The Role of Vitamin D Deficiency in Hepatic Encephalopathy: A Review of Pathophysiology, Clinical Outcomes, and Therapeutic Potential
Abstract
1. Introduction
2. History, Etiology, Clinical Features, and Diagnosis of Hepatic Encephalopathy
3. Pathophysiology of Cirrhosis and Hepatic Encephalopathy and the Role of Oxidative Stress
4. Vitamin D and Its Association with Cirrhosis and Hepatic Encephalopathy
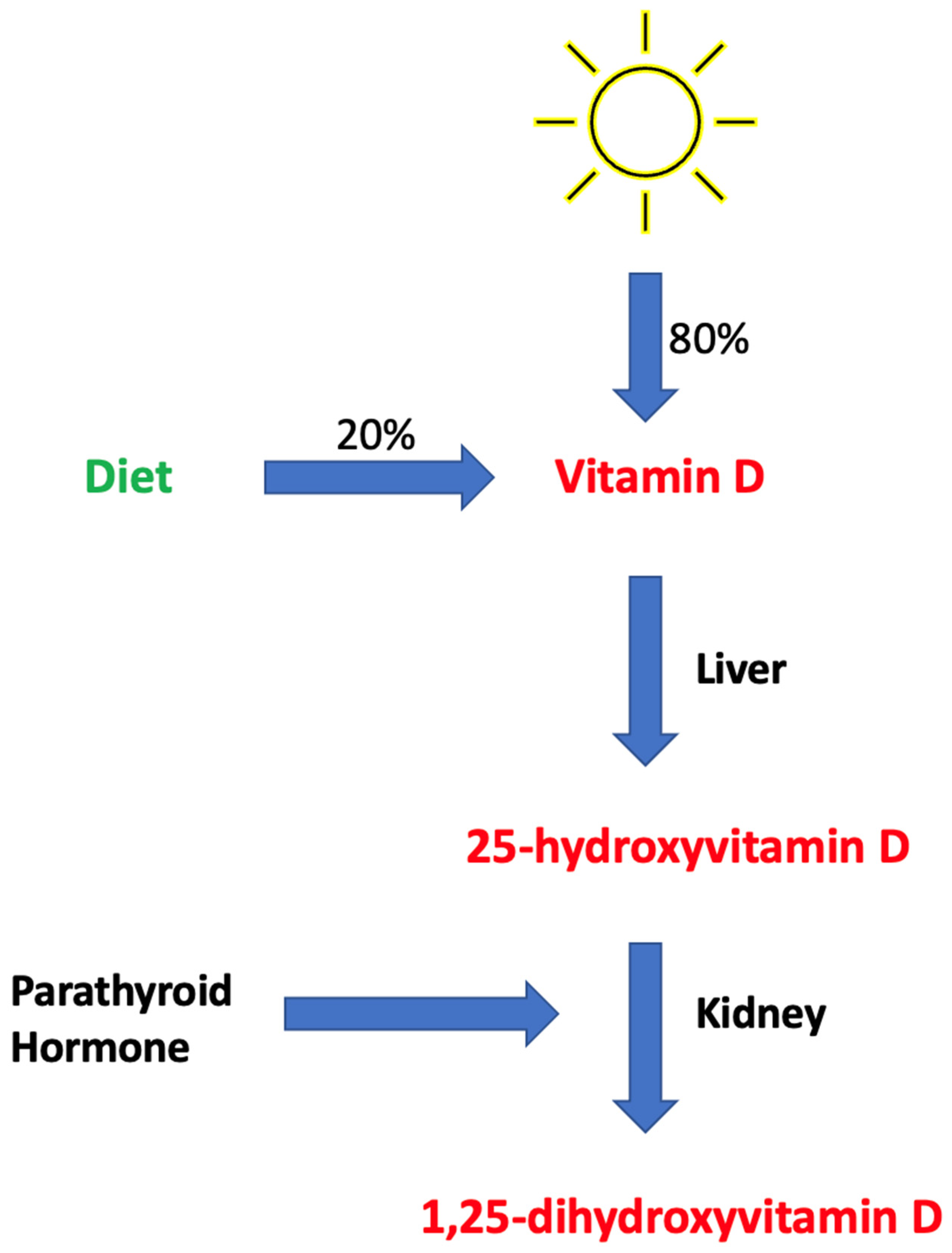
4.1. Brief Overview of Vitamin D Metabolism
4.2. Supplemental Forms of Vitamin D and the Definition of Deficiency
4.3. Vitamin D Levels in Association with Cirrhosis
4.4. Vitamin D Levels in Association with Hepatic Encephalopathy
4.5. Vitamin D Levels in Post-Operative Transjugular Intrahepatic Portosystemic Shunt (TIPS)
4.6. Vitamin D Has Been Shown to Suppress Oxidative Stress
5. Vitamin D Levels in Association with Survival
6. What Should Future Studies Explore?
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sahney, A.; Wadhawan, M. Encephalopathy in Cirrhosis: Prevention and Management. J. Clin. Exp. Hepatol. 2022, 12, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Mandiga, P.; Kommu, S.; Bollu, P.C. Hepatic Encephalopathy. In StatPearls; StatPearls: Treasure Island, FL, USA, 2024. [Google Scholar]
- Poordad, F.F. Review article: The burden of hepatic encephalopathy. Aliment. Pharmacol. Ther. 2007, 25 (Suppl. S1), 3–9. [Google Scholar] [CrossRef] [PubMed]
- Elsaid, M.I.; Rustgi, V.K. Epidemiology of Hepatic Encephalopathy. Clin. Liver Dis. 2020, 24, 157–174. [Google Scholar] [CrossRef] [PubMed]
- Mumit Sarkar, A.; Al Mukit, A.; Bari, T.; Islam, R.; Islam, S.; Sarker, K.; Chowdhury, M.; Rashid, M.H.O.; Alim, A. Association of low serum 25-Hydroxy vitamin D [25(OH)D] with hepatic encephalopathy in patients with decompensated liver cirrhosis. Arab. J. Gastroenterol. 2024, 25, 182–187. [Google Scholar] [CrossRef]
- Afifi, M.A.E.; Hussein, A.M.; Rizk, M. Low Serum 25-Hydroxy Vitamin D (25-OHD) and Hepatic Encephalopathy in HCV-Related Liver Cirrhosis. Int. J. Hepatol. 2021, 2021, 6669527. [Google Scholar] [CrossRef]
- Kalita, S.; Das, J.; Rajkakati, R.; Mili, C. Vitamin D in Patients of Chronic Liver Disease with Hepatic Encephalopathy. J. Assoc. Physicians India 2022, 70, 11–12. [Google Scholar]
- Harris, M.K.; Elliott, D.; Schwendimann, R.N.; Minagar, A.; Jaffe, S.L. Neurologic presentations of hepatic disease. Neurol. Clin. 2010, 28, 89–105. [Google Scholar] [CrossRef]
- Ferenci, P.; Lockwood, A.; Mullen, K.; Tarter, R.; Weissenborn, K.; Blei, A.T. Hepatic encephalopathy—Definition, nomenclature, diagnosis, and quantification: Final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology 2002, 35, 716–721. [Google Scholar] [CrossRef]
- Rudler, M.; Weiss, N.; Bouzbib, C.; Thabut, D. Diagnosis and Management of Hepatic Encephalopathy. Clin. Liver Dis. 2021, 25, 393–417. [Google Scholar] [CrossRef]
- Montoliu, C.; Cauli, O.; Urios, A.; ElMlili, N.; Serra, M.A.; Giner-Duran, R.; González-Lopez, O.; Del Olmo, J.A.; Wassel, A.; Rodrigo, J.M.; et al. 3-nitro-tyrosine as a peripheral biomarker of minimal hepatic encephalopathy in patients with liver cirrhosis. Am. J. Gastroenterol. 2011, 106, 1629–1637. [Google Scholar] [CrossRef]
- Sharma, B.; John, S. Hepatic Cirrhosis. In StatPearls; StatPearls: Treasure Island, FL, USA, 2024. [Google Scholar]
- Shojaie, L.; Iorga, A.; Dara, L. Cell Death in Liver Diseases: A Review. Int. J. Mol. Sci. 2020, 21, 9682. [Google Scholar] [CrossRef] [PubMed]
- Kisseleva, T.; Brenner, D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Premkumar, M.; Anand, A.C. Overview of Complications in Cirrhosis. J. Clin. Exp. Hepatol. 2022, 12, 1150–1174. [Google Scholar] [CrossRef] [PubMed]
- Gressner, O.A.; Lahme, B.; Gressner, A.M. Gc-globulin (vitamin D binding protein) is synthesized and secreted by hepatocytes and internalized by hepatic stellate cells through Ca(2+)-dependent interaction with the megalin/gp330 receptor. Clin. Chim. Acta 2008, 390, 28–37. [Google Scholar] [CrossRef]
- Nardelli, S.; Riggio, O.; Gioia, S.; Puzzono, M.; Pelle, G.; Ridola, L. Spontaneous porto-systemic shunts in liver cirrhosis: Clinical and therapeutical aspects. World J. Gastroenterol. 2020, 26, 1726–1732. [Google Scholar] [CrossRef]
- Lu, K. Cellular Pathogenesis of Hepatic Encephalopathy: An Update. Biomolecules 2023, 13, 396. [Google Scholar] [CrossRef]
- Bai, Y.; Li, K.; Li, X.; Chen, X.; Zheng, J.; Wu, F.; Chen, J.; Li, Z.; Zhang, S.; Wu, K.; et al. Effects of oxidative stress on hepatic encephalopathy pathogenesis in mice. Nat. Commun. 2023, 14, 4456. [Google Scholar] [CrossRef]
- Simicic, D.; Cudalbu, C.; Pierzchala, K. Overview of oxidative stress findings in hepatic encephalopathy: From cellular and ammonium-based animal models to human data. Anal. Biochem. 2022, 654, 114795. [Google Scholar] [CrossRef]
- de la Puente Yague, M.; Yurrita, L.C.; Cabañas, M.J.C.; Cenzual, M.A.C. Role of Vitamin D in Athletes and Their Performance: Current Concepts and New Trends. Nutrients 2020, 12, 579. [Google Scholar] [CrossRef]
- Sizar, O.; Khare, S.; Goyal, A.; Givler, A. Vitamin D Deficiency. In StatPearls; StatPearls: Treasure Island, FL, USA, 2024. [Google Scholar]
- Stevens, C.M.; Jain, S.K. Vitamin D/Bone Mineral Density and Triglyceride Paradoxes Seen in African Americans: A Cross-Sectional Study and Review of the Literature. Int. J. Mol. Sci. 2024, 25, 1305. [Google Scholar] [CrossRef]
- Pludowski, P. Supplementing Vitamin D in Different Patient Groups to Reduce Deficiency. Nutrients 2023, 15, 3725. [Google Scholar] [CrossRef] [PubMed]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Berisha, A.T.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Daroux, M.; Shenouda, M.; Bacri, J.-L.; Lemaitre, V.; Vanhille, P.; Bataille, P. Vitamin D2 versus vitamin D3 supplementation in hemodialysis patients: A comparative pilot study. J. Nephrol. 2013, 26, 152–157. [Google Scholar] [CrossRef]
- Cesareo, R.; Falchetti, A.; Attanasio, R.; Tabacco, G.; Naciu, A.M.; Palermo, A. Hypovitaminosis D: Is It Time to Consider the Use of Calcifediol? Nutrients 2019, 11, 1016. [Google Scholar] [CrossRef]
- Quesada-Gomez, J.M.; Bouillon, R. Is calcifediol better than cholecalciferol for vitamin D supplementation? Osteoporos. Int. 2018, 29, 1697–1711. [Google Scholar] [CrossRef] [PubMed]
- Jodar, E.; Campusano, C.; de Jongh, R.T.; Holick, M.F. Calcifediol: A review of its pharmacological characteristics and clinical use in correcting vitamin D deficiency. Eur. J. Nutr. 2023, 62, 1579–1597. [Google Scholar] [CrossRef]
- Arteh, J.; Narra, S.; Nair, S. Prevalence of vitamin D deficiency in chronic liver disease. Dig. Dis. Sci. 2010, 55, 2624–2628. [Google Scholar] [CrossRef]
- Clements, M.R.; Davies, M.; Hayes, M.E.; Hlckey, C.D.; Lumb, G.A.; Mawer, E.B.; Adams, P.H. The role of 1,25-dihydroxyvitamin D in the mechanism of acquired vitamin D deficiency. Clin. Endocrinol. 1992, 37, 17–27. [Google Scholar] [CrossRef]
- Sitrin, M.D.; Bengoa, J.M. Intestinal absorption of cholecalciferol and 25-hydroxycholecalciferol in chronic cholestatic liver disease. Am. J. Clin. Nutr. 1987, 46, 1011–1015. [Google Scholar] [CrossRef]
- Putz-Bankuti, C.; Pilz, S.; Stojakovic, T.; Scharnagl, H.; Pieber, T.R.; Trauner, M.; Obermayer-Pietsch, B.; Stauber, R.E. Association of 25-hydroxyvitamin D levels with liver dysfunction and mortality in chronic liver disease. Liver Int. 2012, 32, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Stokes, C.S.; Krawczyk, M.; Reichel, C.; Lammert, F.; Grünhage, F. Vitamin D deficiency is associated with mortality in patients with advanced liver cirrhosis. Eur. J. Clin. Investig. 2014, 44, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Anty, R.; Tonohouan, M.; Ferrari-Panaia, P.; Piche, T.; Pariente, A.; Anstee, Q.M.; Gual, P.; Tran, A. Low Levels of 25-Hydroxy Vitamin D are Independently Associated with the Risk of Bacterial Infection in Cirrhotic Patients. Clin. Transl. Gastroenterol. 2014, 5, e56. [Google Scholar] [CrossRef]
- Koop, A.H.; Mousa, O.Y.; Pham, L.E.; Corral-Hurtado, J.E.; Pungpapong, S.; Keaveny, A.P. An Argument for Vitamin D, A, and Zinc Monitoring in Cirrhosis. Ann. Hepatol. 2018, 17, 920–932. [Google Scholar] [CrossRef]
- Kumar, P.; Chaudhry, S.; Dev, N.; Kumar, R.; Singh, G. Serum 25-hydroxyvitamin D level in patients with chronic liver disease and its correlation with hepatic encephalopathy: A cross-sectional study. J. Fam. Med. Prim. Care 2020, 9, 798–803. [Google Scholar]
- Kubesch, A.; Quenstedt, L.; Saleh, M.; Rüschenbaum, S.; Schwarzkopf, K.; Martinez, Y.; Welsch, C.; Zeuzem, S.; Welzel, T.M.; Lange, C.M. Vitamin D deficiency is associated with hepatic decompensation and inflammation in patients with liver cirrhosis: A prospective cohort study. PLoS ONE 2018, 13, e0207162. [Google Scholar] [CrossRef] [PubMed]
- Savic, Z.; Damjanov, D.; Curic, N.; Kovacev-Zavisic, B.; Hadnadjev, L.; Novakovic-Paro, J.; Nikolic, S. Vitamin D status, bone metabolism and bone mass in patients with alcoholic liver cirrhosis. Bratisl. Lek. Listy 2014, 115, 573–578. [Google Scholar] [CrossRef]
- Vidot, H.; Potter, A.; Cheng, R.; Allman-Farinelli, M.; Shackel, N. Serum 25-hydroxyvitamin D deficiency and hepatic encephalopathy in chronic liver disease. World J. Hepatol. 2017, 9, 510–518. [Google Scholar] [CrossRef]
- Jha, A.K.; Jha, S.K.; Kumar, A.; Dayal, V.M.; Jha, S.K. Effect of replenishment of vitamin D on survival in patients with decompensated liver cirrhosis: A prospective study. World J. Gastrointest. Pathophysiol. 2017, 8, 133–141. [Google Scholar] [CrossRef]
- Khan, M.A.; Dar, H.A.; Baba, M.A.; Shah, A.H.; Singh, B.; Shiekh, N.A. Impact of Vitamin D Status in Chronic Liver Disease. J. Clin. Exp. Hepatol. 2019, 9, 574–580. [Google Scholar] [CrossRef]
- Yousif, M.M.; Sadek, A.M.E.M.; Farrag, H.A.; Selim, F.O.; Hamed, E.F.; Salama, R.I. Associated vitamin D deficiency is a risk factor for the complication of HCV-related liver cirrhosis including hepatic encephalopathy and spontaneous bacterial peritonitis. Intern. Emerg. Med. 2019, 14, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Narayanasamy, K.; Karthick, R.; Raj, A.K. High Prevalent Hypovitaminosis D Is Associated with Dysregulation of Calcium-parathyroid Hormone-vitamin D Axis in Patients with Chronic Liver Diseases. J. Clin. Transl. Hepatol. 2019, 7, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Simbrunner, B.; Semmler, G.; Stadlmann, A.; Scheiner, B.; Schwabl, P.; Paternostro, R.; Bucsics, T.; Bauer, D.; Eigenbauer, E.; Pinter, M.; et al. Vitamin A levels reflect disease severity and portal hypertension in patients with cirrhosis. Hepatol. Int. 2020, 14, 1093–1103. [Google Scholar] [CrossRef] [PubMed]
- Casadaban, L.C.; Parvinian, A.; Minocha, J.; Lakhoo, J.; Grant, C.W.; Ray, C.E.; Knuttinen, M.G.; Bui, J.T.; Gaba, R.C. Clearing the Confusion over Hepatic Encephalopathy After TIPS Creation: Incidence, Prognostic Factors, and Clinical Outcomes. Dig. Dis. Sci. 2015, 60, 1059–1066. [Google Scholar] [CrossRef]
- Panchani, N.N.; Colvin, T.; Aryan, M.; Shoreibah, M.G. Nutritional Deficiencies and Clinical Practices in Decompensated Cirrhotics With Hepatic Encephalopathy. Cureus 2022, 14, e25352. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.K.; Stevens, C.M.; Margret, J.J.; Levine, S.N. Alzheimer’s Disease: A Review of Pathology, Current Treatments, and the Potential Therapeutic Effect of Decreasing Oxidative Stress by Combined Vitamin D and l-Cysteine Supplementation. Antioxid. Redox Signal. 2024, 40, 663–678. [Google Scholar] [CrossRef]
- Wenclewska, S.; Szymczak-Pajor, I.; Drzewoski, J.; Bunk, M.; Śliwińska, A. Vitamin D Supplementation Reduces Both Oxidative DNA Damage and Insulin Resistance in the Elderly with Metabolic Disorders. Int. J. Mol. Sci. 2019, 20, 2891. [Google Scholar] [CrossRef]
- Renke, G.; Starling-Soares, B.; Baesso, T.; Petronio, R.; Aguiar, D.; Paes, R. Effects of Vitamin D on Cardiovascular Risk and Oxidative Stress. Nutrients 2023, 15, 769. [Google Scholar] [CrossRef]
- Charoenngam, N.; Holick, M.F. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef]
- Bouillon, R.; Antonio, L.; Olarte, O.R. Calcifediol (25OH Vitamin D(3)) Deficiency: A Risk Factor from Early to Old Age. Nutrients 2022, 14, 1168. [Google Scholar] [CrossRef]
- Wan, Z.; Guo, J.; Pan, A.; Chen, C.; Liu, L.; Liu, G. Association of Serum 25-Hydroxyvitamin D Concentrations With All-Cause and Cause-Specific Mortality Among Individuals With Diabetes. Diabetes Care 2021, 44, 350–357. [Google Scholar] [CrossRef] [PubMed]
| References | Title | Disease (n) | Prevalence of VDD | Finding(s) |
|---|---|---|---|---|
| Putz-Bankuti et al., 2012 [34] | Association of 25-hydroxyvitamin D levels with liver dysfunction and mortality in chronic liver disease | CLD (n = 75) | VDD (<20 ng/mL) in 53(71%) of patients | 25-OHD levels were inversely correlated with CP and MELD scores |
| Savic et al., 2014 [40] | Vitamin D status, bone metabolism and bone mass in patients with alcoholic liver cirrhosis | ALD (n = 30) | VDD (<50 nmol/L) in 67% of patients | VDD had the highest prevalence in CP C class patients |
| Vidot et al., 2017 [41] | Serum 25-hydroxyvitamin D deficiency and hepatic encephalopathy in chronic liver disease | CLD (n = 165) | Moderate to severe 25-OHD deficiency was identified in 49 patients of whom 36 had grade 2–3 HE | 25-OHD deficiency is observed in many patients with CLD |
| Jha et al., 2017 [42] | Effect of replenishment of vitamin D on survival in patients with decompensated liver cirrhosis: A prospective study | Decompensated CLD (n = 153) | VDD (<20 ng/mL) in 129(84%) of patients | VD supplementation may increase survival probability of patients with decompensated liver cirrhosis with improvements in MELD and CP scores |
| Khan et al., 2019 [43] | Impact of Vitamin D Status in Chronic Liver Disease | CLD patients (n = 75) | VDD (<20 ng/dL) was found in 41% out of which 19% suffered from severe VDD (<10 ng/dL) | VDD was associated with CLD and an increased in CP score |
| Yousif et al., 2019 [44] | Associated vitamin D deficiency is a risk factor for the complication of HCV-related liver cirrhosis including hepatic encephalopathy and spontaneous bacterial peritonitis | HCV-related liver cirrhosis w/and w/o HE (n = 90) | 25-OHD level was on average 16.28 ng/mL in control and 6.81 ng/mL in HE group | Low serum levels of 25-OHD were associated with HE in cirrhotic patients |
| Narayanasamy et al., 2019 [45] | High Prevalent Hypovitaminosis D Is Associated with Dysregulation of Calcium-parathyroid Hormone-vitamin D Axis in Patients with Chronic Liver Diseases | CLD patients (n = 236) | VDD (<30 ng/dL) was found in 162 (69%) | VDD is associated with higher CP scores |
| Kumar et al., 2020 [38] | Serum 25-hydroxyvitamin D level in patients with chronic liver disease and its correlation with hepatic encephalopathy: A cross-sectional study | CLD w/and w/o HE (n = 100) | Severe 25-OHD deficiency was seen in 38% of HE group compared to 6% in control group | Serum 25-OHD deficiency was more prevalent in HE group compared to control |
| Simbrunner et al., 2020 [46] | Vitamin A levels reflect disease severity and portal hypertension in patients with cirrhosis | ACLD (n = 234) | VDD was found in 133 (57%) | VDD increased with increasing CP stages |
| Afifi et al., 2021 [6] | Low Serum 25-Hydroxy Vitamin D (25-OHD) and Hepatic Encephalopathy in HCV-Related Liver Cirrhosis | HCV-related liver cirrhosis w/and w/o HE (n = 100) | HE groups w/severe VDD was 16% compared to other group at 6%; HE group w/moderate VDD was 24% compared to other group at 10% | Lower levels of 25-OHD were associated with higher incidence of HE in cirrhotic HCV patients |
| Kalita et al., 2022 [7] | Vitamin D in Patients of Chronic Liver Disease with Hepatic Encephalopathy | CLD w/HE (n = 88) | Mean serum 25-OHD was 24.11, 13.61, 8.41, and 8.00 ng/mL in grades 1–4 HE, respectively | Mean serum 25-OHD deficiency became more severe as HE worsened |
| Sarkar et al., 2024 [5] | Association of low serum 25-Hydroxy vitamin D [25(OH)D] with hepatic encephalopathy in patients with decompensated liver cirrhosis | Decompensated cirrhosis of the liver w/and w/o HE (n = 70) | 91% of HE patients had moderate to severe 25-OHD deficiency compared 51% in control group | Significant association was found between low serum 25-OHD and HE |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson, C.D.; Stevens, C.M.; Bennett, M.R.; Litch, A.B.; Rodrigue, E.M.; Quintanilla, M.D.; Wallace, E.; Allahyari, M. The Role of Vitamin D Deficiency in Hepatic Encephalopathy: A Review of Pathophysiology, Clinical Outcomes, and Therapeutic Potential. Nutrients 2024, 16, 4007. https://doi.org/10.3390/nu16234007
Johnson CD, Stevens CM, Bennett MR, Litch AB, Rodrigue EM, Quintanilla MD, Wallace E, Allahyari M. The Role of Vitamin D Deficiency in Hepatic Encephalopathy: A Review of Pathophysiology, Clinical Outcomes, and Therapeutic Potential. Nutrients. 2024; 16(23):4007. https://doi.org/10.3390/nu16234007
Chicago/Turabian StyleJohnson, Coplen D., Christopher M. Stevens, Matthew R. Bennett, Adam B. Litch, Eugenie M. Rodrigue, Maria D. Quintanilla, Eric Wallace, and Massoud Allahyari. 2024. "The Role of Vitamin D Deficiency in Hepatic Encephalopathy: A Review of Pathophysiology, Clinical Outcomes, and Therapeutic Potential" Nutrients 16, no. 23: 4007. https://doi.org/10.3390/nu16234007
APA StyleJohnson, C. D., Stevens, C. M., Bennett, M. R., Litch, A. B., Rodrigue, E. M., Quintanilla, M. D., Wallace, E., & Allahyari, M. (2024). The Role of Vitamin D Deficiency in Hepatic Encephalopathy: A Review of Pathophysiology, Clinical Outcomes, and Therapeutic Potential. Nutrients, 16(23), 4007. https://doi.org/10.3390/nu16234007





