Benefits of Camel Milk over Cow and Goat Milk for Infant and Adult Health in Fighting Chronic Diseases: A Review
Highlights
- Therapeutic Superiority: Camel milk (CAM) contains insulin-like proteins, lactoferrin, and antioxidants that contribute to improved glycemic control and cardiovascular health, thus constituting a promising dietary intervention for diabetes and CVD management.
- Comparative Nutrition: CAM offers a distinct nutritional profile with higher levels of vitamin C, iron, and zinc and lower levels of allergenic proteins compared to cow (COM) and goat milk (GOM), supporting its digestibility and tolerability in sensitive individuals.
- Bioactivity and Immunity: The unique bioactive compounds in CAM exhibit antimicrobial, anti-inflammatory, and immunomodulatory activities, demonstrating potential in chronic disease prevention.
- Infant and Adult Health: CAM is better tolerated in lactose-intolerant individuals, forms softer curds during digestion, and mimics the digestibility of human milk, indicating its suitability for both infants and adults.
Abstract
1. Introduction
2. Study Selection Criteria
2.1. Inclusion Criteria
- -
- Studies published within the last six years (2018–2024) to reflect the most recent developments in the research on CAM, COM, and GOM.
- -
- Peer-reviewed articles focused on the nutritional composition, hypoglycemic, lipid-lowering, antioxidant, or antimicrobial effects of CAM, COM, and GOM, particularly concerning diabetes, CVD, and other health conditions.
- -
- Various study designs, including clinical trials, animal studies, and in vitro experiments, were considered to provide a comprehensive understanding of the potential health impacts of these milk types.
- -
- Articles written in English are available in full-text format.
2.2. Exclusion Criteria
- -
- Studies published before 2018 unless they provided critical data or insights foundational to the reviewed topics.
- -
- Articles that did not specifically address the health effects or nutritional comparisons of CAM, COM, and GOM.
- -
- Reviews and meta-analyses that did not offer new insights or perspectives beyond those found in more recent primary research.
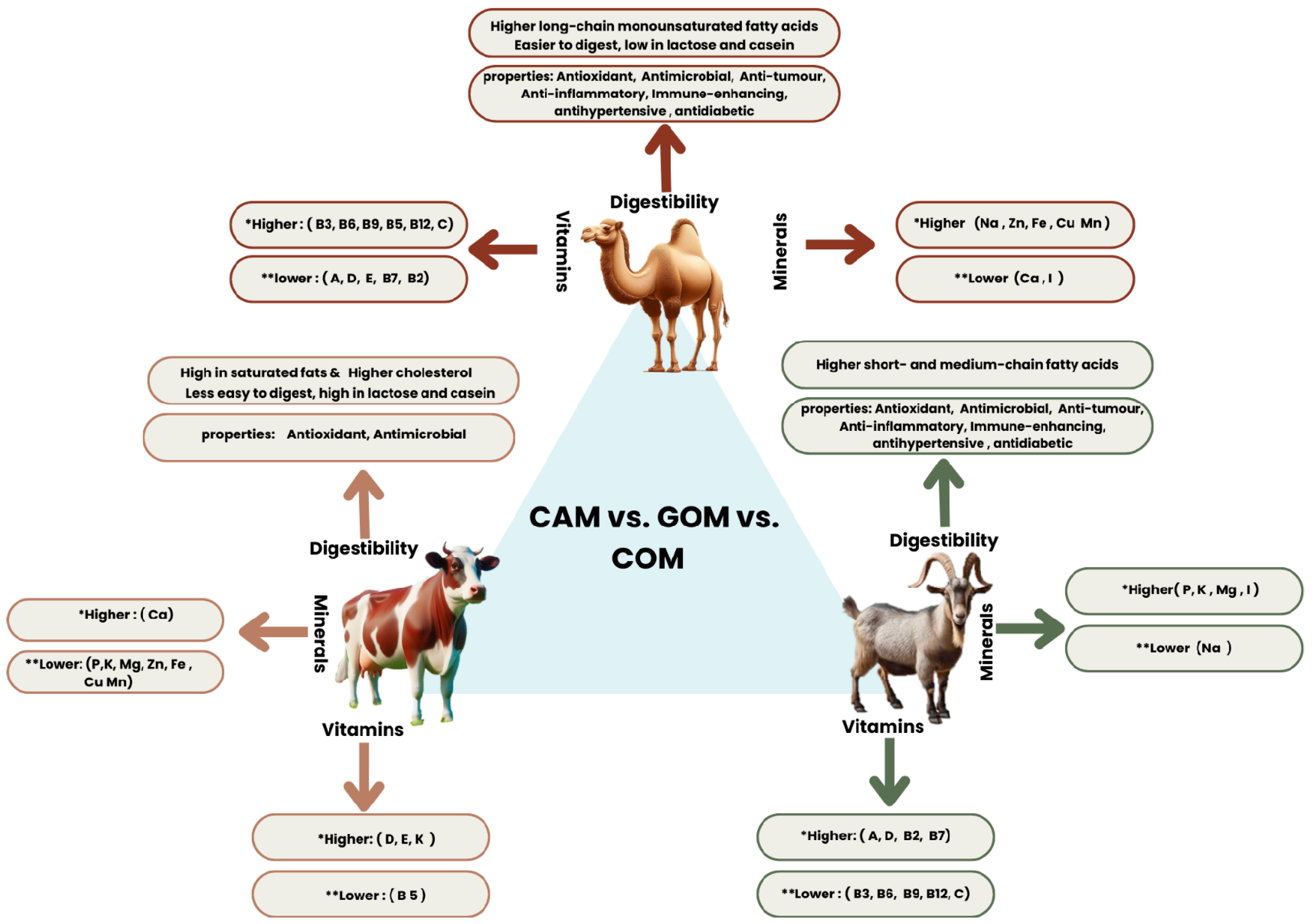
3. Nutritional Composition of Camel Milk
3.1. Protein
3.2. Lactose
3.3. Fat
3.4. Minerals and Vitamins
4. Milk Digestion
4.1. Infant Milk Digestion
4.2. Childhood Allergy to CAM
4.3. Adult Milk Digestion
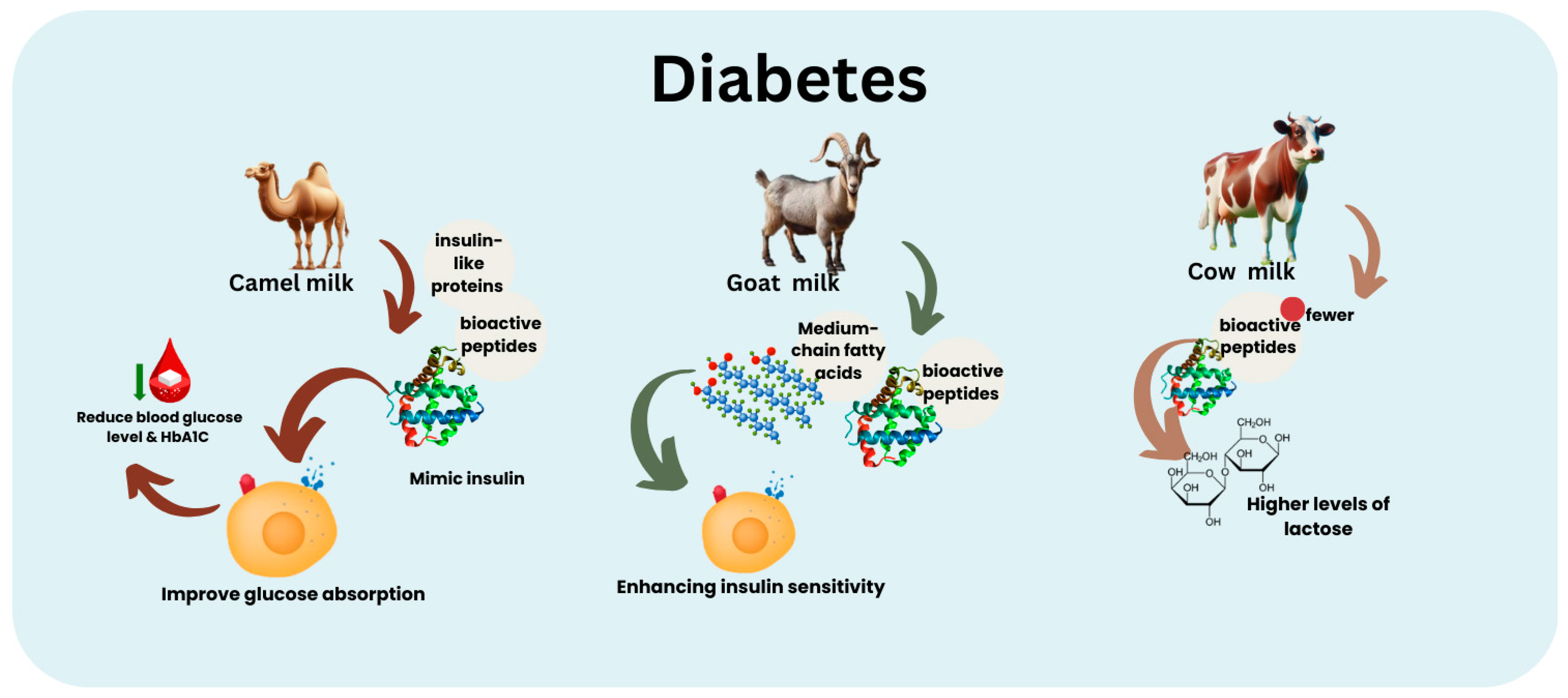
5. CAM and Metabolic Disorders in Adulthood
5.1. CAM and Diabetes
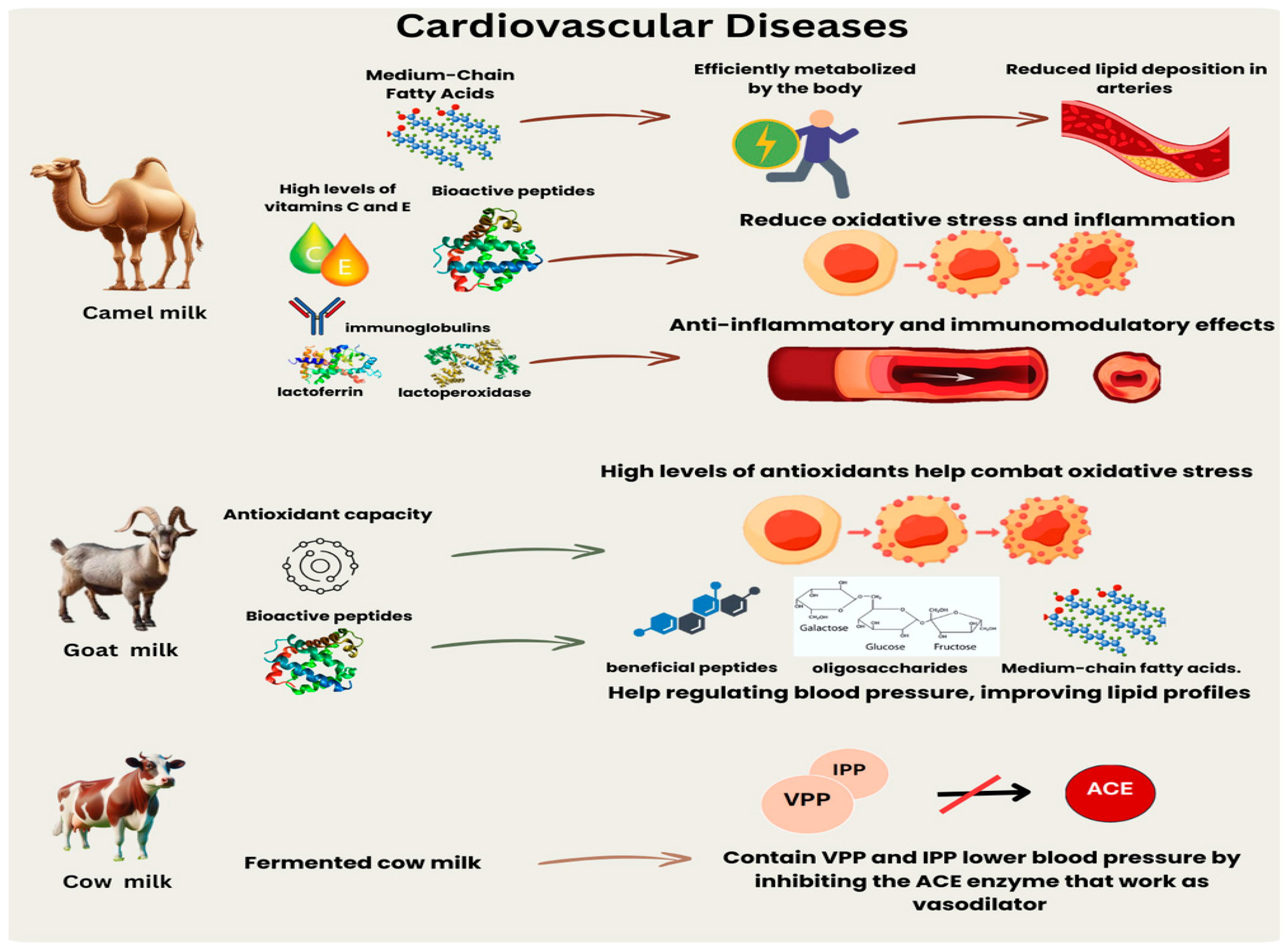
5.2. Camel Milk and CVD
6. CAM Antioxidant, Anti-Inflammatory, and Antimicrobial Properties
7. CAM and Gut Microbiota
8. Molecular Mechanisms in Beneficial Health Effects of CAM, COM, and GOM
9. Comparative Analysis of Human Milk, CAM, GOM, and COM: Nutritional Composition and Digestibility
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Miller, R.G.; Costacou, T.; Orchard, T.J. Risk factor modeling for cardiovascular disease in type 1 diabetes in the Pittsburgh epidemiology of diabetes complications (EDC) Study: A comparison with the diabetes control and complications trial/epidemiology of diabetes interventions and complications study (DCCT/EDIC). Diabetes 2019, 68, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Zahra, A.; Lee, E.-W.; Sun, L.-y.; Park, J.-H. Cardiovascular disease and diabetes mortality, and their relation to socio-economical, environmental, and health behavioral factors in worldwide view. Public Health 2015, 129, 385–395. [Google Scholar] [CrossRef]
- Pennells, L.; Kaptoge, S.; Wood, A.; Sweeting, M.; Zhao, X.; White, I.; Burgess, S.; Willeit, P.; Bolton, T.; Moons, K.G.M.; et al. Equalization of four cardiovascular risk algorithms after systematic recalibration: Individual-participant meta-analysis of 86 prospective studies. Eur. Heart J. 2018, 40, 621–663. [Google Scholar] [CrossRef] [PubMed]
- Jan, B.; Dar, M.I.; Choudhary, B.; Basist, P.; Khan, R.; Alhalmi, A. Cardiovascular diseases among Indian older adults: A comprehensive review. Cardiovasc. Ther. 2024, 2024, 6894693. [Google Scholar] [CrossRef]
- Dong, C.; Wu, G.; Li, H.; Qiao, Y.; Gao, S. Type 1 and Type 2 diabetes mortality burden: Predictions for 2030 based on Bayesian age-period-cohort analysis of China and global mortality burden from 1990 to 2019. J. Diabetes Investig. 2024, 15, 623–633. [Google Scholar] [CrossRef]
- Ismail, L.C.; Osaili, T.M.; Mohamad, M.N.; Zakaria, H.; Ali, A.; Tarek, A.; Ashfaq, A.; Al Abdouli, M.A.; Saleh, S.T.; Al Daour, R.; et al. Camel milk consumption patterns and perceptions in the UAE: A cross-sectional study. J. Nutr. Sci. 2022, 11, e59. [Google Scholar] [CrossRef]
- Mirmiran, P.; Ejtahed, H.S.; Angoorani, P.; Eslami, F.; Azizi, F. Camel milk has beneficial effects on diabetes mellitus: A systematic review. Int. J. Endocrinol. Metab. 2017, 15, e42150. [Google Scholar] [CrossRef] [PubMed]
- Mohammadabadi, T.; Ur Rehman, A.; Jain, R. Mechanism of camel milk on diabetes complications and cardiovascular disorders. Pak. Heart J. 2022, 55, 311–317. [Google Scholar] [CrossRef]
- Ayoub, M.A.; Palakkott, A.R.; Ashraf, A.; Iratni, R. The molecular basis of the anti-diabetic properties of camel milk. Diabetes Res. Clin. Pr. 2018, 146, 305–312. [Google Scholar] [CrossRef]
- Hussain, H.; Wattoo, F.H.; Wattoo, M.H.S.; Gulfraz, M.; Masud, T.; Shah, I.; Ali, S.; Alavi, S.E. Camel milk as an alternative treatment regimen for diabetes therapy. Food Sci. Nutr. 2021, 9, 1347–1356. [Google Scholar] [CrossRef]
- Mohammaddin, A.; Rohra, D.K.; Mortaja, S.; Abanmi, S.; Al Saati, O.; Cahusac, P.M.B.; Khawaja, R.A.; Al-Selaihem, A.; Al-Omran, Y. Effects of camel milk in dyslipidaemia: A randomized clinical trial. Int. Dairy J. 2018, 84, 79–84. [Google Scholar] [CrossRef]
- Muthukumaran, M.S.; Mudgil, P.; Baba, W.N.; Ayoub, M.A.; Maqsood, S. A comprehensive review on health benefits, nutritional composition and processed products of camel milk. Food Rev. Int. 2023, 39, 3080–3116. [Google Scholar] [CrossRef]
- Alkaisy, Q.H.; Al-Saadi, J.S.; AL-Rikabi, A.K.J.; Altemimi, A.B.; Hesarinejad, M.A.; Abedelmaksoud, T.G. Exploring the health benefits and functional properties of goat milk proteins. Food Sci. Nutr. 2023, 11, 5641–5656. [Google Scholar] [CrossRef]
- Antunes, I.C.; Bexiga, R.; Pinto, C.; Roseiro, L.C.; Quaresma, M.A.G. Cow’s milk in human nutrition and the emergence of plant-based milk alternatives. Foods 2022, 12, 99. [Google Scholar] [CrossRef]
- El-Hatmi, H.E.-H.; Jrad, Z.; Salhi, I.; Aguibi, A.; Nadri, A.; Khorchani, T. Comparison of composition and whey protein fractions of human, camel, donkey, goat, and cow milk. Mljekarstvo 2015, 65, 159–167. [Google Scholar] [CrossRef]
- Mohamed, H.; Johansson, M.; Lundh, Å.; Nagy, P.; Kamal-Eldin, A. Short communication: Caseins and α-lactalbumin content of camel milk (Camelus dromedarius) determined by capillary electrophoresis. J. Dairy Sci. 2020, 103, 11094–11099. [Google Scholar] [CrossRef] [PubMed]
- Konuspayeva, G.; Faye, B.; Loiseau, G. The composition of camel milk: A meta-analysis of the literature data. J. Food Compos. Anal. 2009, 22, 95–101. [Google Scholar] [CrossRef]
- Ho, T.M.; Zou, Z.; Bansal, N. Camel milk: A review of its nutritional value, heat stability, and potential food products. Food Res. Int. 2022, 153, 110870. [Google Scholar] [CrossRef]
- Guetouache, M.; Guessas, B.; Medjekal, S. Composition and nutritional value of raw milk. Issues Biol. Sci. Pharm. Res. 2014, 2, 115–122. [Google Scholar] [CrossRef]
- Seifu, E. Recent advances on camel milk: Nutritional and health benefits and processing implications—A review. AIMS Agric. Food 2022, 7, 777–804. [Google Scholar] [CrossRef]
- Turkmen, N. The nutritional value and health benefits of goat milk components. In Nutrients in Dairy and Their Implications on Health and Disease; Watson, R.R., Collier, R.J., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 441–449. [Google Scholar] [CrossRef]
- Konuspayeva, G.; Faye, B.; Bengoumi, M. Mineral status in camel milk: A critical review. Anim. Front. 2022, 12, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Li, R.-R.; Yue, H.-T.; Shi, Z.-Y.; Shen, T.; Yao, H.-B.; Zhang, J.-W.; Gao, Y.; Yang, J. Protein profile of whole camel milk resulting from commercial thermal treatment. LWT 2020, 134, 110256. [Google Scholar] [CrossRef]
- Ye, A.; Cui, J.; Carpenter, E.; Prosser, C.; Singh, H. Dynamic in vitro gastric digestion of infant formulae made with goat milk and cow milk: Influence of protein composition. Int. Dairy J. 2019, 97, 76–85. [Google Scholar] [CrossRef]
- Arain, M.A.; Salman, H.M.; Ali, M.; Khaskheli, G.B.; Barham, G.S.; Marghazani, I.B.; Ahmed, S. A Review on camel milk composition, techno-functional properties and processing constraints. Food Sci. Anim. Resour. 2024, 44, 739–757. [Google Scholar] [CrossRef]
- Prosser, C.G. Compositional and functional characteristics of goat milk and relevance as a base for infant formula. J. Food Sci. 2021, 86, 257–265. [Google Scholar] [CrossRef]
- Wang, L.; Wu, T.; Zhang, Y.; Yang, K.; He, Y.; Deng, K.; Liang, C.; Gu, Y. Comparative studies on the nutritional and physicochemical properties of yoghurts from cows’, goats’, and camels’ milk powder. Int. Dairy J. 2023, 138, 105542. [Google Scholar] [CrossRef]
- Vincenzetti, S.; Cammertoni, N.; Rapaccetti, R.; Santini, G.; Klimanova, Y.; Zhang, J.-J.; Polidori, P. Nutraceutical and functional properties of camelids’ milk. Beverages 2022, 8, 12. [Google Scholar] [CrossRef]
- Mohamed, H.; Ayyash, M.; Kamal-Eldin, A. Effect of heat treatments on camel milk proteins—A review. Int. Dairy J. 2022, 133, 105404. [Google Scholar] [CrossRef]
- Habtegebriel, H.; Wawire, M.; Gaukel, V.; Taboada, M.L. Comparison of the viscosity of camel milk with model milk systems in relation to their atomization properties. J. Food Sci. 2020, 85, 3459–3466. [Google Scholar] [CrossRef]
- Roy, D.; Ye, A.; Moughan, P.J.; Singh, H. Gelation of milks of different species (dairy cattle, goat, sheep, red deer, and water buffalo) using glucono-δ-lactone and pepsin. J. Dairy Sci. 2020, 103, 5844–5862. [Google Scholar] [CrossRef]
- Ingham, B.; Smialowska, A.; Kirby, N.M.; Wang, C.; Carr, A.J. A structural comparison of casein micelles in cow, goat, and sheep milk using X-ray scattering. Soft Matter 2018, 14, 3336–3343. [Google Scholar] [CrossRef]
- Hailu, Y.; Hansen, E.B.; Seifu, E.; Eshetu, M.; Ipsen, R.; Kappeler, S. Functional and technological properties of camel milk proteins: A review. J. Dairy Res. 2016, 83, 422–429. [Google Scholar] [CrossRef]
- Albenzio, M.; Campanozzi, A.; D’Apolito, M.; Santillo, A.; Mantovani, M.P.; Sevi, A. Differences in protein fraction from goat and cow milk and their role on cytokine production in children with cow’s milk protein allergy. Small Rumin. Res. 2012, 105, 202–205. [Google Scholar] [CrossRef]
- Almaas, H.; Cases, A.-L.; Devold, T.G.; Holm, H.; Langsrud, T.; Aabakken, L.; Aadnoey, T.; Vegarud, G.E. In vitro digestion of bovine and caprine milk by human gastric and duodenal enzymes. Int. Dairy J. 2006, 16, 961–968. [Google Scholar] [CrossRef]
- Nayik, G.A.; Jagdale, Y.D.; Gaikwad, S.A.; Devkatte, A.N.; Dar, A.H.; Ansari, M.J. Nutritional profile, processing and potential products: A comparative review of goat milk. Dairy 2022, 3, 622–647. [Google Scholar] [CrossRef]
- Nowier, A.M.; Ramadan, S.I. Association of β-casein gene polymorphism with milk composition traits of Egyptian Maghrebi camels (Camelus dromedarius). Arch. Anim. Breed. 2020, 63, 493–500. [Google Scholar] [CrossRef]
- Rahmatalla, S.A.; Arends, D.; Brockmann, G.A. Review: Genetic and protein variants of milk caseins in goats. Front. Genet. 2022, 13, 995349. [Google Scholar] [CrossRef]
- Cieślińska, A.; Fiedorowicz, E.; Zwierzchowski, G.; Kordulewska, N.; Jarmołowska, B.; Kostyra, E. Genetic polymorphism of β-casein gene in polish red cattle-Preliminary study of a1 and a2 frequency in genetic conservation herd. Animals 2019, 9, 377. [Google Scholar] [CrossRef]
- Moatsou, G.; Moschopoulou, E.; Mollé, D.; Gagnaire, V.; Kandarakis, I.; Léonil, J. Comparative study of the protein fraction of goat milk from the indigenous Greek breed and from international breeds. Food Chem. 2008, 106, 509–520. [Google Scholar] [CrossRef]
- Letaief, N.; Bedhiaf-Romdhani, S.; Ben Salem, W.; Mohammed, A.A.S.; Gaspa, G.; Pauciullo, A. Tunisian camel casein gene characterization reveals similarities and differences with Sudanese and Nigerian populations. J. Dairy Sci. 2022, 105, 6783–6794. [Google Scholar] [CrossRef]
- Amandykova, M.; Dossybayev, K.; Mussayeva, A.; Bekmanov, B.; Saitou, N. Comparative analysis of the polymorphism of the casein genes in camels bred in Kazakhstan. Diversity 2022, 14, 285. [Google Scholar] [CrossRef]
- Navarrete-Rodríguez, E.M.; Ríos-Villalobos, L.A.; Alcocer-Arreguín, C.R.; Del-Rio-Navarro, B.E.; Del Rio-Chivardi, J.M.; Saucedo-Ramírez, O.J.; Sienra-Monge, J.J.L.; Frias, R.V. Cross-over clinical trial for evaluating the safety of camel’s milk intake in patients who are allergic to cow’s milk protein. Allergol. Immunopathol. 2018, 46, 149–154. [Google Scholar] [CrossRef]
- Khalesi, M.; Salami, M.; Moslehishad, M.; Winterburn, J.; Moosavi-Movahedi, A.A. Biomolecular content of camel milk: A traditional superfood towards future healthcare industry. Trends Food Sci. Technol. 2017, 62, 49–58. [Google Scholar] [CrossRef]
- Attaie, R.; Richter, R.L. Size distribution of fat globules in goat milk. J. Dairy Sci. 2000, 83, 940–944. [Google Scholar] [CrossRef]
- Kumar, D.; Verma, A.K.; Chatli, M.K.; Singh, R.; Kumar, P.; Mehta, N.; Malav, O.P. Camel milk: Alternative milk for human consumption and its health benefits. Nutr. Food Sci. 2016, 46, 217–227. [Google Scholar] [CrossRef]
- Seifu, E. Camel milk products: Innovations, limitations and opportunities. Food Prod. Process Nutr. 2023, 5, 15. [Google Scholar] [CrossRef]
- Bakry, I.A.; Yang, L.; Farag, M.A.; Korma, S.A.; Khalifa, I.; Cacciotti, I.; Ziedan, N.I.; Jin, J.; Jin, Q.; Wei, W.; et al. A comprehensive review of the composition, nutritional value, and functional properties of camel milk fat. Foods 2021, 10, 2158. [Google Scholar] [CrossRef]
- Medhammar, E.; Wijesinha-Bettoni, R.; Stadlmayr, B.; Nilsson, E.; Charrondiere, U.R.; Burlingame, B. Composition of milk from minor dairy animals and buffalo breeds: A biodiversity perspective. J. Sci. Food Agric. 2012, 92, 445–474. [Google Scholar] [CrossRef]
- Zou, Z.; Liu, Y.; Wang, J.; Zhang, L.; Chen, Z. Digestibility of proteins in camel milk in comparison to bovine and human milk using an in vitro infant gastrointestinal digestion system. Food Chem. 2022, 374, 131704. [Google Scholar] [CrossRef]
- Ménard, O.; Bourlieu, C.; De Oliveira, S.C.; Dellarosa, N.; Laghi, L.; Carrière, F.; Capozzi, F.; Dupont, D.; Deglaire, A. A first step towards a consensus static in vitro model for simulating full-term infant digestion. Food Chem. 2018, 240, 338–345. [Google Scholar] [CrossRef]
- He, T.; Rombouts, W.; Einerhand, A.W.C.; Hotrum, N.; van de Velde, F. Gastric protein digestion of goat and cow milk infant formula and human milk under simulated infant conditions. Int. J. Food Sci. Nutr. 2022, 73, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Liu, L.; Liu, T.; Shen, Q.; Liu, C.; Zhou, H.; Ren, F. Simulated in vitro infant gastrointestinal digestion of yak milk fat globules: A comparison with cow milk fat globules. Food Chem. 2020, 314, 126160. [Google Scholar] [CrossRef]
- Meena, S.; Rajput, Y.S.; Sharma, R. Comparative fat digestibility of goat, camel, cow, and buffalo milk. Int. Dairy J. 2014, 35, 153–156. [Google Scholar] [CrossRef]
- Albenzio, M.; d’Angelo, F.; Santillo, A. Role of goat milk in infant health and nutrition. In Goat Science-Environment, Health, And Economy; Kukovics, S., Ed.; Intech Open: London, UK, 2021. [Google Scholar] [CrossRef]
- Jiang, H.; Gallier, S.; Feng, L.; Han, J.; Liu, W. Development of the digestive system in early infancy and nutritional management of digestive problems in breastfed and formula-fed infants. Food Funct. 2022, 13, 1062–1077. [Google Scholar] [CrossRef]
- Al-Hammadi, S.; El-Hassan, T.; Al-Reyami, L. Anaphylaxis to camel milk in an atopic child. Allergy Eur. J. Allergy Clin. Immunol. 2010, 65, 1623–1625. [Google Scholar] [CrossRef]
- Ehlayel, M.; Bener, A. Camel’s milk allergy. Allergy Asthma Proc. 2018, 39, 384–388. [Google Scholar] [CrossRef]
- Restani, P.; Fiocchi, A.; Galli, C.; Conti, A.; Azzini, A.; Sforza, S.; Ricci, G. Cross-reactivity between milk proteins from different animal species. Clin. Exp. Allergy 1999, 29, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- El-Agamy, E.I.; Nawar, M.; Shamsia, S.M.; Awad, S.; Haenlein, G.F.W. Are camel milk proteins convenient to the nutrition of cow milk allergic children? Small Rumin. Res. 2009, 82, 1–6. [Google Scholar] [CrossRef]
- Leite Júnior, B.R.D.C.; Tribst, A.A.L.; Cristianini, M. High pressure homogenization of porcine pepsin protease: Effects on enzyme activity, stability, milk coagulation profile and gel development. PLOS One 2015, 10, e0125061. [Google Scholar] [CrossRef]
- Suwareh, O.; Moghaddam, M.S.; Jabeen, A.; Gill, A.; Hussain, A.; Zhi, S.; Al Shahrani, H.; Anwar, M. Statistical modeling of in vitro pepsin specificity. Food Chem. 2021, 362, 130098. [Google Scholar] [CrossRef]
- Roy, D.; Ye, A.; Moughan, P.J.; Singh, H. Composition, structure, and digestive dynamics of milk from different species: A review. Front. Nutr. 2020, 7, 577759. [Google Scholar] [CrossRef] [PubMed]
- Maqsood, S.; Al-Dowaila, A.; Mudgil, P.; Kamal, H.; Jobe, B.; Hassan, H.M. Comparative characterization of protein and lipid fractions from camel and cow milk, their functionality, antioxidant and antihypertensive properties upon simulated gastro-intestinal digestion. Food Chem. 2019, 279, 328–338. [Google Scholar] [CrossRef]
- Oselu, S.; Ebere, R.; Arimi, J.M. Camels, camel milk, and camel milk product situation in Kenya in relation to the world. Int. J. Food Sci. 2022, 2022, 1–15. [Google Scholar] [CrossRef]
- Fukuda, K. Camel milk. In Milk And Dairy Products in Human Nutrition; Park, Y.W., Haenlein, G.F.W., Eds.; John Wiley & Sons: New York, NY, USA, 2013; pp. 578–593. [Google Scholar] [CrossRef]
- Pratelli, G.; Fabbri, A.; Sargenti, A.; Manzoli, L.; Polilli, E.; Piana, A. Cow’s milk: A benefit for human health? Omics tools and precision nutrition for lactose intolerance management. Nutrients 2024, 16, 320. [Google Scholar] [CrossRef]
- O’Sullivan, D.; Arora, T.; Durif, C.; Uriot, O.; Brun, M.; Riu, M.; Foguet-Romero, E.; Samarra, I.; Domingo-Almenara, X.; Gahan, C.; et al. Impact of western diet on enterohemorrhagic Escherichia coli colonization in the human in vitro mucosal artificial colon as mediated by gut microbiota. Nutrients 2024, 16, 2046. [Google Scholar] [CrossRef] [PubMed]
- Abdisa, K.B.; Szerdahelyi, E.; Molnár, M.A.; Friedrich, L.; Lakner, Z.; Koris, A.; Toth, A.; Nath, A. Metabolic syndrome and biotherapeutic activity of dairy (cow and buffalo) milk proteins and peptides: Fast food-induced obesity perspective—A narrative review. Biomolecules 2024, 14, 478. [Google Scholar] [CrossRef] [PubMed]
- Darma, A.; Sumitro, K.R.; Jo, J.; Sitorus, N. Lactose intolerance versus cow’s milk allergy in infants: A clinical dilemma. Nutrients 2024, 16, 414. [Google Scholar] [CrossRef]
- Salari, F.; Altomonte, I.; Ribeiro, N.L.; Ribeiro, M.N.; Bozzi, R.; Martini, M. Effects of season on the quality of Garfagnina goat milk. Ital. J. Anim. Sci. 2016, 15, 568–575. [Google Scholar] [CrossRef]
- Zanchini, R.; Di Vita, G.; Panzone, L.; Brun, F. What is the value of a ‘mountain product’ claim? a ranking conjoint experiment on goat’s milk yoghurt. Foods 2023, 12, 2059. [Google Scholar] [CrossRef]
- Vargas-Bello-Pérez, E.; Tajonar, K.; Foggi, G.; Mele, M.; Simitzis, P.; Mavrommatis, A.; Tsiplakou, E.; Habib, M.R.; Gonzalez-Ronquillo, M.; Toro-Mujica, P. Consumer attitudes toward dairy products from sheep and goats: A cross-continental perspective. J. Dairy Sci. 2022, 105, 8718–8733. [Google Scholar] [CrossRef]
- Shori, A.B. Camel milk as a potential therapy for controlling diabetes and its complications: A review of in vivo studies. J. Food Drug Anal. 2015, 23, 609–618. [Google Scholar] [CrossRef]
- AlNohair, S.F. Medical benefits of camel’s milk: A comprehensive review. J. Pak. Med. Assoc. 2020, 71, 1–15. [Google Scholar] [CrossRef]
- Benmeziane-Derradji, F. Evaluation of camel milk: Gross composition—A scientific overview. Trop. Anim. Health Prod. 2021, 53, 308. [Google Scholar] [CrossRef]
- Swelum, A.A.; Alhussain, M.N.; Alshehri, F.; Abouheif, M.M.; El-Tarabily, K.A.; Alzahrani, H.A.; Alharthi, A.A. Nutritional, antimicrobial and medicinal properties of camel’s milk: A review. Saudi J. Biol. Sci. 2021, 28, 3126–3136. [Google Scholar] [CrossRef]
- Liu, C.; Liu, L.-X.; Yang, J.; Liu, Y.-G. Exploration and analysis of the composition and mechanism of efficacy of camel milk. Food Biosci. 2023, 53, 102564. [Google Scholar] [CrossRef]
- Han, B.; Zhang, L.; Hou, Y.; Zhong, J.; Hettinga, K.; Zhou, P. Phosphoproteomics reveals that camel and goat milk improve glucose homeostasis in HDF/STZ-induced diabetic rats through activation of hepatic AMPK and GSK3-GYS axis. Food Res. Int. 2022, 157, 111254. [Google Scholar] [CrossRef]
- Amr, M.; Farid, A. Impact of cow, buffalo, goat or camel milk consumption on oxidative stress, inflammation and immune response post weaning time. Sci. Rep. 2024, 14, 9967. [Google Scholar] [CrossRef]
- Chauhan, S.; Powar, P.; Mehra, R. A review on nutritional advantages and nutraceutical properties of cow and goat milk. Int. J. Appl. Res. 2021, 7, 101–105. [Google Scholar] [CrossRef]
- Alharbi, Y.M.; Sakr, S.S.; Albarrak, S.M.; Almundarij, T.I.; Barakat, H.; Hassan, M.F.Y. Antioxidative, antidiabetic, and hypolipidemic properties of probiotic-enriched fermented camel milk combined with Salvia officinalis leaves hydroalcoholic extract in streptozotocin-induced diabetes in rats. Antioxidants 2022, 11, 668. [Google Scholar] [CrossRef]
- Sboui, A.; Atig, C.; Khabir, A.; Hammadi, M.; Khorchani, T. Camel milk used as an adjuvant therapy to treat type 2 diabetic patients: Effects on blood glucose, HbA1c, cholesterol, and TG levels. J. Chem. 2022, 2022, 5860162. [Google Scholar] [CrossRef]
- Behrouz, S.; Saadat, S.; Memarzia, A.; Sarir, H.; Folkerts, G.; Boskabady, M.H. The antioxidant, anti-inflammatory and immunomodulatory effects of camel milk. Front. Immunol. 2022, 13, 855342. [Google Scholar] [CrossRef] [PubMed]
- Khalid, N.; Abdelrahim, D.N.; Hanach, N.; AlKurd, R.; Khan, M.; Mahrous, L.; Radwan, H.; Naja, F.; Madkour, M.; Obaideen, K.; et al. Effect of camel milk on lipid profile among patients with diabetes: A systematic review, meta-analysis, and meta-regression of randomized controlled trials. BMC Complement. Med. Ther. 2023, 23, 438. [Google Scholar] [CrossRef] [PubMed]
- Hamed, N.S.; Mbye, M.; Ayyash, M.; Ulusoy, B.H.; Kamal-Eldin, A. Camel milk: Antimicrobial agents, fermented products, and shelf life. Foods 2024, 13, 381. [Google Scholar] [CrossRef]
- El-Kattawy, A.M.; Rashed, A.; Youness, E.R.; Fadhl, B.M.; Morsy, M.A.; Khedher, N.B.; Marzouk, K.M.; Ahmed, M. Therapeutic potential of camel milk exosomes against HepaRG cells with potent apoptotic, anti-inflammatory, and anti-angiogenesis effects for colostrum exosomes. Biomed. Pharmacother. 2021, 143, 112220. [Google Scholar] [CrossRef]
- Soleymanzadeh, N.; Mirdamadi, S.; Kianirad, M. Antioxidant activity of camel and bovine milk fermented by lactic acid bacteria isolated from traditional fermented camel milk (Chal). Dairy Sci. Technol. 2016, 96, 443–457. [Google Scholar] [CrossRef]
- Hao, S.; Sun, J.; Zhao, J.; Liu, Y.; Huang, Z.; Zhao, M.; Huang, C.; Zhang, C.; Li, Z. Modulatory effect of camel milk on intestinal microbiota of mice with non-alcoholic fatty liver disease. Front. Nutr. 2022, 9, 1072133. [Google Scholar] [CrossRef]
- AlKurd, R.; Abdalla, H.; Al Naimi, A.; Ameen, S.; Youssef, F.; Khaireddin, Y.; Alzahrani, S.; Zaki, A.; Asfour, M.; Fadhl, A.; et al. Effect of camel milk on glucose homeostasis in patients with diabetes: A systematic review and meta-analysis of randomized controlled trials. Nutrients 2022, 14, 1245. [Google Scholar] [CrossRef]
- Ayoub, M.A.; Benkhelifa, S.; Ouezzani, A.; Allouche, N.; Sidhoum, R.; Ould Djemia, M.; Bouchard, J.-F.; Ghaffari, S.; Attia, H.; Taanoubi, K.; et al. Invited review: Camel milk–derived bioactive peptides and diabetes—Molecular view and perspectives. J. Dairy Sci. 2024, 107, 649–668. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M. Bioactive peptides in preventative healthcare: An overview of bioactivities and suggested methods to assess potential applications. Curr. Pharm. Des. 2021, 27, 1332–1341. [Google Scholar] [CrossRef]
- Aslam, M.Z.; Firdos, S.; Zhousi, L.; Wang, X.; Liu, Y.; Qin, X.; Yang, S.; Ma, Y.; Zhang, B.; Dong, Q. Managing hypertension by exploiting microelements and fermented dairy products. CYTA J Food 2022, 20, 327–342. [Google Scholar] [CrossRef]
- Salvo, E.D.; Conte, F.; Casciaro, M.; Gangemi, S.; Cicero, N. Bioactive natural products in donkey and camel milk: A perspective review. Nat. Prod. Res. 2023, 37, 2098–2112. [Google Scholar] [CrossRef] [PubMed]
- Saeed, A.; Abubakar, M.; Kul, O. (Eds.) Modification of animal products for fat and other characteristics. In The Role of Biotechnology in Improvement of Livestock; Springer: Berlin/Heidelberg, Germany, 2015; pp. 55–89. [Google Scholar] [CrossRef]
- Coelho, M.S.; Fernandes, S.S.; de las Mercedes Salas-Mellado, M. Association between diet, health, and the presence of bioactive compounds in foods. In Bioactive Compounds; Segura, M.R., Ed.; Woodhead Publishing: Sawston, UK, 2019; pp. 159–183. [Google Scholar] [CrossRef]
- Samtiya, M.; Samtiya, S.; Badgujar, P.C.; Puniya, A.K.; Dhewa, T.; Aluko, R.E. Health-promoting and therapeutic attributes of milk-derived bioactive peptides. Nutrients 2022, 14, 3001. [Google Scholar] [CrossRef] [PubMed]
- Almoraie, N.M.; Shatwan, I.M. Effect of camel milk on the physicochemical, rheological, and sensory qualities of bread. J. Food Qual. 2021, 2021, 8889406. [Google Scholar] [CrossRef]
- Zhou, X.; Chai, L.; Wu, Q.; Wang, Y.; Li, S.; Chen, J. Anti-diabetic properties of bioactive components from fish and milk. J. Funct. Foods. 2021, 85, 104669. [Google Scholar] [CrossRef]
- Amaya-Farfan, J.; Moura, C.S.; Morato, P.N.; Lollo, P.C.B. Dietary whey protein and type 2 diabetes: Molecular aspects. In Molecular Nutrition and Diabetes; Mauricio, D., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 211–220. [Google Scholar] [CrossRef]
- Acquah, C.; Dzuvor, C.K.O.; Tosh, S.; Agyei, D. Anti-diabetic effects of bioactive peptides: Recent advances and clinical implications. Crit. Rev. Food Sci. Nutr. 2022, 62, 2158–2171. [Google Scholar] [CrossRef]
- Marya, H.; Khan, S.M.; Nabavi, S.M.; Habtemariam, S. Anti-diabetic potential of peptides: Future prospects as therapeutic agents. Life Sci. 2018, 193, 153–158. [Google Scholar] [CrossRef]
- Jäkälä, P.; Vapaatalo, H. Antihypertensive peptides from milk proteins. Pharmaceuticals 2010, 3, 251–272. [Google Scholar] [CrossRef]
- Basak, S.; Duttaroy, A.K. Conjugated linoleic acid and its beneficial effects in obesity, cardiovascular disease, and cancer. Nutrients 2020, 12, 1913. [Google Scholar] [CrossRef]
- Melini, F.; Melini, V.; Luziatelli, F.; Ficca, A.G.; Ruzzi, M. Health-promoting components in fermented foods: An up-to-date systematic review. Nutrients 2019, 11, 1189. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, J.; Tian, Z.; Si, Y.; Chen, H.; Gan, J. The mechanisms of the potential probiotic Lactiplantibacillus plantarum against cardiovascular disease and the recent developments in its fermented foods. Foods 2022, 11, 2549. [Google Scholar] [CrossRef]
- Zhang, W.; Al-Wraikata, M.; Li, L.; Liu, Y. Physicochemical properties, antioxidant and antidiabetic activities of different hydrolysates of goat milk protein. J. Dairy Sci. 2024, in press. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, Z.; Niu, H.; Tian, X.; Tian, H.; Yao, W.; He, H.; Shi, H.; Li, C.; Luo, J. Goat milk improves glucose metabolism in type 2 diabetic mice and protects pancreatic β-cell functions. Mol. Nutr. Food Res. 2024, 68, 2200842. [Google Scholar] [CrossRef] [PubMed]
- Freire, F.C.; Adorno, M.A.T.; Sakamoto, I.K.; Antoniassi, R.; Chaves, A.C.S.D.; dos Santos, K.M.O.; Sivieri, K. Impact of multi-functional fermented goat milk beverage on gut microbiota in a dynamic colon model. Food Res. Int. 2017, 99, 315–327. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, F. Comparison of whole goat milk and its major fractions regarding the modulation of gut microbiota. J. Sci. Food Agric. 2022, 102, 3618–3627. [Google Scholar] [CrossRef]
- Liu, Y.; Cai, J.; Zhang, F. Functional comparison of breast milk, cow milk and goat milk based on changes in the intestinal flora of mice. LWT 2021, 150, 111976. [Google Scholar] [CrossRef]
- van Leeuwen, S.S.; te Poele, E.M.; Chatziioannou, A.C.; Benjamins, E.; Haandrikman, A.; Dijkhuizen, L. Goat milk oligosaccharides: Their diversity, quantity, and functional properties in comparison to human milk oligosaccharides. J. Agric. Food Chem. 2020, 68, 13469–13485. [Google Scholar] [CrossRef]
- Bakry, I.A.; Wei, W.; Farag, M.A.; Korma, S.A.; Khalifa, I.; Ziedan, N.I.; Mahdi, H.K.; Jin, J.; Wang, X. How does camel milk fat profile compare with that of human milk fat to serve as a substitute for human milk? Int. Dairy J. 2023, 146, 105738. [Google Scholar] [CrossRef]
- Al-Awadi, F.M.; Srikumar, T.S. Trace elements and their distribution in protein fractions of camel milk in comparison to other commonly consumed milks. J. Dairy Res. 2001, 68, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Tannock, G.W.; Lawley, B.; Munro, K.; Pathmanathan, S.G.; Zhou, S.J.; Makrides, M.; Gibson, R.A.; Sullivan, T.; Prosser, C.G.; Lowry, D.; et al. Comparison of the compositions of the stool microbiotas of infants fed goat milk formula, cow milk-based formula, or breast milk. Appl. Environ. Microbiol. 2013, 79, 3040–3048. [Google Scholar] [CrossRef]
- Marshall, N.E.; Lau, B.; Purnell, J.Q.; Thornburg, K.L. Impact of maternal obesity and breastfeeding intention on lactation intensity and duration. Matern. Child Nutr. 2018, 14, e12732. [Google Scholar] [CrossRef]
- Leng, G. Oxytocin in lactation and parturition. In Neuroendocrine Regulation of Mammalian Pregnancy and Lactation; Brunton, P.J., Grattan, D.R., Eds.; Springer: Cham, Switzerland, 2024; pp. 155–179. [Google Scholar] [CrossRef]
- Di Benedetto, M.G.; Bottanelli, C.; Cattaneo, A.; Pariante, C.M.; Borsini, A. Nutritional and immunological factors in breast milk: A role in the intergenerational transmission from maternal psychopathology to child development. Brain Behav. Immun. 2020, 85, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Fernandez, J.; Ochoa, J.J.; Lopez-Frias, M.; Diaz-Castro, J. Impact of early nutrition, physical activity and sleep on the fetal programming of disease in the pregnancy: A narrative review. Nutrients 2020, 12, 3900. [Google Scholar] [CrossRef] [PubMed]
- El-Hatmi, H.; Girardet, J.-M.; Gaillard, J.-L.; Yahyaoui, M.H.; Attia, H. Characterization of whey proteins of camel (Camelus dromedarius) milk and colostrum. Small Rumin. Res. 2007, 70, 267–271. [Google Scholar] [CrossRef]
| Component | Camel Milk | Cow Milk | Goat Milk | Reference |
|---|---|---|---|---|
| Fat (%) | 3.82–5.4 | 3.0–4.0 | 3.4–4.2 | [17,18] |
| Protein (%) | 2.15–4.90 | 3.2–3.4 | 3.1–3.8 | [17,18] |
| Casein (%) | 1.63–2.76 | 2.5–2.7 | 2.31–2.64 | [23,24] |
| Whey Protein (%) | 0.6–0.7 | 0.6–0.7 | 0.66–0.99 | [23,24] |
| Ash (%) | 0.79–0.81 | 0.75 | 0.82 | [17,18] |
| Calcium (mg/100 mL) | 114–116 | 120–130 | 134 | [17,18] |
| Vitamin C (mg/100 mL) | 33 | 2 | 1.29 | [17,18] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almasri, R.S.; Bedir, A.S.; Ranneh, Y.K.; El-Tarabily, K.A.; Al Raish, S.M. Benefits of Camel Milk over Cow and Goat Milk for Infant and Adult Health in Fighting Chronic Diseases: A Review. Nutrients 2024, 16, 3848. https://doi.org/10.3390/nu16223848
Almasri RS, Bedir AS, Ranneh YK, El-Tarabily KA, Al Raish SM. Benefits of Camel Milk over Cow and Goat Milk for Infant and Adult Health in Fighting Chronic Diseases: A Review. Nutrients. 2024; 16(22):3848. https://doi.org/10.3390/nu16223848
Chicago/Turabian StyleAlmasri, Razan S., Alaa S. Bedir, Yazan K. Ranneh, Khaled A. El-Tarabily, and Seham M. Al Raish. 2024. "Benefits of Camel Milk over Cow and Goat Milk for Infant and Adult Health in Fighting Chronic Diseases: A Review" Nutrients 16, no. 22: 3848. https://doi.org/10.3390/nu16223848
APA StyleAlmasri, R. S., Bedir, A. S., Ranneh, Y. K., El-Tarabily, K. A., & Al Raish, S. M. (2024). Benefits of Camel Milk over Cow and Goat Milk for Infant and Adult Health in Fighting Chronic Diseases: A Review. Nutrients, 16(22), 3848. https://doi.org/10.3390/nu16223848








