Dose–Response Associations Between Diet and Risk of Rheumatoid Arthritis: A Meta-Analysis of Prospective Cohort Studies
Highlights
- This comprehensive meta-analysis evaluated 32 dietary factors and their associations with the risk of rheumatoid arthritis (RA).
- Low-to-moderate alcohol consumption and higher intake of fruit and cereals were associated with a reduced RA risk, while tea and caffeinated coffee were associated with an increased risk, supported by moderate-certainty evidence.
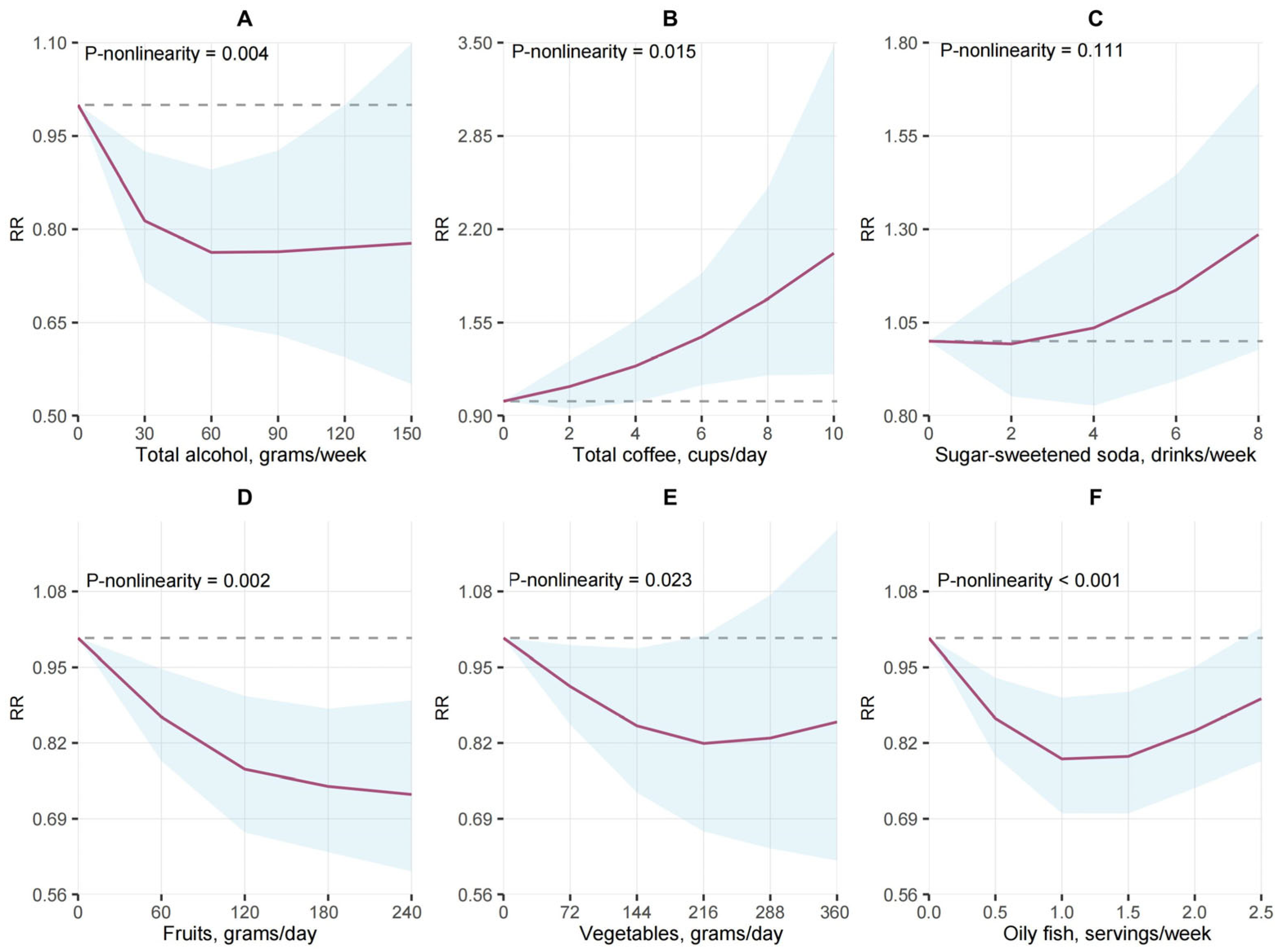
- Dose–response analyses revealed non-linear relationships for several dietary factors (e.g., total coffee, vegetables, oily fish, vitamin D supplements), providing insight into optimal consumption levels for RA prevention.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion Criteria and Study Selection
2.3. Data Extraction and Quality Assessment
2.4. Statistical Methods
3. Results
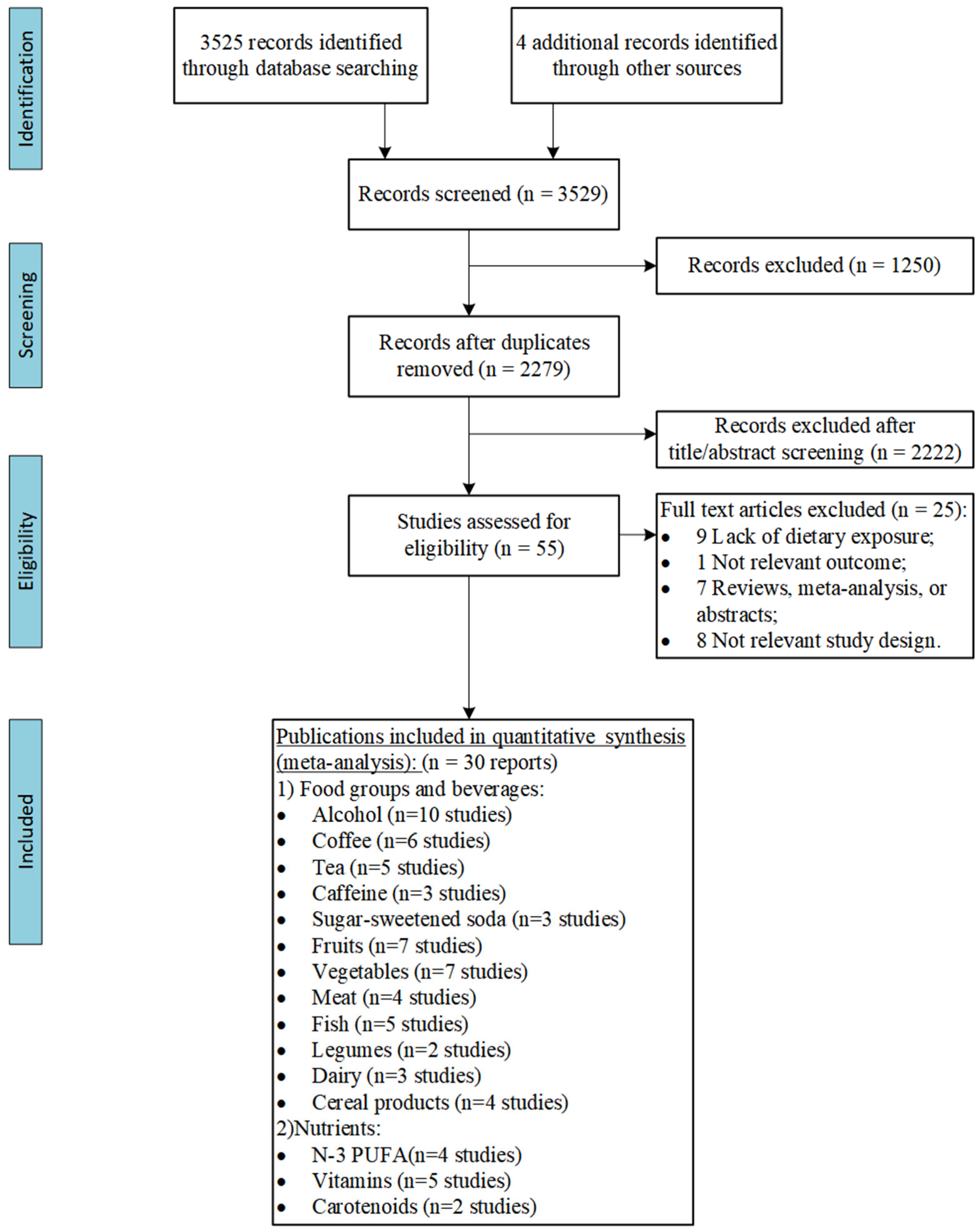
3.1. Literature Search
3.2. Characteristics of Included Studies
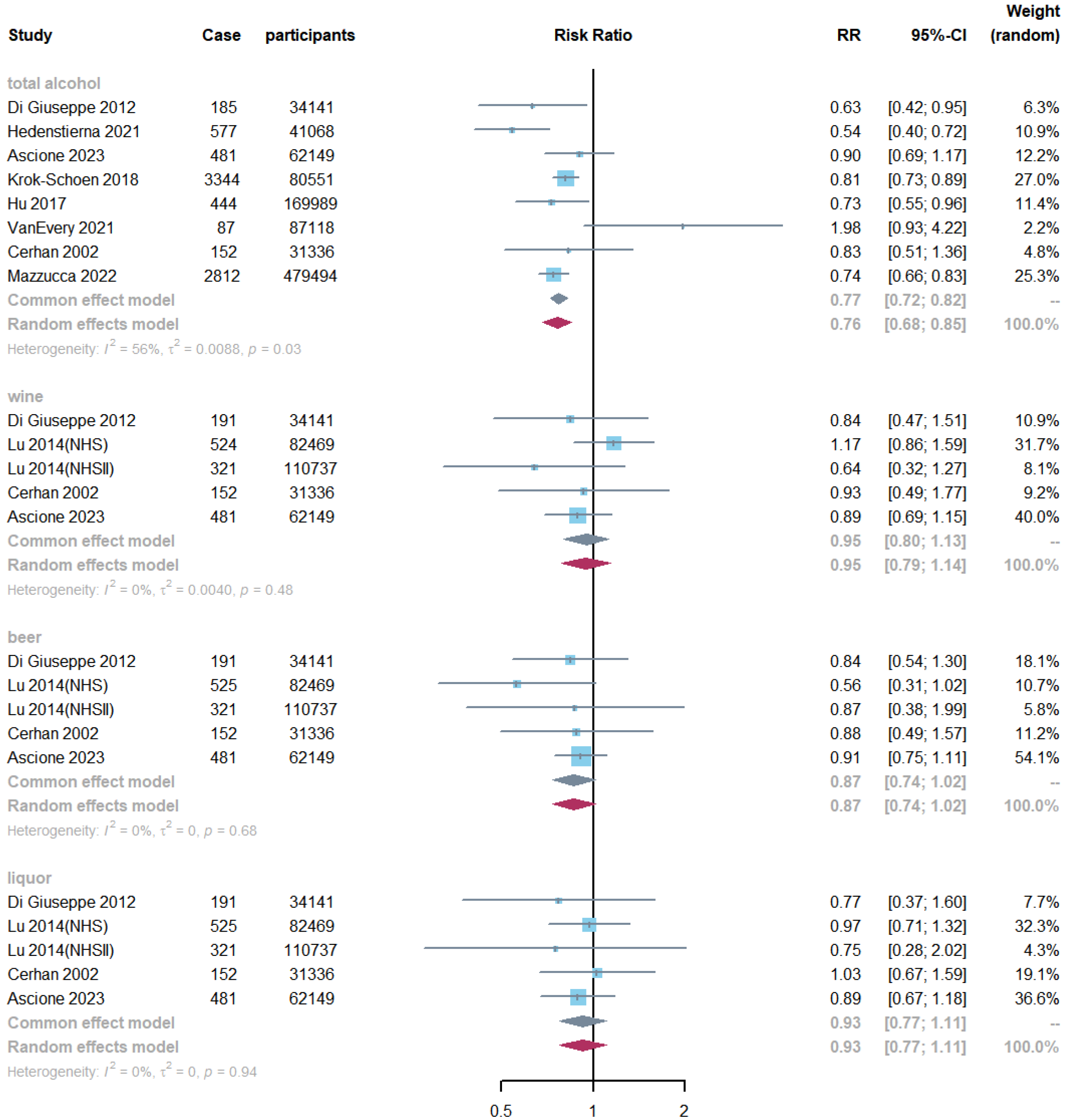
3.3. Alcohol Consumption and RA Risk
3.4. Non-Alcoholic Beverages and RA Risk
3.5. Fruit, Vegetables, and RA Risk
3.6. Meat, Fish, and RA Risk
3.7. Other Food Components and RA Risk
3.8. Nutrients and RA Risk
3.8.1. Dietary Macronutrients
3.8.2. Dietary Micronutrients
3.9. Subgroup Analysis and Bias Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- van Delft, M.A.M.; Huizinga, T.W.J. An overview of autoantibodies in rheumatoid arthritis. J. Autoimmun. 2020, 110, 102392. [Google Scholar] [CrossRef]
- Finckh, A.; Gilbert, B.; Hodkinson, B.; Bae, S.C.; Thomas, R.; Deane, K.D.; Alpizar-Rodriguez, D.; Lauper, K. Global epidemiology of rheumatoid arthritis. Nat. Rev. Rheumatol. 2022, 18, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Ren, Y.; Mu, Y.; Zhang, L.; Chen, B.; Li, S.; Fang, Q.; Zhang, Z.; Zhang, K.; Li, S.; et al. Microbial imbalance in Chinese children with diarrhea or constipation. Sci. Rep. 2024, 14, 13516. [Google Scholar] [CrossRef] [PubMed]
- Romão, V.C.; Fonseca, J.E. Etiology and Risk Factors for Rheumatoid Arthritis: A State-of-the-Art Review. Front. Med. 2021, 8, 689698. [Google Scholar] [CrossRef] [PubMed]
- Manzel, A.; Muller, D.N.; Hafler, D.A.; Erdman, S.E.; Linker, R.A.; Kleinewietfeld, M. Role of "Western diet" in inflammatory autoimmune diseases. Curr. Allergy Asthma Rep. 2014, 14, 404. [Google Scholar] [CrossRef]
- Pedersen, M.; Jacobsen, S.; Klarlund, M.; Pedersen, B.V.; Wiik, A.; Wohlfahrt, J.; Frisch, M. Environmental risk factors differ between rheumatoid arthritis with and without auto-antibodies against cyclic citrullinated peptides. Arthritis Res. Ther. 2006, 8, R133. [Google Scholar] [CrossRef]
- Menzel, J.; Biemann, R.; Longree, A.; Isermann, B.; Mai, K.; Schulze, M.B.; Abraham, K.; Weikert, C. Associations of a vegan diet with inflammatory biomarkers. Sci. Rep. 2020, 10, 1933. [Google Scholar] [CrossRef]
- Al-Saoodi, H.; Kolahdooz, F.; Andersen, J.R.; Jalili, M. Effect of vitamin D on inflammatory and clinical outcomes in patients with rheumatoid arthritis: A systematic review and dose-response meta-analysis of randomized controlled trials. Nutr. Rev. 2024, 82, 600–611. [Google Scholar] [CrossRef]
- Mölzer, C.; Wilson, H.M.; Kuffova, L.; Forrester, J.V. A Role for Folate in Microbiome-Linked Control of Autoimmunity. J. Immunol. Res. 2021, 2021, 9998200. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Missbach, B.; König, J.; Hoffmann, G. Adherence to a Mediterranean diet and risk of diabetes: A systematic review and meta-analysis. Public Health Nutr. 2015, 18, 1292–1299. [Google Scholar] [CrossRef]
- Shen, J.; Wilmot, K.A.; Ghasemzadeh, N.; Molloy, D.L.; Burkman, G.; Mekonnen, G.; Gongora, M.C.; Quyyumi, A.A.; Sperling, L.S. Mediterranean dietary patterns and cardiovascular health. Annu. Rev. Nutr. 2015, 35, 425–449. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Solomon, D.H.; Costenbader, K.H.; Karlson, E.W. Alcohol consumption and risk of incident rheumatoid arthritis in women: A prospective study. Arthritis Rheumatol. 2014, 66, 1998–2005. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Xiang, C.; Cai, Q.; Wei, X.; He, J. Alcohol consumption as a preventive factor for developing rheumatoid arthritis: A dose-response meta-analysis of prospective studies. Ann. Rheum. Dis. 2014, 73, 1962–1967. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Li, J.; Gan, Y.; Liu, J.; Zhao, X.; Chen, J.; Zhang, R.; Zhong, Y.; Chen, X.; Wu, L.; et al. Tea consumption is associated with decreased disease activity of rheumatoid arthritis in a real-world, large-scale study. Ann. Nutr. Metab. 2020, 76, 54–61. [Google Scholar] [CrossRef]
- Westerlind, H.; Palmqvist, I.; Saevarsdottir, S.; Alfredsson, L.; Klareskog, L.; Di Giuseppe, D. Is tea consumption associated with reduction of risk of rheumatoid arthritis? A Swedish case-control study. Arthritis Res. Ther. 2021, 23, 209. [Google Scholar] [CrossRef]
- Mazzucca, C.B.; Scotti, L.; Cappellano, G.; Barone-Adesi, F.; Chiocchetti, A. Nutrition and Rheumatoid Arthritis Onset: A Prospective Analysis Using the UK Biobank. Nutrients 2022, 14, 1554. [Google Scholar] [CrossRef]
- Daien, C.; Czernichow, S.; Letarouilly, J.G.; Nguyen, Y.; Sanchez, P.; Sigaux, J.; Beauvais, C.; Desouches, S.; Le Puillandre, R.; Rigalleau, V.; et al. Dietary recommendations of the French Society for Rheumatology for patients with chronic inflammatory rheumatic diseases. Jt. Bone Spine 2022, 89, 105319. [Google Scholar] [CrossRef]
- Gkiouras, K.; Grammatikopoulou, M.G.; Myrogiannis, I.; Papamitsou, T.; Rigopoulou, E.I.; Sakkas, L.I.; Bogdanos, D.P. Efficacy of n-3 fatty acid supplementation on rheumatoid arthritis’ disease activity indicators: A systematic review and meta-analysis of randomized placebo-controlled trials. Crit. Rev. Food Sci. Nutr. 2024, 64, 16–30. [Google Scholar] [CrossRef]
- Martu, M.A.; Maftei, G.A.; Luchian, I.; Stefanescu, O.M.; Scutariu, M.M.; Solomon, S.M. The Effect of Acknowledged and Novel Anti-Rheumatic Therapies on Periodontal Tissues-A Narrative Review. Pharmaceuticals 2021, 14, 1209. [Google Scholar] [CrossRef]
- Bäcklund, R.; Drake, I.; Bergström, U.; Compagno, M.; Sonestedt, E.; Turesson, C. Diet and the risk of rheumatoid arthritis—A systematic literature review. Semin. Arthritis Rheum. 2023, 58, 152118. [Google Scholar] [CrossRef]
- Guan, C.M.; Beg, S. Diet as a risk factor for rheumatoid arthritis. Cureus 2023, 15, e39273. [Google Scholar] [CrossRef] [PubMed]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Schardt, C.; Adams, M.B.; Owens, T.; Keitz, S.; Fontelo, P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med. Inform. Decis. Mak. 2007, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.; Glasziou, P.; Del Mar, C.; Bannach-Brown, A.; Stehlik, P.; Scott, A.M. A full systematic review was completed in 2 weeks using automation tools: A case study. J. Clin. Epidemiol. 2020, 121, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Arnett, F.C.; Edworthy, S.M.; Bloch, D.A.; McShane, D.J.; Fries, J.F.; Cooper, N.S.; Healey, L.A.; Kaplan, S.R.; Liang, M.H.; Luthra, H.S.; et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988, 31, 315–324. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Knüppel, S.; Schwedhelm, C.; Hoffmann, G.; Missbach, B.; Stelmach-Mardas, M.; Dietrich, S.; Eichelmann, F.; Kontopantelis, E.; Iqbal, K.; et al. Perspective: NutriGrade: A Scoring System to Assess and Judge the Meta-Evidence of Randomized Controlled Trials and Cohort Studies in Nutrition Research. Adv. Nutr. 2016, 7, 994–1004. [Google Scholar] [CrossRef]
- Greenland, S.; Longnecker, M.P. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am. J. Epidemiol. 1992, 135, 1301–1309. [Google Scholar] [CrossRef]
- Orsini, N.; Li, R.; Wolk, A.; Khudyakov, P.; Spiegelman, D. Meta-analysis for linear and nonlinear dose-response relations: Examples, an evaluation of approximations, and software. Am. J. Epidemiol. 2012, 175, 66–73. [Google Scholar] [CrossRef]
- Orsini, N. From floated to conventional confidence intervals for the relative risks based on published dose-response data. Comput. Methods Programs Biomed. 2010, 98, 90–93. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clin. Res. Ed.) 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Krok-Schoen, J.L.; Brasky, T.M.; Hunt, R.P.; Rohan, T.E.; Baker, T.A.; Li, W.; Carbone, L.; Mackey, R.H.; Snetselaar, L.; Lustberg, M.B.; et al. Dietary Long-Chain n-3 Fatty Acid Intake and Arthritis Risk in the Women’s Health Initiative. J. Acad. Nutr. Diet. 2018, 118, 2057–2069. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Sparks, J.A.; Malspeis, S.; Costenbader, K.H.; Hu, F.B.; Karlson, E.W.; Lu, B. Long-term dietary quality and risk of developing rheumatoid arthritis in women. Ann. Rheum. Dis. 2017, 76, 1357–1364. [Google Scholar] [CrossRef]
- Cerhan, J.R.; Saag, K.G.; Merlino, L.A.; Mikuls, T.R.; Criswell, L.A. Antioxidant micronutrients and risk of rheumatoid arthritis in a cohort of older women. Am. J. Epidemiol. 2003, 157, 345–354. [Google Scholar] [CrossRef]
- Nguyen, Y.; Salliot, C.; Mariette, X.; Boutron-Ruault, M.-C.; Seror, R. Fish consumption and risk of rheumatoid arthritis: Findings from the E3N cohort study. Nutrients 2022, 14, 861. [Google Scholar] [CrossRef]
- Hedenstierna, L.; Bellocco, R.; Ye, W.; Adami, H.O.; Åkerstedt, T.; Trolle Lagerros, Y.; Hedström, A.K. Effects of alcohol consumption and smoking on risk for RA: Results from a Swedish prospective cohort study. RMD Open 2021, 7, e001379. [Google Scholar] [CrossRef]
- Nguyen, Y.; Salliot, C.; Gelot, A.; Gambaretti, J.; Mariette, X.; Boutron-Ruault, M.C.; Seror, R. Mediterranean diet and risk of rheumatoid arthritis: Findings from the French E3N-EPIC cohort study. Arthritis Rheumatol. 2021, 73, 69–77. [Google Scholar] [CrossRef]
- Costenbader, K.H.; Feskanich, D.; Holmes, M.; Karlson, E.W.; Benito-Garcia, E. Vitamin D intake and risks of systemic lupus erythematosus and rheumatoid arthritis in women. Ann. Rheum. Dis. 2008, 67, 530–535. [Google Scholar] [CrossRef]
- Costenbader, K.H.; Kang, J.H.; Karlson, E.W. Antioxidant intake and risks of rheumatoid arthritis and systemic lupus erythematosus in women. Am. J. Epidemiol. 2010, 172, 205–216. [Google Scholar] [CrossRef]
- Di Giuseppe, D.; Alfredsson, L.; Bottai, M.; Askling, J.; Wolk, A. Long term alcohol intake and risk of rheumatoid arthritis in women: A population based cohort study. BMJ (Clin. Res. Ed.) 2012, 345, e4230. [Google Scholar] [CrossRef] [PubMed]
- Sundström, B.; Ljung, L.; Di Giuseppe, D. Consumption of Meat and Dairy Products Is Not Associated with the Risk for Rheumatoid Arthritis among Women: A Population-Based Cohort Study. Nutrients 2019, 11, 2825. [Google Scholar] [CrossRef] [PubMed]
- Di Giuseppe, D.; Wallin, A.; Bottai, M.; Askling, J.; Wolk, A. Long-term intake of dietary long-chain n-3 polyunsaturated fatty acids and risk of rheumatoid arthritis: A prospective cohort study of women. Ann. Rheum. Dis. 2014, 73, 1949–1953. [Google Scholar] [CrossRef] [PubMed]
- Hiraki, L.T.; Munger, K.L.; Costenbader, K.H.; Karlson, E.W. Dietary intake of vitamin D during adolescence and risk of adult-onset systemic lupus erythematosus and rheumatoid arthritis. Arthritis Care Res. 2012, 64, 1829–1836. [Google Scholar] [CrossRef]
- Merlino, L.A.; Curtis, J.; Mikuls, T.R.; Cerhan, J.R.; Criswell, L.A.; Saag, K.G. Vitamin D intake is inversely associated with rheumatoid arthritis: Results from the Iowa Women’s Health Study. Arthritis Rheum. 2004, 50, 72–77. [Google Scholar] [CrossRef]
- Karlson, E.W.; Mandl, L.A.; Aweh, G.N.; Grodstein, F. Coffee consumption and risk of rheumatoid arthritis. Arthritis Rheum. 2003, 48, 3055–3060. [Google Scholar] [CrossRef]
- Lamichhane, D.; Collins, C.; Constantinescu, F.; Walitt, B.; Pettinger, M.; Parks, C.; Howard, B.V. Coffee and Tea Consumption in Relation to Risk of Rheumatoid Arthritis in the Women’s Health Initiative Observational Cohort. J. Clin. Rheumatol. Pract. Rep. Rheum. Musculoskelet. Dis. 2019, 25, 127–132. [Google Scholar] [CrossRef]
- Lahiri, M.; Luben, R.N.; Morgan, C.; Bunn, D.K.; Marshall, T.; Lunt, M.; Verstappen, S.M.; Symmons, D.P.; Khaw, K.T.; Wareham, N.; et al. Using lifestyle factors to identify individuals at higher risk of inflammatory polyarthritis (results from the European Prospective Investigation of Cancer-Norfolk and the Norfolk Arthritis Register--the EPIC-2-NOAR Study). Ann. Rheum. Dis. 2014, 73, 219–226. [Google Scholar] [CrossRef]
- Pedersen, M.; Stripp, C.; Klarlund, M.; Olsen, S.F.; Tjønneland, A.M.; Frisch, M. Diet and risk of rheumatoid arthritis in a prospective cohort. J. Rheumatol. 2005, 32, 1249–1252. [Google Scholar]
- Rubin, K.H.; Rasmussen, N.F.; Petersen, I.; Kopp, T.I.; Stenager, E.; Magyari, M.; Hetland, M.L.; Bygum, A.; Glintborg, B.; Andersen, V. Intake of dietary fibre, red and processed meat and risk of late-onset Chronic Inflammatory Diseases: A prospective Danish study on the diet, cancer and health cohort. Int. J. Med. Sci. 2020, 17, 2487–2495. [Google Scholar] [CrossRef]
- VanEvery, H.; Yang, W.; Olsen, N.; Bao, L.; Lu, B.; Wu, S.; Cui, L.; Gao, X. Alcohol Consumption and Risk of Rheumatoid Arthritis among Chinese Adults: A Prospective Study. Nutrients 2021, 13, 2231. [Google Scholar] [CrossRef] [PubMed]
- Heliövaara, M.; Aho, K.; Knekt, P.; Impivaara, O.; Reunanen, A.; Aromaa, A. Coffee consumption, rheumatoid factor, and the risk of rheumatoid arthritis. Ann. Rheum. Dis. 2000, 59, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Ro, J.; Kim, S.H.; Kim, H.R.; Lee, S.H.; Min, H.K. Impact of lifestyle and comorbidities on seropositive rheumatoid arthritis risk from Korean health insurance data. Sci. Rep. 2022, 12, 2201. [Google Scholar] [CrossRef] [PubMed]
- Ascione, S.; Barde, F.; Artaud, F.; Nguyen, Y.; Macdonald, C.; Mariette, X.; Boutron-Ruault, M.C.; Salliot, C.; Seror, R. Association between beverage consumption and risk of rheumatoid arthritis: A prospective study from the French E3N Cohort. Rheumatology 2023, 62, 1814–1823. [Google Scholar] [CrossRef] [PubMed]
- Mikuls, T.R.; Cerhan, J.R.; Criswell, L.A.; Merlino, L.; Mudano, A.S.; Burma, M.; Folsom, A.R.; Saag, K.G. Coffee, tea, and caffeine consumption and risk of rheumatoid arthritis: Results from the Iowa Women’s Health Study. Arthritis Rheum. 2002, 46, 83–91. [Google Scholar] [CrossRef]
- Sparks, J.A.; O’Reilly É, J.; Barbhaiya, M.; Tedeschi, S.K.; Malspeis, S.; Lu, B.; Willett, W.C.; Costenbader, K.H.; Karlson, E.W. Association of fish intake and smoking with risk of rheumatoid arthritis and age of onset: A prospective cohort study. BMC Musculoskelet. Disord. 2019, 20, 2. [Google Scholar] [CrossRef]
- Cerhan, J.R.; Saag, K.G.; Criswell, L.A.; Merlino, L.A.; Mikuls, T.R. Blood transfusion, alcohol use, and anthropometric risk factors for rheumatoid arthritis in older women. J. Rheumatol. 2002, 29, 246–254. [Google Scholar]
- Benito-Garcia, E.; Feskanich, D.; Hu, F.B.; Mandl, L.A.; Karlson, E.W. Protein, iron, and meat consumption and risk for rheumatoid arthritis: A prospective cohort study. Arthritis Res. Ther. 2007, 9, R16. [Google Scholar] [CrossRef]
- Hu, Y.; Costenbader, K.H.; Gao, X.; Al-Daabil, M.; Sparks, J.A.; Solomon, D.H.; Hu, F.B.; Karlson, E.W.; Lu, B. Sugar-sweetened soda consumption and risk of developing rheumatoid arthritis in women. Am. J. Clin. Nutr. 2014, 100, 959–967. [Google Scholar] [CrossRef]
- Eun, Y.; Jeon, K.H.; Han, K.; Kim, D.; Kim, H.; Lee, J.; Lee, D.Y.; Yoo, J.E.; Shin, D.W. Menopausal factors and risk of seropositive rheumatoid arthritis in postmenopausal women: A nationwide cohort study of 1.36 million women. Sci. Rep. 2020, 10, 20793. [Google Scholar] [CrossRef]
- WHO. Increasing Fruit and Vegetable Consumption to Reduce the Risk of Noncommunicable Diseases. 2023. Available online: https://www.who.int/tools/elena/interventions/fruit-vegetables-ncds (accessed on 1 May 2024).
- Li, F.; Liu, X.; Wang, W.; Zhang, D. Consumption of vegetables and fruit and the risk of inflammatory bowel disease:A meta-analysis. Eur. J. Gastroenterol. Hepatol. 2015, 27, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Fotros, D.; Noormohammadi, M.; Razeghi Jahromi, S.; Abdolkarimi, M. Fruits and vegetables intake may be associated with a reduced odds of multiple sclerosis: A systematic review and dose–response meta-analysis of observational studies. Nutr. Neurosci. 2024, 27, 887–898. [Google Scholar] [CrossRef] [PubMed]
- Carter, P.; Gray, L.J.; Troughton, J.; Khunti, K.; Davies, M. Fruit and vegetable intake and incidence of type 2 diabetes mellitus: Systematic review and meta-analysis. BMJ 2010, 341, c4229. [Google Scholar] [CrossRef]
- Gualtieri, P.; Marchetti, M.; Frank, G.; Smeriglio, A.; Trombetta, D.; Colica, C.; Cianci, R.; De Lorenzo, A.; Di Renzo, L. Antioxidant-Enriched Diet on Oxidative Stress and Inflammation Gene Expression: A Randomized Controlled Trial. Genes 2023, 14, 206. [Google Scholar] [CrossRef]
- van der Merwe, M.; Moore, D.; Hill, J.L.; Keating, F.H.; Buddington, R.K.; Bloomer, R.J.; Wang, A.; Bowman, D.D. The Impact of a Dried Fruit and Vegetable Supplement and Fiber Rich Shake on Gut and Health Parameters in Female Healthcare Workers: A Placebo-Controlled, Double-Blind, Randomized Clinical Trial. Microorganisms 2021, 9, 843. [Google Scholar] [CrossRef]
- Gibson, A.; Edgar, J.D.; Neville, C.E.; Gilchrist, S.E.; McKinley, M.C.; Patterson, C.C.; Young, I.S.; Woodside, J.V. Effect of fruit and vegetable consumption on immune function in older people: A randomized controlled trial. Am. J. Clin. Nutr. 2012, 96, 1429–1436. [Google Scholar] [CrossRef]
- Hosseini, B.; Berthon, B.S.; Saedisomeolia, A.; Starkey, M.R.; Collison, A.; Wark, P.A.B.; Wood, L.G. Effects of fruit and vegetable consumption on inflammatory biomarkers and immune cell populations: A systematic literature review and meta-analysis. Am. J. Clin. Nutr. 2018, 108, 136–155. [Google Scholar] [CrossRef]
- Ecarnot, F.; Maggi, S. The impact of the Mediterranean diet on immune function in older adults. Aging Clin. Exp. Res. 2024, 36, 117. [Google Scholar] [CrossRef]
- Liu, X.; Tedeschi, S.K.; Lu, B.; Zaccardelli, A.; Speyer, C.B.; Costenbader, K.H.; Karlson, E.W.; Sparks, J.A. Long-term physical activity and subsequent risk for rheumatoid arthritis among women: A prospective cohort study. Arthritis Rheumatol. 2019, 71, 1460–1471. [Google Scholar] [CrossRef]
- Feng, Q.; Kim, J.H.; Omiyale, W.; Bešević, J.; Conroy, M.; May, M.; Yang, Z.; Wong, S.Y.; Tsoi, K.K.; Allen, N.; et al. Raw and Cooked Vegetable Consumption and Risk of Cardiovascular Disease: A Study of 400,000 Adults in UK Biobank. Front. Nutr. 2022, 9, 831470. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; O’Keefe, J.H. Importance of maintaining a low omega-6/omega-3 ratio for reducing inflammation. Open Heart 2018, 5, e000946. [Google Scholar] [CrossRef] [PubMed]
- Arnotti, K.; Bamber, M. Fruit and vegetable consumption in overweight or obese individuals: A meta-analysis. West. J. Nurs. Res. 2020, 42, 306–314. [Google Scholar] [CrossRef]
- Aune, D.; Keum, N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: Systematic review and dose-response meta-analysis of prospective studies. BMJ 2016, 353, i2716. [Google Scholar] [CrossRef]
- Aune, D.; Norat, T.; Romundstad, P.; Vatten, L.J. Whole grain and refined grain consumption and the risk of type 2 diabetes: A systematic review and dose–response meta-analysis of cohort studies. Eur. J. Epidemiol. 2013, 28, 845–858. [Google Scholar] [CrossRef]
- Garutti, M.; Nevola, G.; Mazzeo, R.; Cucciniello, L.; Totaro, F.; Bertuzzi, C.A.; Caccialanza, R.; Pedrazzoli, P.; Puglisi, F. The impact of cereal grain composition on the health and disease outcomes. Front. Nutr. 2022, 9, 888974. [Google Scholar] [CrossRef]
- Scott, I.C.; Tan, R.; Stahl, D.; Steer, S.; Lewis, C.M.; Cope, A.P. The protective effect of alcohol on developing rheumatoid arthritis: A systematic review and meta-analysis. Rheumatology 2013, 52, 856–867. [Google Scholar] [CrossRef]
- Hedström, A.K.; Hössjer, O.; Klareskog, L.; Alfredsson, L. Interplay between alcohol, smoking and HLA genes in RA aetiology. RMD Open 2019, 5, e000893. [Google Scholar] [CrossRef]
- Boatca, R.M.; Scutariu, M.M.; Rudnic, I.; Stefanache, M.A.M.; Hurjui, L.; Rezus, E.; Martu, S. Evolution of inflammatory biochemical markers within periodontal therapy to patients with rheumatoid arthritis. Rev. De Chim. 2016, 67, 741–744. [Google Scholar]
- Carvalho, J.F.; Lerner, A. Resveratrol in Rheumatological Diseases: A Systematic Review. Eur. J. Rheumatol. 2023, 10, 163–168. [Google Scholar] [CrossRef]
- Dey, M.; Cutolo, M.; Nikiphorou, E. Beverages in Rheumatoid Arthritis: What to Prefer or to Avoid. Nutrients 2020, 12, 3155. [Google Scholar] [CrossRef]
- Lee, Y.H.; Bae, S.C.; Song, G.G. Coffee or tea consumption and the risk of rheumatoid arthritis: A meta-analysis. Clin. Rheumatol. 2014, 33, 1575–1583. [Google Scholar] [CrossRef] [PubMed]
- Asoudeh, F.; Dashti, F.; Jayedi, A.; Hemmati, A.; Fadel, A.; Mohammadi, H. Caffeine, Coffee, Tea and Risk of Rheumatoid Arthritis: Systematic Review and Dose-Response Meta-analysis of Prospective Cohort Studies. Front. Nutr. 2022, 9, 822557. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, E.; Sadanaga, T.; Okada, T.; Minagawa, T.; Akiba, J. Does caffeine have a double-edged sword role in inflammation and carcinogenesis in the colon? Intest. Res. 2023, 21, 306–317. [Google Scholar] [CrossRef]
- Ma, X.; Nan, F.; Liang, H.; Shu, P.; Fan, X.; Song, X.; Hou, Y.; Zhang, D. Excessive intake of sugar: An accomplice of inflammation. Front. Immunol. 2022, 13, 988481. [Google Scholar] [CrossRef] [PubMed]
- French, M.T.; Popovici, I.; Maclean, J.C. Do alcohol consumers exercise more? Findings from a national survey. Am. J. Health Promot. AJHP 2009, 24, 2–10. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Y.; Greenwood, D.C.; Webster, J.; Uzokwe, C.; Tao, J.; Hardie, L.J.; Cade, J.E. Dose–Response Associations Between Diet and Risk of Rheumatoid Arthritis: A Meta-Analysis of Prospective Cohort Studies. Nutrients 2024, 16, 4050. https://doi.org/10.3390/nu16234050
Dong Y, Greenwood DC, Webster J, Uzokwe C, Tao J, Hardie LJ, Cade JE. Dose–Response Associations Between Diet and Risk of Rheumatoid Arthritis: A Meta-Analysis of Prospective Cohort Studies. Nutrients. 2024; 16(23):4050. https://doi.org/10.3390/nu16234050
Chicago/Turabian StyleDong, Yuanyuan, Darren C. Greenwood, James Webster, Chinwe Uzokwe, Jinhui Tao, Laura J. Hardie, and Janet E. Cade. 2024. "Dose–Response Associations Between Diet and Risk of Rheumatoid Arthritis: A Meta-Analysis of Prospective Cohort Studies" Nutrients 16, no. 23: 4050. https://doi.org/10.3390/nu16234050
APA StyleDong, Y., Greenwood, D. C., Webster, J., Uzokwe, C., Tao, J., Hardie, L. J., & Cade, J. E. (2024). Dose–Response Associations Between Diet and Risk of Rheumatoid Arthritis: A Meta-Analysis of Prospective Cohort Studies. Nutrients, 16(23), 4050. https://doi.org/10.3390/nu16234050







