Circulating Phylloquinone and the Risk of Four Female-Specific Cancers: A Mendelian Randomization Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source and Selection of Genetic Instruments
2.2. Cancer Outcomes
2.3. Statistical Analysis
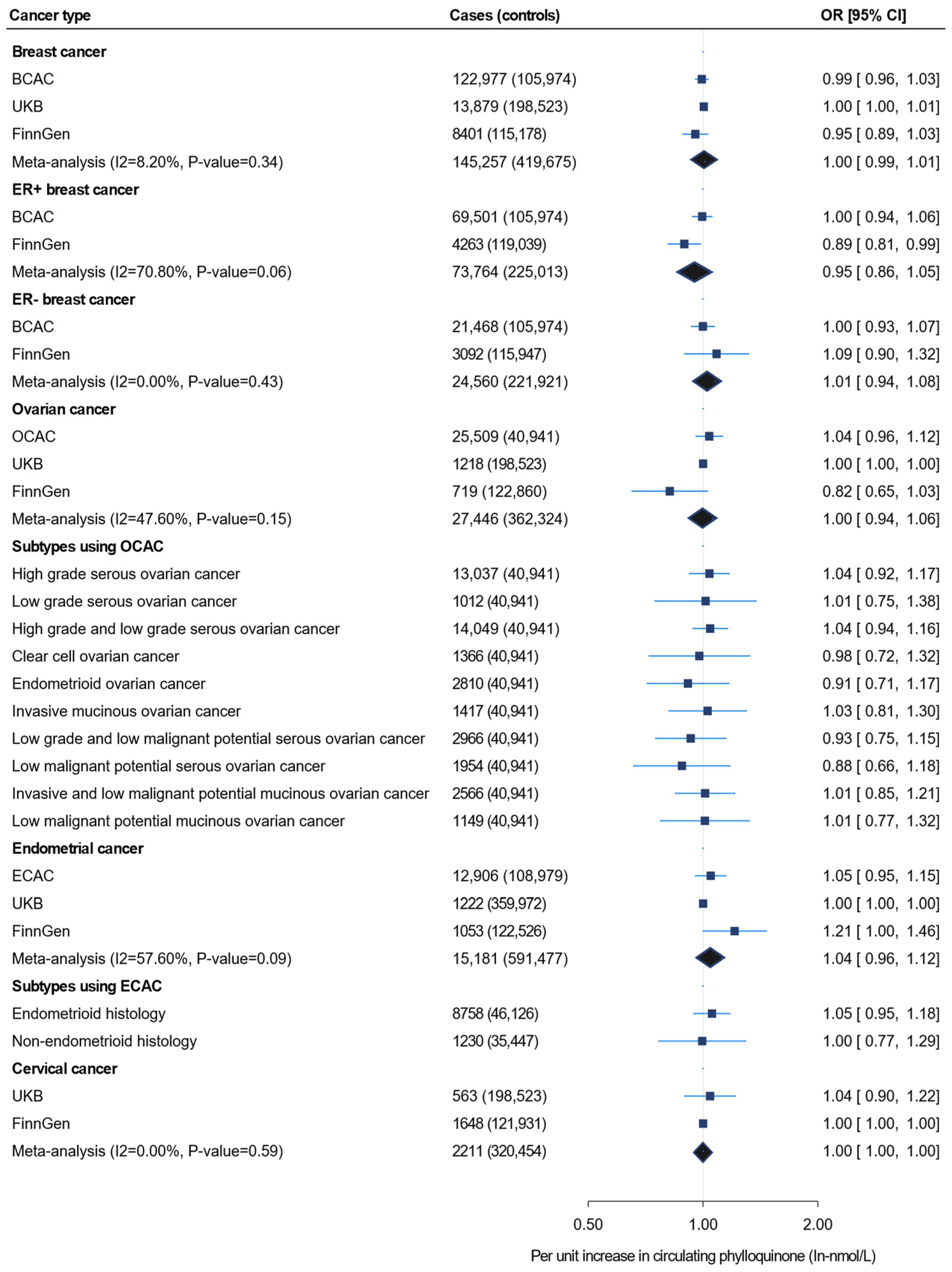
3. Results
3.1. Instrument Strength and Validation
3.2. Mendelian Randomization Analysis
3.3. Power Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schwalfenberg, G.K. Vitamins K1 and K2: The emerging group of vitamins required for human health. J. Nutr. Metab. 2017, 2017, 6254836. [Google Scholar] [CrossRef] [PubMed]
- Booth, S.L. Vitamin K: Food composition and dietary intakes. Food Nutr. Res. 2012, 56, 5505. [Google Scholar] [CrossRef]
- Cheung, A.M.; Tile, L.; Lee, Y.; Tomlinson, G.; Hawker, G.; Scher, J.; Hu, H.; Vieth, R.; Thompson, L.; Jamal, S.; et al. Vitamin K supplementation in postmenopausal women with osteopenia (ECKO trial): A randomized controlled trial. PLoS Med. 2008, 5, e196. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Booth, S.L.; Suttie, J.W. Dietary Intake and Adequacy of Vitamin K1. J. Nutr. 1998, 128, 785–788. [Google Scholar] [CrossRef] [PubMed]
- Kamao, M.; Suhara, Y.; Tsugawa, N.; Uwano, M.; Yamaguchi, N.; Uenishi, K.; Ishida, H.; Sasaki, S.; Okano, T. Vitamin K content of foods and dietary vitamin K intake in Japanese young women. J. Nutr. Sci. Vitaminol. 2007, 53, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Hill, M. Intestinal flora and endogenous vitamin synthesis. Eur. J. Cancer Prev. 1997, 6, S43–S45. [Google Scholar] [CrossRef]
- Fernandez, F.; Collins, M.D. Vitamin K composition of anaerobic gut bacteria. FEMS Microbiol. Lett. 1987, 41, 175–180. [Google Scholar] [CrossRef]
- Walther, B.; Karl, J.P.; Booth, S.L.; Boyaval, P. Menaquinones, bacteria, and the food supply: The relevance of dairy and fermented food products to vitamin K requirements. Adv. Nutr. 2013, 4, 463–473. [Google Scholar] [CrossRef]
- Dashti, H.S.; Shea, M.K.; Smith, C.E.; Tanaka, T.; Hruby, A.; Richardson, K.; Wang, T.J.; Nalls, M.A.; Guo, X.; Liu, Y. Meta-analysis of genome-wide association studies for circulating phylloquinone concentrations. Am. J. Clin. Nutr. 2014, 100, 1462–1469. [Google Scholar] [CrossRef]
- Thijssen, H.; Drittij-Reijnders, M. Vitamin K distribution in rat tissues: Dietary phylloquinone is a source of tissue menaquinone-4. Br. J. Nutr. 1994, 72, 415–425. [Google Scholar] [CrossRef]
- Al Rajabi, A.; Booth, S.L.; Peterson, J.W.; Choi, S.W.; Suttie, J.W.; Shea, M.K.; Miao, B.; Grusak, M.A.; Fu, X. Deuterium-labeled phylloquinone has tissue-specific conversion to menaquinone-4 among Fischer 344 male rats. J. Nutr. 2012, 142, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Girolami, A.; Ferrari, S.; Cosi, E.; Santarossa, C.; Randi, M.L. Vitamin K-dependent coagulation factors that may be responsible for both bleeding and thrombosis (FII, FVII, and FIX). Clin. Appl. Thromb./Hemost. 2018, 24, 42S–47S. [Google Scholar] [CrossRef]
- Berkner, K.; Runge, K. The physiology of vitamin K nutriture and vitamin K-dependent protein function in atherosclerosis. J. Thromb. Haemost. 2004, 2, 2118–2132. [Google Scholar] [CrossRef]
- Ferland, G. The discovery of vitamin K and its clinical applications. Ann. Nutr. Metab. 2012, 61, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Beulens, J.W.; van der, A.D.; Grobbee, D.E.; Sluijs, I.; Spijkerman, A.M.; van der Schouw, Y.T. Dietary phylloquinone and menaquinones intakes and risk of type 2 diabetes. Diabetes Care 2010, 33, 1699–1705. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ibarrola-Jurado, N.; Salas-Salvadó, J.; Martínez-González, M.A.; Bulló, M. Dietary phylloquinone intake and risk of type 2 diabetes in elderly subjects at high risk of cardiovascular disease. Am. J. Clin. Nutr. 2012, 96, 1113–1118. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.-W.; Li, Q.-J.; Cheng, L.; Yang, P.-F.; Sun, W.-P.; Peng, Y.; Hu, J.-J.; Wu, J.-J.; Gong, J.-P.; Zhong, G.-C. Dietary vitamin K intake and the risk of pancreatic cancer: A prospective study of 101,695 American adults. Am. J. Epidemiol. 2021, 190, 2029–2041. [Google Scholar] [CrossRef]
- Bellinge, J.W.; Dalgaard, F.; Murray, K.; Connolly, E.; Blekkenhorst, L.C.; Bondonno, C.P.; Lewis, J.R.; Sim, M.; Croft, K.D.; Gislason, G. Vitamin K Intake and atherosclerotic cardiovascular disease in the danish diet cancer and health study. J. Am. Heart Assoc. 2021, 10, e020551. [Google Scholar] [CrossRef]
- Schultz, C.J.; Dalgaard, F.; Bellinge, J.W.; Murray, K.; Sim, M.; Connolly, E.; Blekkenhorst, L.C.; Bondonno, C.P.; Lewis, J.R.; Gislason, G.H. Dietary Vitamin K1 Intake and Incident Aortic Valve Stenosis. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 513–521. [Google Scholar] [CrossRef]
- Dupuy, M.; Radavelli-Bagatini, S.; Zhong, L.; Dalla Via, J.; Zhu, K.; Blekkenhorst, L.C.; Bondonno, N.P.; Linneberg, A.; Bellinge, J.W.; Schultz, C.; et al. Vitamin K1 intake is associated with lower risk for all-cause and cardiovascular disease mortality in community-dwelling older Australian women. Nutr. Metab. Cardiovasc. Dis. 2024, 34, 1189–1197. [Google Scholar] [CrossRef]
- Palmer, C.R.; Bellinge, J.W.; Dalgaard, F.; Sim, M.; Murray, K.; Connolly, E.; Blekkenhorst, L.C.; Bondonno, C.P.; Croft, K.D.; Gislason, G.; et al. Association between vitamin K1 intake and mortality in the Danish Diet, Cancer, and Health cohort. Eur. J. Epidemiol. 2021, 36, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Juanola-Falgarona, M.; Salas-Salvadó, J.; Martínez-González, M.; Corella, D.; Estruch, R.; Ros, E.; Fitó, M.; Arós, F.; Gómez-Gracia, E.; Fiol, M.; et al. Dietary intake of vitamin K is inversely associated with mortality risk. J. Nutr. 2014, 144, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Nimptsch, K.; Rohrmann, S.; Kaaks, R.; Linseisen, J. Dietary vitamin K intake in relation to cancer incidence and mortality: Results from the Heidelberg cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Heidelberg). Am. J. Clin. Nutr. 2010, 91, 1348–1358. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wu, Q.; Li, Z.; Reger, M.K.; Xiong, Y.; Zhong, G.; Li, Q.; Zhang, X.; Li, H.; Foukakis, T.; et al. Vitamin K intake and breast cancer incidence and death: Results from a prospective cohort study. Clin. Nutr. 2021, 40, 3370–3378. [Google Scholar] [CrossRef] [PubMed]
- Shurrab, M.; Quinn, K.L.; Kitchlu, A.; Jackevicius, C.A.; Ko, D.T. Long-Term Vitamin K Antagonists and Cancer Risk: A Systematic Review and Meta-Analysis. Am. J. Clin. Oncol. 2019, 42, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Pengo, V.; Noventa, F.; Denas, G.; Pengo, M.F.; Gallo, U.; Grion, A.M.; Iliceto, S.; Prandoni, P. Long-term use of vitamin K antagonists and incidence of cancer: A population-based study. Blood J. Am. Soc. Hematol. 2011, 117, 1707–1709. [Google Scholar] [CrossRef]
- Pottegård, A.; Friis, S.; Hallas, J. Cancer risk in long-term users of vitamin K antagonists: A population-based case–control study. Int. J. Cancer 2013, 132, 2606–2612. [Google Scholar] [CrossRef]
- Wu, F.Y.H.; Liao, W.-C.; Chang, H.-M. Comparison of antitumor activity of vitamins K1, K2 and K3 on human tumor cells by two (MTT and SRB) cell viability assays. Life Sci. 1993, 52, 1797–1804. [Google Scholar] [CrossRef]
- Habu, D.; Shiomi, S.; Tamori, A.; Takeda, T.; Tanaka, T.; Kubo, S.; Nishiguchi, S. Role of vitamin K2 in the development of hepatocellular carcinoma in women with viral cirrhosis of the liver. JAMA 2004, 292, 358–361. [Google Scholar] [CrossRef]
- Haruna, Y.; Yakushijin, T.; Kawamoto, S. Efficacy and safety of sorafenib plus vitamin K treatment for hepatocellular carcinoma: A phase II, randomized study. Cancer Med. 2021, 10, 914–922. [Google Scholar] [CrossRef]
- Markowska, A.; Antoszczak, M.; Markowska, J.; Huczyński, A. Role of vitamin K in selected malignant neoplasms in women. Nutrients 2022, 14, 3401. [Google Scholar] [CrossRef] [PubMed]
- Orlando, A.; Linsalata, M.; Tutino, V.; D′ Attoma, B.; Notarnicola, M.; Russo, F. Vitamin K1 exerts antiproliferative effects and induces apoptosis in three differently graded human colon cancer cell lines. BioMed Res. Int. 2015, 2015, 296721. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, D.A.; Smith, G.D.; Bruckdorfer, K.R.; Kundu, D.; Ebrahim, S. Those confounded vitamins: What can we learn from the differences between observational versus randomised trial evidence? Lancet 2004, 363, 1724–1727. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Traylor, M.; Markus, H.S. Circulating vitamin K1 levels in relation to ischemic stroke and its subtypes: A Mendelian randomization study. Nutrients 2018, 10, 1575. [Google Scholar] [CrossRef]
- Schooling, C. Plasma levels of vitamin K and the risk of ischemic heart disease: A Mendelian randomization study. J. Thromb. Haemost. 2016, 14, 1211–1215. [Google Scholar] [CrossRef]
- Zwakenberg, S.R.; Remmelzwaal, S.; Beulens, J.W.; Booth, S.L.; Burgess, S.; Dashti, H.S.; Imamura, F.; Feskens, E.J.; van der Schouw, Y.T.; Sluijs, I. Circulating phylloquinone concentrations and risk of type 2 diabetes: A mendelian randomization study. Diabetes 2019, 68, 220–225. [Google Scholar] [CrossRef]
- Edson, K.Z.; Prasad, B.; Unadkat, J.D.; Suhara, Y.; Okano, T.; Guengerich, F.P.; Rettie, A.E. Cytochrome P450-dependent catabolism of vitamin K: ω-hydroxylation catalyzed by human CYP4F2 and CYP4F11. Biochemistry 2013, 52, 8276–8285. [Google Scholar] [CrossRef]
- McDonald, M.G.; Rieder, M.J.; Nakano, M.; Hsia, C.K.; Rettie, A.E. CYP4F2 is a vitamin K1 oxidase: An explanation for altered warfarin dose in carriers of the V433M variant. Mol. Pharmacol. 2009, 75, 1337–1346. [Google Scholar] [CrossRef]
- Zheng, H.-F.; Duncan, E.L.; Yerges-Armstrong, L.M.; Eriksson, J.; Bergström, U.; Leo, P.J.; Leslie, W.D.; Goltzman, D.; Blangero, J.; Hanley, D.A. Meta-analysis of genome-wide studies identifies MEF2C SNPs associated with bone mineral density at forearm. J. Med. Genet. 2013, 50, 473–478. [Google Scholar] [CrossRef]
- Pietzner, M.; Wheeler, E.; Carrasco-Zanini, J.; Raffler, J.; Kerrison, N.D.; Oerton, E.; Auyeung, V.P.W.; Luan, J.; Finan, C.; Casas, J.P.; et al. Genetic architecture of host proteins involved in SARS-CoV-2 infection. Nat. Commun. 2020, 11, 6397. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Michailidou, K.; Lindström, S.; Dennis, J.; Beesley, J.; Hui, S.; Kar, S.; Lemaçon, A.; Soucy, P.; Glubb, D.; Rostamianfar, A.; et al. Association analysis identifies 65 new breast cancer risk loci. Nature 2017, 551, 92–94. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Phelan, C.M.; Kuchenbaecker, K.B.; Tyrer, J.P.; Kar, S.P.; Lawrenson, K.; Winham, S.J.; Dennis, J.; Pirie, A.; Riggan, M.J.; Chornokur, G.; et al. Identification of 12 new susceptibility loci for different histotypes of epithelial ovarian cancer. Nat. Genet. 2017, 49, 680–691. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- O’Mara, T.A.; Glubb, D.M.; Amant, F.; Annibali, D.; Ashton, K.; Attia, J.; Auer, P.L.; Beckmann, M.W.; Black, A.; Bolla, M.K.; et al. Identification of nine new susceptibility loci for endometrial cancer. Nat. Commun. 2018, 9, 3166. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Elsworth, B.; Lyon, M.; Alexander, T.; Liu, Y.; Matthews, P.; Hallett, J.; Bates, P.; Palmer, T.; Haberland, V.; Smith, G.D. The MRC IEU OpenGWAS data infrastructure. BioRxiv 2020. [Google Scholar] [CrossRef]
- Burgess, S.; Thompson, S.G.; Collaboration, C.C.G. Avoiding bias from weak instruments in Mendelian randomization studies. Int. J. Epidemiol. 2011, 40, 755–764. [Google Scholar] [CrossRef]
- Burgess, S. Sample size and power calculations in Mendelian randomization with a single instrumental variable and a binary outcome. Int. J. Epidemiol. 2014, 43, 922–929. [Google Scholar] [CrossRef]
- Bowden, J.; Del Greco M, F.; Minelli, C.; Davey Smith, G.; Sheehan, N.; Thompson, J. A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Stat. Med. 2017, 36, 1783–1802. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Haycock, P.C.; Burgess, S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hartwig, F.P.; Davey Smith, G.; Bowden, J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int. J. Epidemiol. 2017, 46, 1985–1998. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bowden, J.; Davey Smith, G.; Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015, 44, 512–525. [Google Scholar] [CrossRef]
- Verbanck, M.; Chen, C.-Y.; Neale, B.; Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018, 50, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Beaudin, S.; Kokabee, L.; Welsh, J. Divergent effects of vitamins K1 and K2 on triple negative breast cancer cells. Oncotarget 2019, 10, 2292. [Google Scholar] [CrossRef] [PubMed]
| SNPs | Chr | Nearest Gene | Effect Allele | Alternative Allele | Phylloquinone | Coagulation Factor IX | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta a | SE | p-Value | R2 | F-Statistics | Beta b | SE | p-Value | |||||
| rs2192574 | 2 | CTNAA2 | C | T | 0.28 | 0.06 | 1.82 × 10−6 ** | 0.010 | 21.78 | 0.020 | 0.020 | 0.34 |
| rs4122275 | 5 | CDO1 | G | A | 0.68 | 0.17 | 4.76 × 10−5 *** | 0.007 | 16.00 | 0.078 | 0.051 | 0.13 |
| rs4645543 | 8 | KCNK9 | C | T | 0.42 | 0.08 | 2.0 × 10−7 | 0.013 | 27.56 | 0.046 | 0.031 | 0.14 |
| rs2108622 | 19 | CYP4F2 | T | C | 0.16 | 0.03 | 8.78 × 10−7 | 0.013 | 28.44 | 0.015 | 0.015 | 0.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yalew, M.; Mulugeta, A.; Lumsden, A.L.; Madakkatel, I.; Lee, S.H.; Oehler, M.K.; Mäenpää, J.; Hyppönen, E. Circulating Phylloquinone and the Risk of Four Female-Specific Cancers: A Mendelian Randomization Study. Nutrients 2024, 16, 3680. https://doi.org/10.3390/nu16213680
Yalew M, Mulugeta A, Lumsden AL, Madakkatel I, Lee SH, Oehler MK, Mäenpää J, Hyppönen E. Circulating Phylloquinone and the Risk of Four Female-Specific Cancers: A Mendelian Randomization Study. Nutrients. 2024; 16(21):3680. https://doi.org/10.3390/nu16213680
Chicago/Turabian StyleYalew, Melaku, Anwar Mulugeta, Amanda L. Lumsden, Iqbal Madakkatel, S. Hong Lee, Martin K. Oehler, Johanna Mäenpää, and Elina Hyppönen. 2024. "Circulating Phylloquinone and the Risk of Four Female-Specific Cancers: A Mendelian Randomization Study" Nutrients 16, no. 21: 3680. https://doi.org/10.3390/nu16213680
APA StyleYalew, M., Mulugeta, A., Lumsden, A. L., Madakkatel, I., Lee, S. H., Oehler, M. K., Mäenpää, J., & Hyppönen, E. (2024). Circulating Phylloquinone and the Risk of Four Female-Specific Cancers: A Mendelian Randomization Study. Nutrients, 16(21), 3680. https://doi.org/10.3390/nu16213680







