.8 (−1.4, −0.3) | |
| p-value a | 0.005 | |
| BCSS breathlessness score | ||
| At week 12, mean ± SD | 0.4 ± 0.6 | 0.6 ± 0.6 |
| Change from baseline to week 12, mean | −0.7 ± 0.8 | −0.4 ± 0.8 |
| p-value within groups | <0.001 | <0.001 |
| p-value between groups | 0.009 | |
| Change from baseline to week 12, LS mean ± SE | −0.5 ± 0.2 | −0.2 ± 0.2 |
| Difference in change from baseline to week 12, LS mean, (95% CI) | −0.4 (−0.6, −0.1) | |
| p-value a | 0.011 | |
| BCSS cough score | ||
| At week 12, mean ± SD | 0.6 ± 0.6 | 0.9 ± 0.7 |
| Change from baseline to week 12, mean ± SD | −1.3 ± 0.8 | −0.9 ± 0.9 |
| p-value within groups | <0.001 | <0.001 |
| p-value between groups | 0.011 | |
| Change from baseline to week 12, LS mean ± SE | −0.8 ± 0.2 | −0.5 ± 0.2 |
| Difference in change from baseline to week 12, LS mean, (95% CI) | −0.3 (−0.6, −0.1) | |
| p-value a | 0.020 | |
| BCSS sputum score | ||
| At week 12, mean ± SD | 0.6 ± 0.6 | 0.8 ± 0.7 |
| Change from baseline to week 12, mean ± SD | −0.9 ± 0.7 | −0.8 ± 0.8 |
| p-value within groups | <0.001 | <0.001 |
| p-value between groups | 0.540 | |
| Change from baseline to week 12, LS mean ± SE | −1.0 ± 0.2 | −0.9 ± 0.2 |
| Difference in change from baseline to week 12, LS mean, (95% CI) | −0.1 (−0.4, 0.2) | |
| p-value a | 0.380 | |
| ETB-F01 (n = 77) | Placebo (n = 73) | p-Value | |
|---|---|---|---|
| No. of patients with adverse events (%) | 12 (15.6) | 14 (19.2) | 0.357 |
| Pharyngitis | 4 (5.2) | 2 (2.7) | 0.883 |
| Musculoskeletal pain | 0 (0) | 3 (4.1) | 0.113 |
| Leukocytosis | 0 (0) | 2 (2.7) | 0.235 |
| Lymphopenia | 0 (0) | 2 (2.7) | 0.235 |
| Dermatitis | 0 (0) | 2 (2.7) | 0.235 |
| Respiratory disorder | 1 (1.3) | 0 (0.0) | 1.000 |
| Hematuria | 1 (1.3) | 0 (0.0) | 1.000 |
| Agitation | 1 (1.3) | 0 (0.0) | 1.000 |
| Amenorrhea | 0 (0) | 1 (1.4) | 0.487 |
| Dysmenorrhea | 0 (0) | 1 (1.4) | 0.487 |
| Otitis media | 0 (0) | 1 (1.4) | 0.487 |
| Dyspepsia | 2 (2.6) | 2 (2.7) | 0.670 |
| Gastritis | 1 (1.3) | 0 (0.0) | 1.000 |
| No. of patients with serious adverse events (%) | 0 (0) | 0 (0) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.W.; Lee, S.-N.; Seo, J.-G.; Koo, Y.; Kang, S.-Y.; Choi, C.W.; Park, S.-Y.; Lee, S.-Y.; Kim, S.-R.; Kim, J.-H.; et al. Efficacy of ETB-F01, Heat-Killed Akkermansia muciniphila Strain EB-AMDK19, in Patients with Respiratory Symptoms: A Multicenter Clinical Trial. Nutrients 2024, 16, 4113. https://doi.org/10.3390/nu16234113
Lee HW, Lee S-N, Seo J-G, Koo Y, Kang S-Y, Choi CW, Park S-Y, Lee S-Y, Kim S-R, Kim J-H, et al. Efficacy of ETB-F01, Heat-Killed Akkermansia muciniphila Strain EB-AMDK19, in Patients with Respiratory Symptoms: A Multicenter Clinical Trial. Nutrients. 2024; 16(23):4113. https://doi.org/10.3390/nu16234113
Chicago/Turabian StyleLee, Hyun Woo, Sang-Nam Lee, Jae-Gu Seo, Yemo Koo, Sung-Yoon Kang, Cheon Woong Choi, So-Young Park, Suh-Young Lee, Sung-Ryeol Kim, Joo-Hee Kim, and et al. 2024. "Efficacy of ETB-F01, Heat-Killed Akkermansia muciniphila Strain EB-AMDK19, in Patients with Respiratory Symptoms: A Multicenter Clinical Trial" Nutrients 16, no. 23: 4113. https://doi.org/10.3390/nu16234113
APA StyleLee, H. W., Lee, S.-N., Seo, J.-G., Koo, Y., Kang, S.-Y., Choi, C. W., Park, S.-Y., Lee, S.-Y., Kim, S.-R., Kim, J.-H., & Choi, H. S. (2024). Efficacy of ETB-F01, Heat-Killed Akkermansia muciniphila Strain EB-AMDK19, in Patients with Respiratory Symptoms: A Multicenter Clinical Trial. Nutrients, 16(23), 4113. https://doi.org/10.3390/nu16234113





