Propionate Production by Infant Fecal Microbiota Is Inversely Correlated with the Protein Glycation Level of Supplemented Infant Formula Ex Vivo
Abstract
1. Introduction
2. Materials and Methods
2.1. Breast Milk
2.2. Infant Formula Preparation
2.3. In Vitro Simulated Upper Gastrointestinal Digestion of Infant Formula
2.4. In Vitro Fecal Fermentation
2.5. Determination of Short- and Branched-Chain Fatty Acids (SCFAs/BCFAs)
2.6. DNA Extraction, 16S rRNA Gene Amplicon Sequencing and Bioinformatics
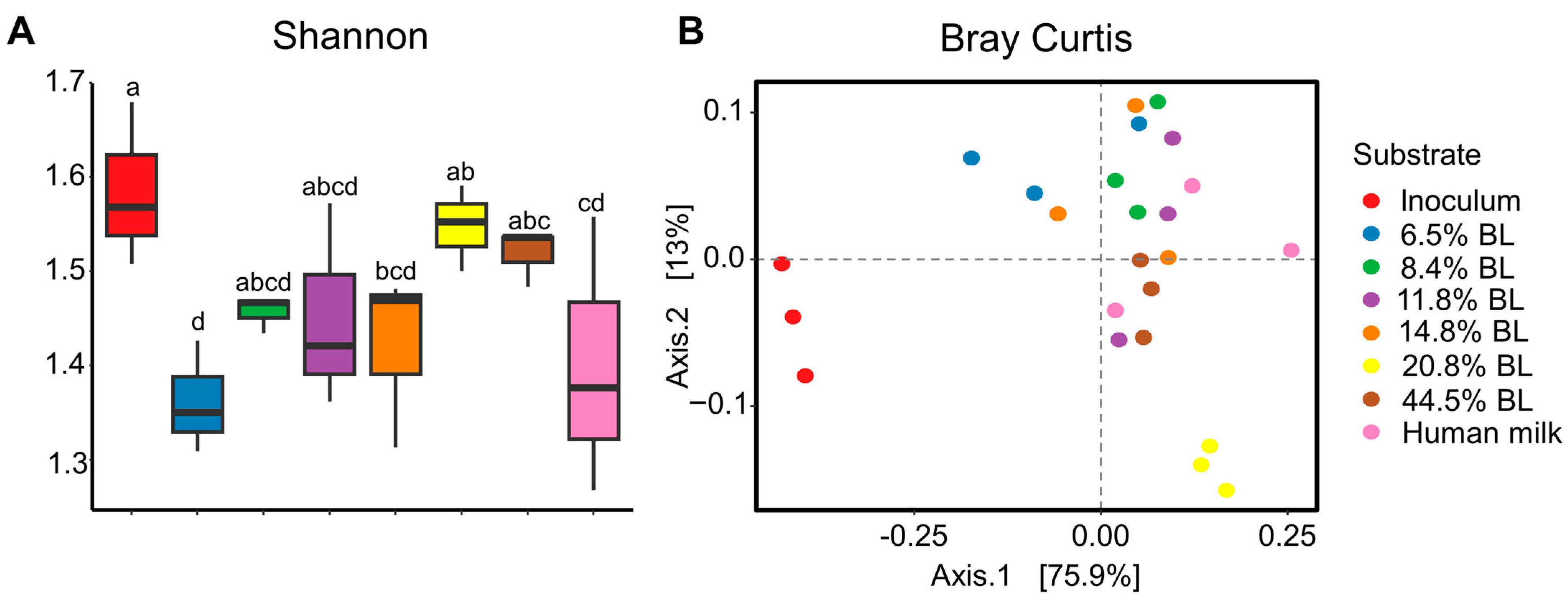
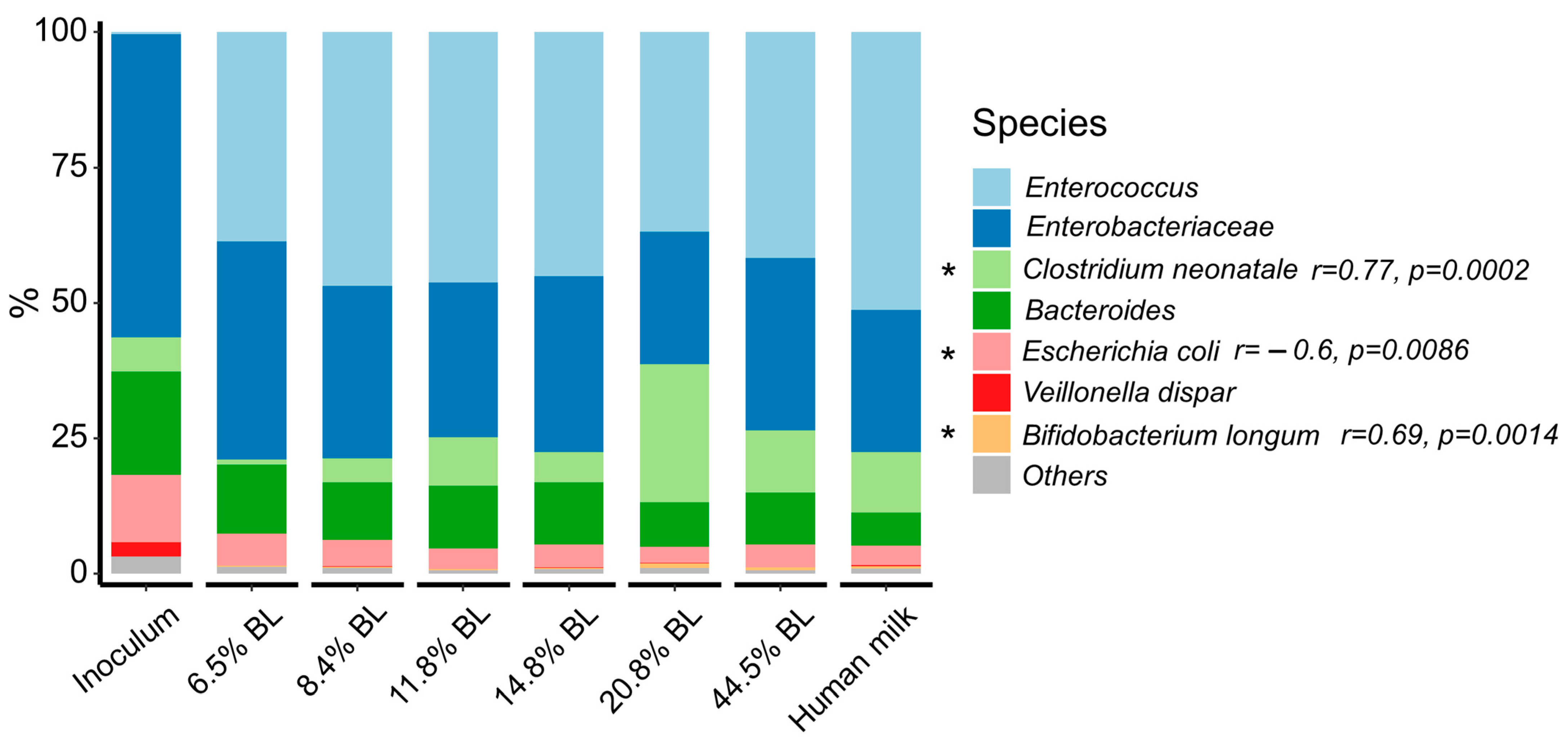
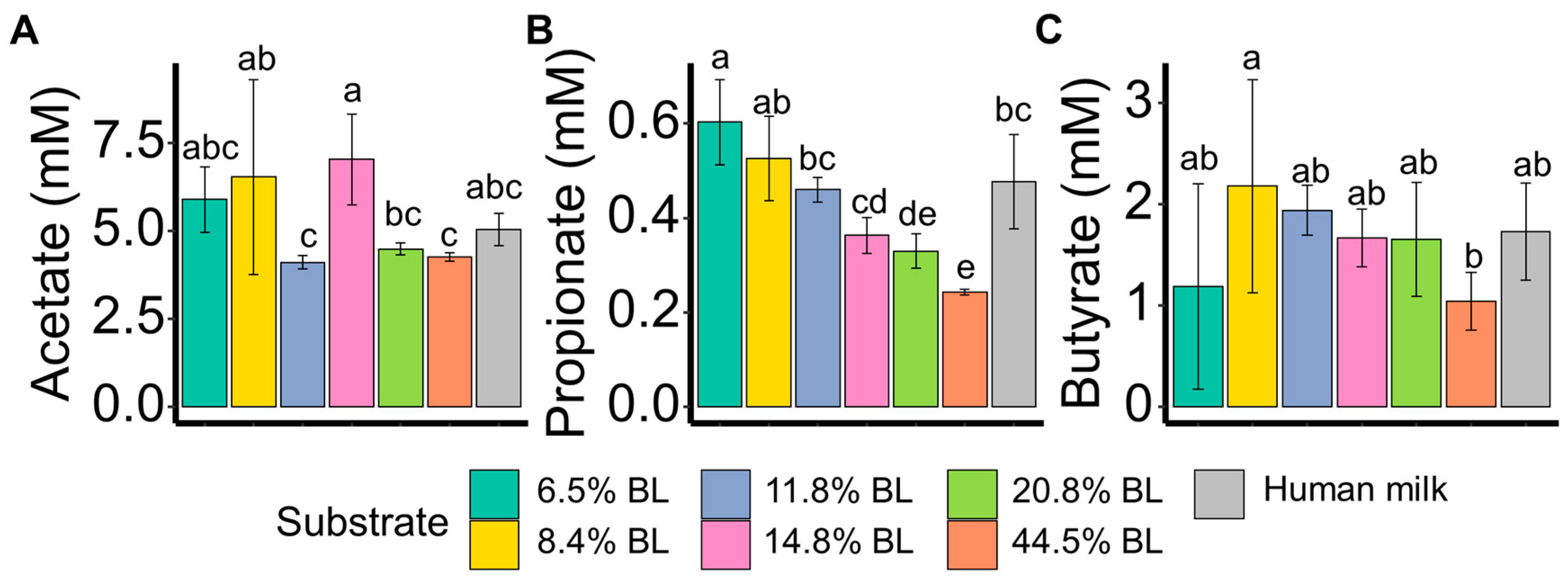
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. 2013, 60, 49–74. [Google Scholar] [CrossRef]
- Kunz, C.; Rudloff, S.; Baier, W.; Klein, N.; Strobel, S. Oligosaccharides in human milk: Structural, functional, and metabolic aspects. Ann. Rev. Nutr. 2000, 20, 699–722. [Google Scholar] [CrossRef] [PubMed]
- Gallier, S.P.; Van den Abbeele, P.; Prosser, C. Comparison of the bifidogenic effects of goat and cow milk-based infant formulas to human breast milk in an in vitro gut model for 3-month-old infants. Front. Nutr. 2020, 7, 304. [Google Scholar] [CrossRef] [PubMed]
- Schrander, J.J.; Van den Bogart, J.P.; Forget, P.P.; Schrander-Stumpel, C.T.; Kuijten, R.H.; Kester, A.D. Cow’s milk protein intolerance in infants under 1 year of age: A prospective epidemiological study. Eur. J. Pediatr. 1993, 152, 640–644. [Google Scholar] [CrossRef]
- Van de Heijning, B.J.; Berton, A.; Bouritius, H.; Goulet, O. GI symptoms in infants are a potential target for fermented infant milk formulae: A review. Nutrients 2014, 6, 3942–3967. [Google Scholar] [CrossRef]
- Schmitz-Schug, I.; Foerst, P.; Kulozik, U. Impact of the spray drying conditions and residence time distribution on lysine loss in spray dried infant formula. Dairy Sci. Technol. 2013, 93, 443–462. [Google Scholar] [CrossRef]
- Van Boekel, M. Effect of heating on Maillard reactions in milk. Food Chem. 1998, 62, 403–414. [Google Scholar] [CrossRef]
- Moughan, P.J.; Rutherfurd, S.M. Available lysine in foods: A brief historical overview. J. AOAC Int. 2008, 91, 901–906. [Google Scholar] [CrossRef]
- Meade, S.J.; Reid, E.A.; Gerrard, J.A. The impact of processing on the nutritional quality of food proteins. J. AOAC Int. 2005, 88, 904–922. [Google Scholar] [CrossRef]
- Pischetsrieder, M.; Henle, T. Glycation products in infant formulas: Chemical, analytical, and physiological aspects. Amino Acids 2012, 42, 1111–1118. [Google Scholar] [CrossRef]
- Mehta, B.M.; Deeth, H.C. Blocked lysine in dairy products: Formation, occurrence, analysis, and nutritional implications. Compr. Rev. Food Sci. Food Saf. 2016, 15, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Finot, P.A.; Bujard, E.; Mottu, F.; Mauron, J. Availability of the true Schiff’s bases of lysine. Chemical evaluation of the Schiff’s base between lysine and lactose in milk. Adv. Exp. Med. Biol. 1977, 86B, 343–365. [Google Scholar] [CrossRef] [PubMed]
- Nyakayiru, J.; Van Lieshout, G.A.A.; Trommelen, J.; Van Kranenburg, J.; Verdijk, L.B.; Bragt, M.C.E.; Van Loon, L.J.C. The glycation level of milk protein strongly modulates the post-prandial lysine availability in humans. Br. J. Nutr. 2020, 123, 545–552. [Google Scholar] [CrossRef]
- Efsa, N.D.A. EFSA Panel on Dietetic Products, Nutrition and Allergies, Scientific Opinion on the essential composition of infant and follow-on formulae. EFSA J. 2014, 12, 3760. [Google Scholar] [CrossRef]
- Consultation, F.E. Dietary protein quality evaluation in human nutrition. Report of an FAO Expert Consultation. FAO Food Nutr. Pap. 2011, 92, 1–66. [Google Scholar]
- Sheng, X.Y.; Buthmanaban, V.; Van Lieshout, G.A.A.; Parikh, P. Reduced Occurrence of Gastrointestinal Symptoms in Chinese Infants Fed Minimally Processed Commercially Available Formula: A Cross-Sectional Observational Study. J. Nutr. Metab. 2020, 2020, 1807397. [Google Scholar] [CrossRef]
- Eggesbø, M.; Moen, B.; Peddada, S.; Baird, D.; Rugtveit, J.; Midtvedt, T.; Bushel, P.R.; Sekelja, M.; Rudi, K. Development of gut microbiota in infants not exposed to medical interventions. APMIS 2011, 119, 17–35. [Google Scholar] [CrossRef]
- Portune, K.J.; Beaumont, M.; Davila, A.; Tomé, D.; Blachier, F.; Sanz, Y. Gut microbiota role in dietary protein metabolism and health-related outcomes: The two sides of the coin. Trends Food Sci. Technol. 2016, 57, 213–232. [Google Scholar] [CrossRef]
- Korpola, K. Diet, Microbiota, and metabolic health: Trade-off between saccharolytic and proteolytic fermentation. Ann. Rev. Food Sci. Technol. 2018, 9, 65–84. [Google Scholar] [CrossRef]
- Zenker, H.E.; Van Lieshout, G.A.A.; Van Gool, M.P.; Bragt, M.C.E.; Hettinga, K.A. Lysine blockage of milk proteins in infant formula impairs overall protein digestibility and peptide release. Food Func. 2020, 11, 358–369. [Google Scholar] [CrossRef]
- Ménard, O.; Bourlieu, C.; De Oliveira, S.C.; Dellarosa, N.; Laghi, L.; Carrière, F.; Capozzi, F.; Dupont, D.; Deglaire, A. A first step towards a consensus static in vitro model for simulating full-term infant digestion. Food Chem. 2018, 240, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Wiese, M.; Khakimov, B.; Nielsen, S.; Sørensen, H.; Van de Berg, F.; Nielsen, D.S. CoMiniGut-a small volume in vitro colon model for the screening of gut microbial fermentation processes. PeerJ 2018, 6, e4268. [Google Scholar] [CrossRef] [PubMed]
- Hui, Y.; Tamez-Hidalgo, P.; Cieplak, T.; Satessa, G.T.; Kot, W.; Kjaerulff, S.; Nielsen, M.O.; Nielsen, D.S.; Krych, L. Supplementation of a lacto-fermented rapeseed-seaweed blend promotes gut microbial- and gut immune-modulation in weaner piglets. J. Anim. Sci. Biotechnol. 2021, 12, 85. [Google Scholar] [CrossRef] [PubMed]
- Davis, E.C.; Castagna, V.P.; Sela, D.A.; Hillard, M.A.; Lindberg, S.; Mantis, N.J.; Seppo, A.E.; Järvinen, K.M. Gut microbiome and breast-feeding: Implications for early immune regulation. J. Allergy Clin Immnol. 2022, 150, 523–534. [Google Scholar] [CrossRef]
- Vatanen, T.; Kostic, A.D.; d’Hennezel, E.; Siljander, H.; Franzosa, E.A.; Yassour, M.; Kolde, R.; Vlamakis, H.; Arthur, T.D.; Hämäläinen, A.-M.; et al. Variation in Microbiome LPS Immunogenicity Contributes to Autoimmunity in Humans. Cell 2016, 165, 842–853. [Google Scholar] [CrossRef]
- Hosny, M.; Baptiste, E.; Levasseur, A.; La Scola, B. Molecular epidemiology of Clostridium neonatale and its relationship with the occurrence of necrotizing enterocolitis in preterm neonates. New Microb. New Infect. 2019, 32, 100612. [Google Scholar] [CrossRef]
- Bernard, K.; Burdz, T.; Wiebe, D.; Alfa, M.; Bernier, A.-M. Clostridium neonatale sp. nov. linked to necrotizing enterocolitis in neonates and the clarification of species assignable to the genus Clostridium (Prazmowski 1880) emend. Lawson and Rainey 2016. Int. J. Syst. Evol. Microbiol. 2018, 68, 2416–2423. [Google Scholar] [CrossRef]
- Cassir, N.; Grandvuillemin, I.; Boxberger, M.; Jardot, P.; Boubred, F.; La Scola, B. Case Report: Clostridium neonatale Bacteremia in a Preterm Neonate With Necrotizing Enterocolitis. Front Pediatr. 2021, 9, 771467. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Smith, E.A.; Macfarlane, G.T. Dissimilatory amino acid metabolism in human colonic bacteria. Anearobe 1997, 3, 327–337. [Google Scholar] [CrossRef]
- Heath, A.M.; Haszard, J.J.; Galland, B.C.; Lawley, B.; Rehrer, N.J.; Drummond, L.N.; Sims, I.M.; Taylor, R.W.; Otal, A.; Taylor, B.; et al. Association between the faecal short-chain fatty acid propionate and infant sleep. Eur. J. Clin. Nutr. 2020, 74, 1362–1365. [Google Scholar] [CrossRef]
- Wang, Y.; Van de Wouw, M.; Drogos, L.; Vaghef-Mehrabani, E.; Reimer, R.A.; Tomfohr-Madsen, L.; Giesbrecht, G.F. Sleep and the gut microbiota in preschool-aged children. Sleep J. 2022, 45, zsac020. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouillon, G.A.; Xie, Z.; Nielsen, D.S.; Wiese, M.; Nauta, A. Propionate Production by Infant Fecal Microbiota Is Inversely Correlated with the Protein Glycation Level of Supplemented Infant Formula Ex Vivo. Nutrients 2024, 16, 4047. https://doi.org/10.3390/nu16234047
Bouillon GA, Xie Z, Nielsen DS, Wiese M, Nauta A. Propionate Production by Infant Fecal Microbiota Is Inversely Correlated with the Protein Glycation Level of Supplemented Infant Formula Ex Vivo. Nutrients. 2024; 16(23):4047. https://doi.org/10.3390/nu16234047
Chicago/Turabian StyleBouillon, Grégoire A., Zhuqing Xie, Dennis S. Nielsen, Maria Wiese, and Arjen Nauta. 2024. "Propionate Production by Infant Fecal Microbiota Is Inversely Correlated with the Protein Glycation Level of Supplemented Infant Formula Ex Vivo" Nutrients 16, no. 23: 4047. https://doi.org/10.3390/nu16234047
APA StyleBouillon, G. A., Xie, Z., Nielsen, D. S., Wiese, M., & Nauta, A. (2024). Propionate Production by Infant Fecal Microbiota Is Inversely Correlated with the Protein Glycation Level of Supplemented Infant Formula Ex Vivo. Nutrients, 16(23), 4047. https://doi.org/10.3390/nu16234047






