Amelioration of Cancer Cachexia by Dalbergia odorifera Extract Through AKT Signaling Pathway Regulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Antibodies
2.2. DOE Preparation
2.3. Cell Culture
2.4. CT26 Conditioned Medium Preparation
2.5. Cell Viability Assay
2.6. Immunocytochemistry (ICC)
2.7. LADD Staining
2.8. Oil Red O Staining
2.9. Glycerol Assay
2.10. Animal Study
2.11. Hematoxylin and Eosin (H&E) Staining
2.12. Western Blot Analysis
2.13. Reverse-Transcription Quantitative Polymerase Chain Reaction (qPCR)
2.14. Statistical Analysis
3. Results
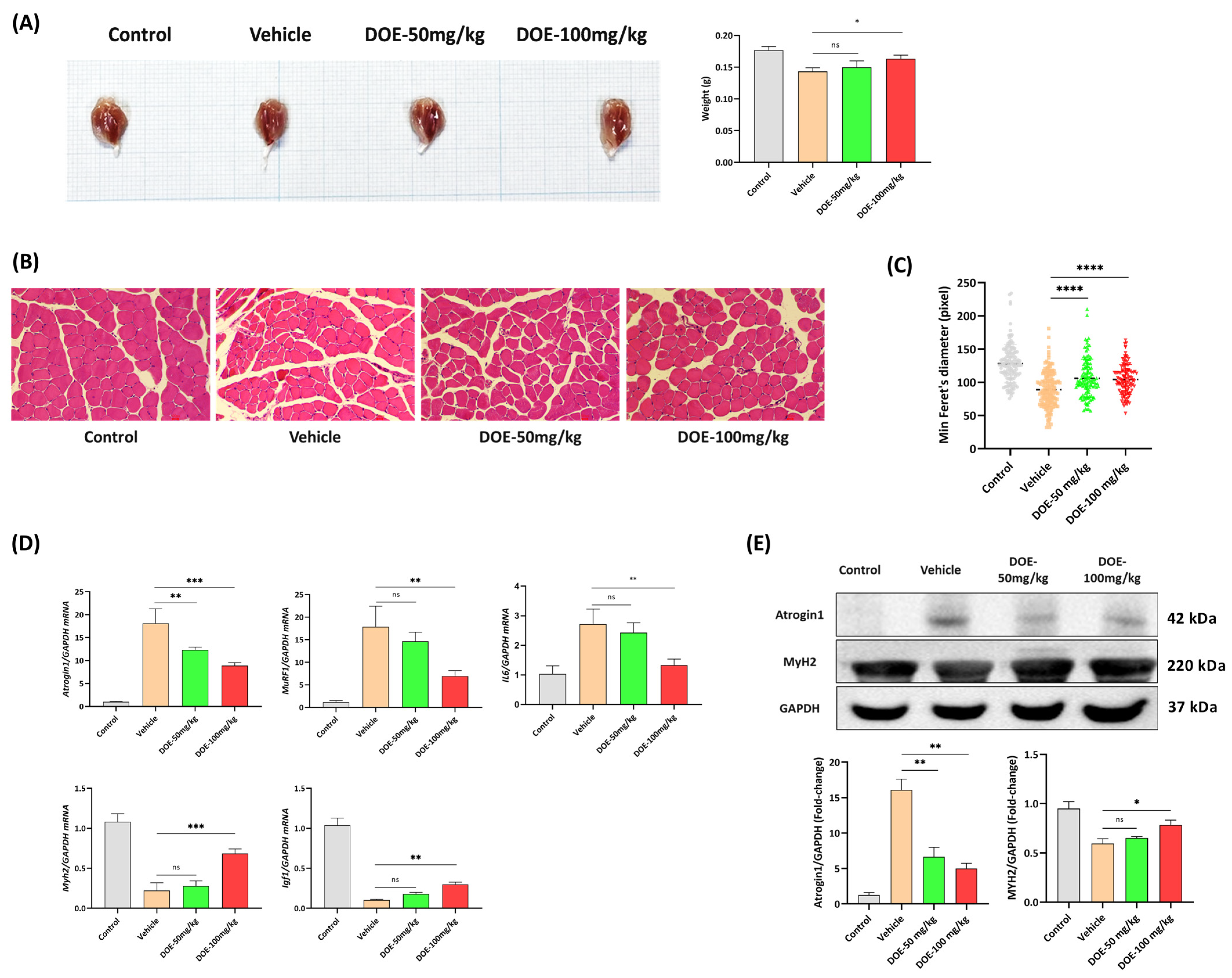
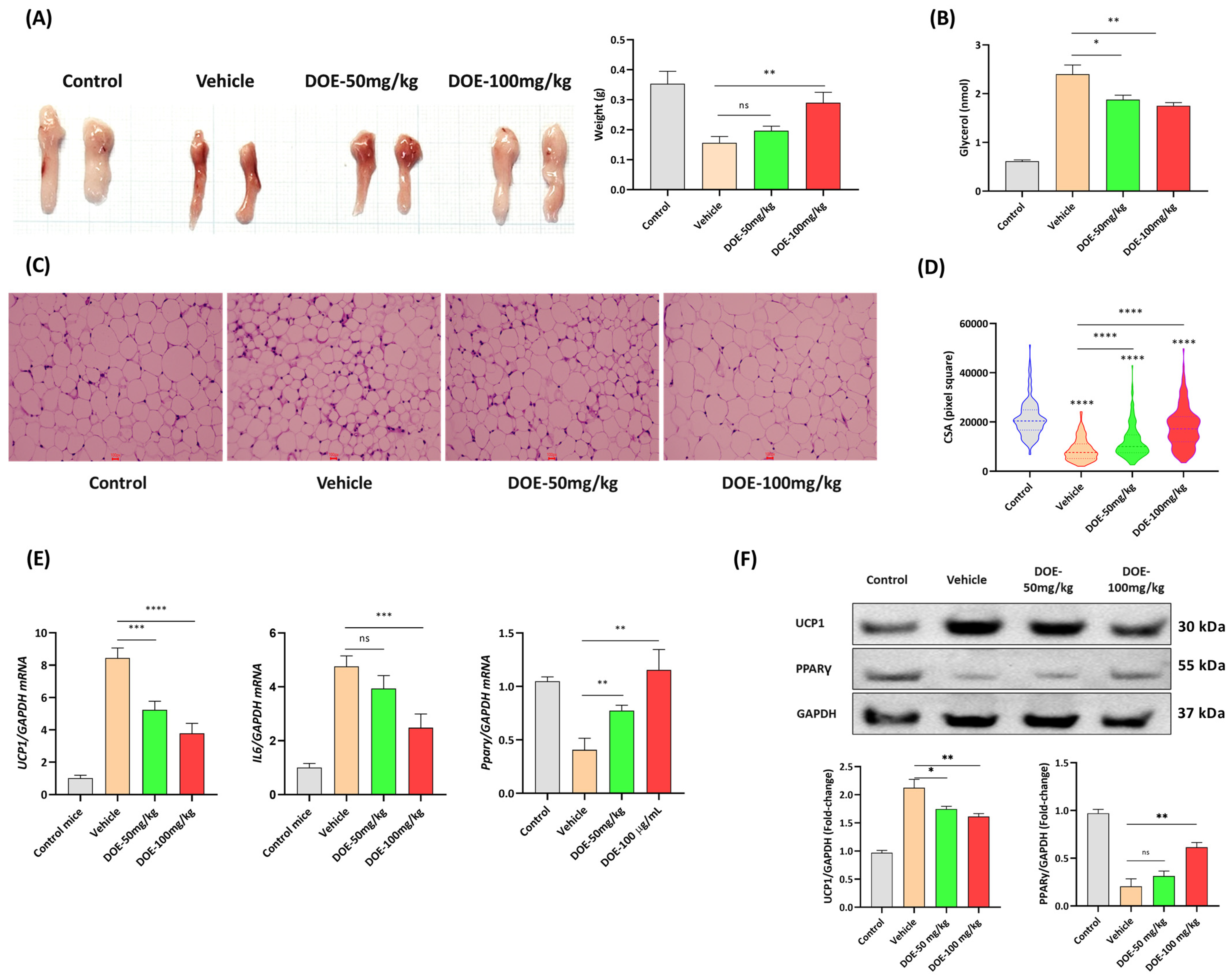
3.1. DOE Improved the Loss of Body Weight, Food Intake, and Releasing of Glycerol in the Serum Samples of CT26-Bearing Mice
3.2. DOE Attenuates Skeletal Muscle Atrophy in CT26-Bearing Mice
3.3. DOE Prevents the Loss of Adipose Tissue in CT26-Bearing Mice
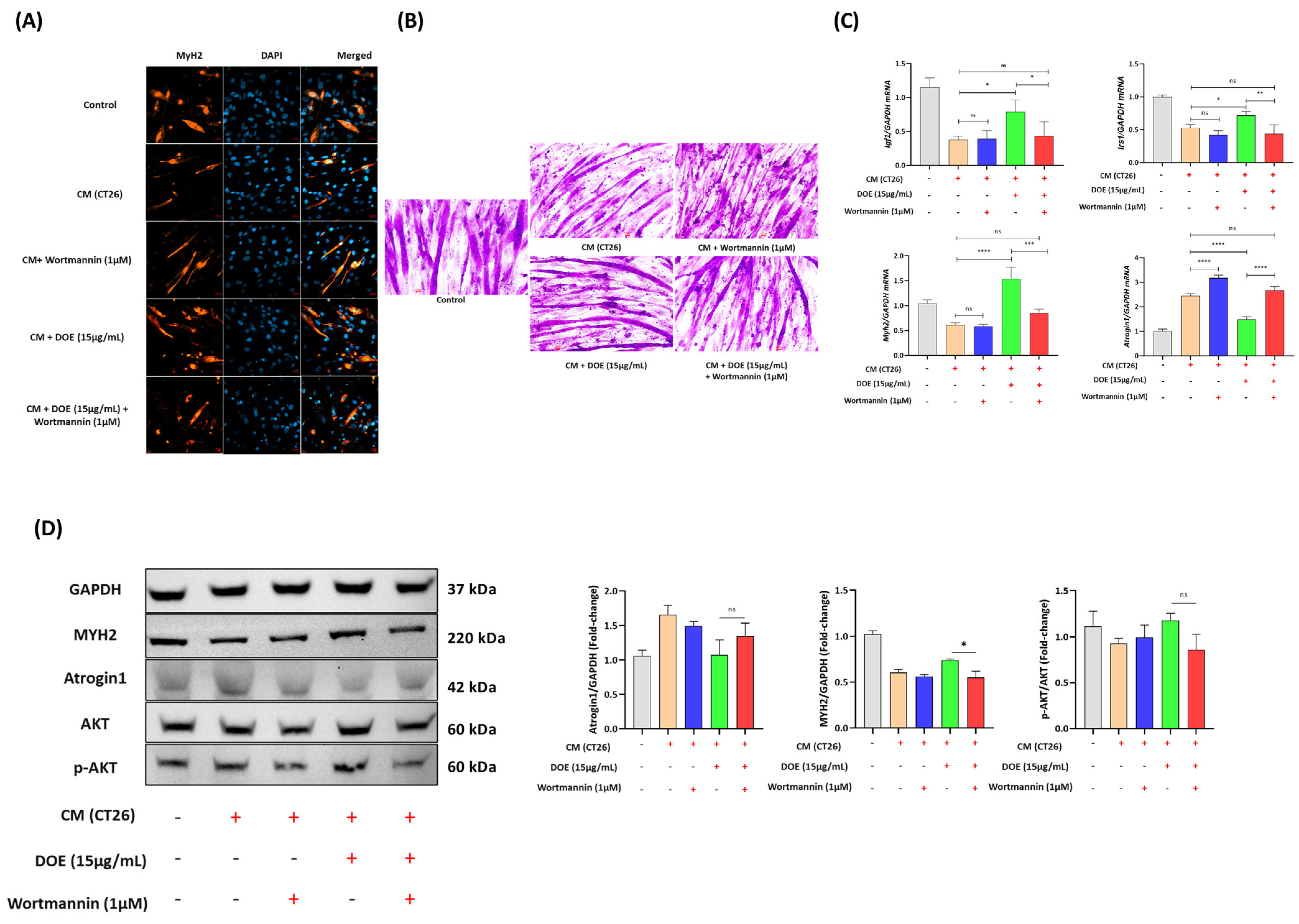
3.4. DOE Prevents Muscle Atrophy (In C2C12 Myotubes) Induced by Cancer Cachexia
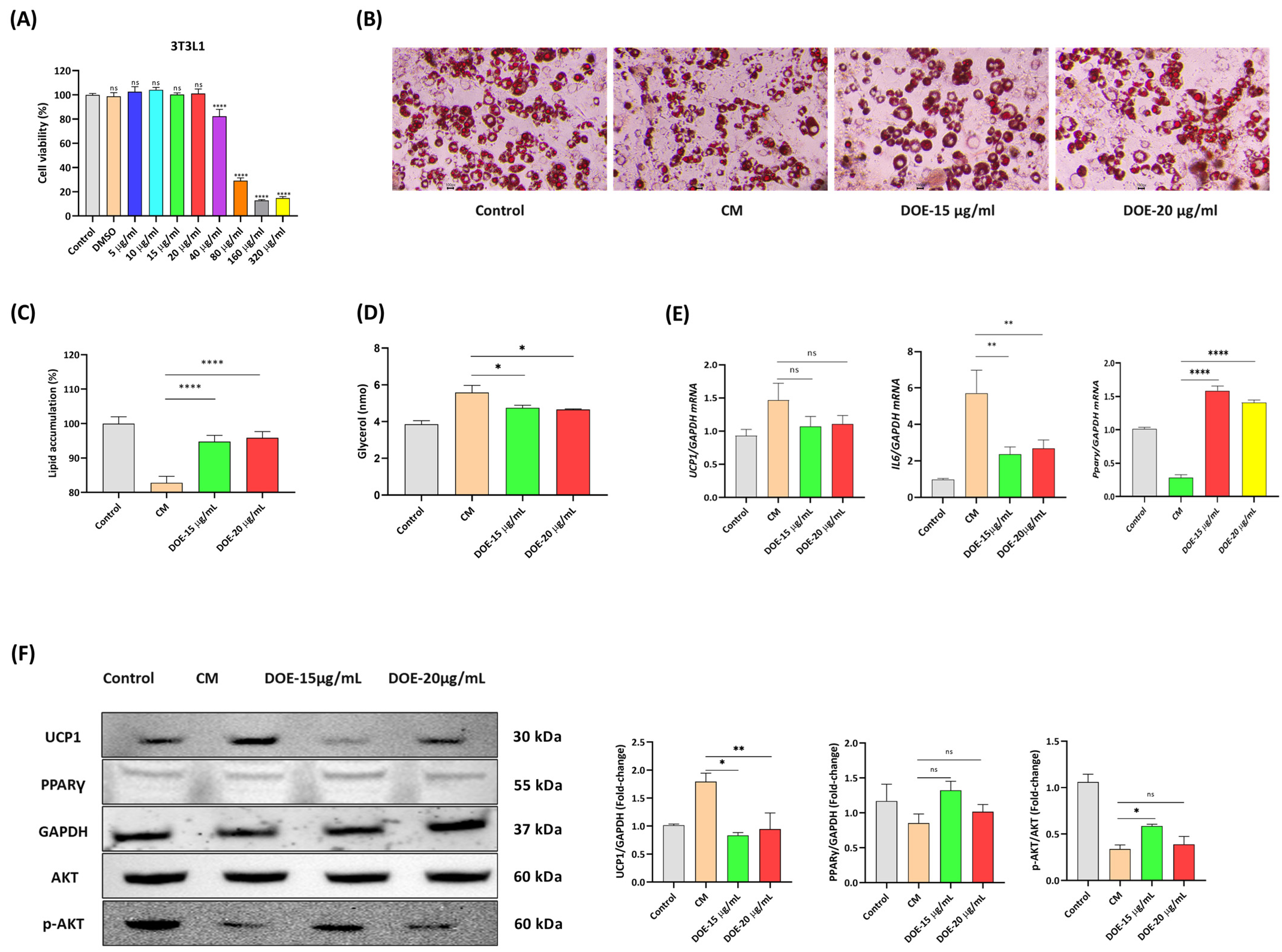
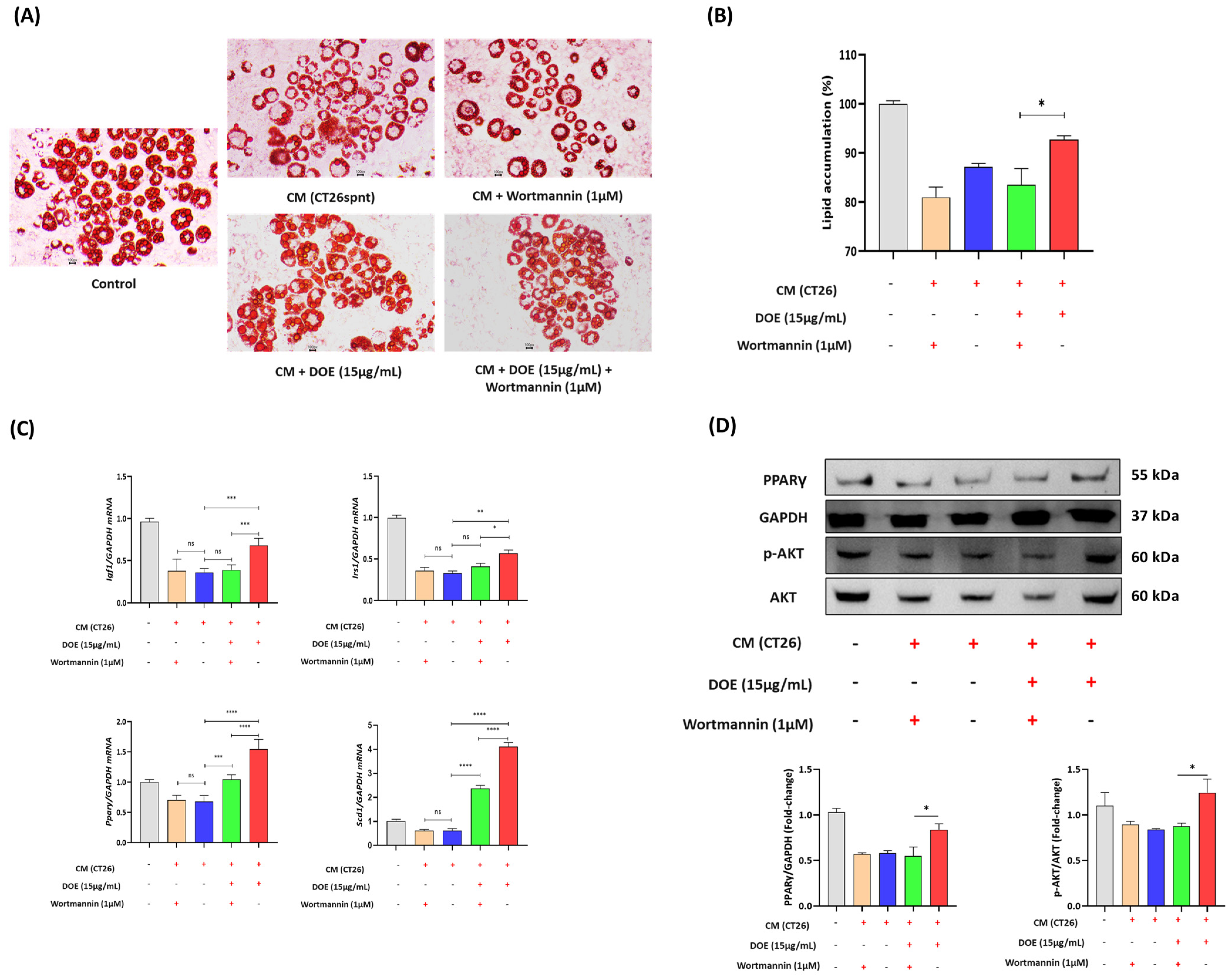
3.5. DOE Prevents Lipolysis (In 3T3L1 Adipocytes) Induced by Cancer Cachexia
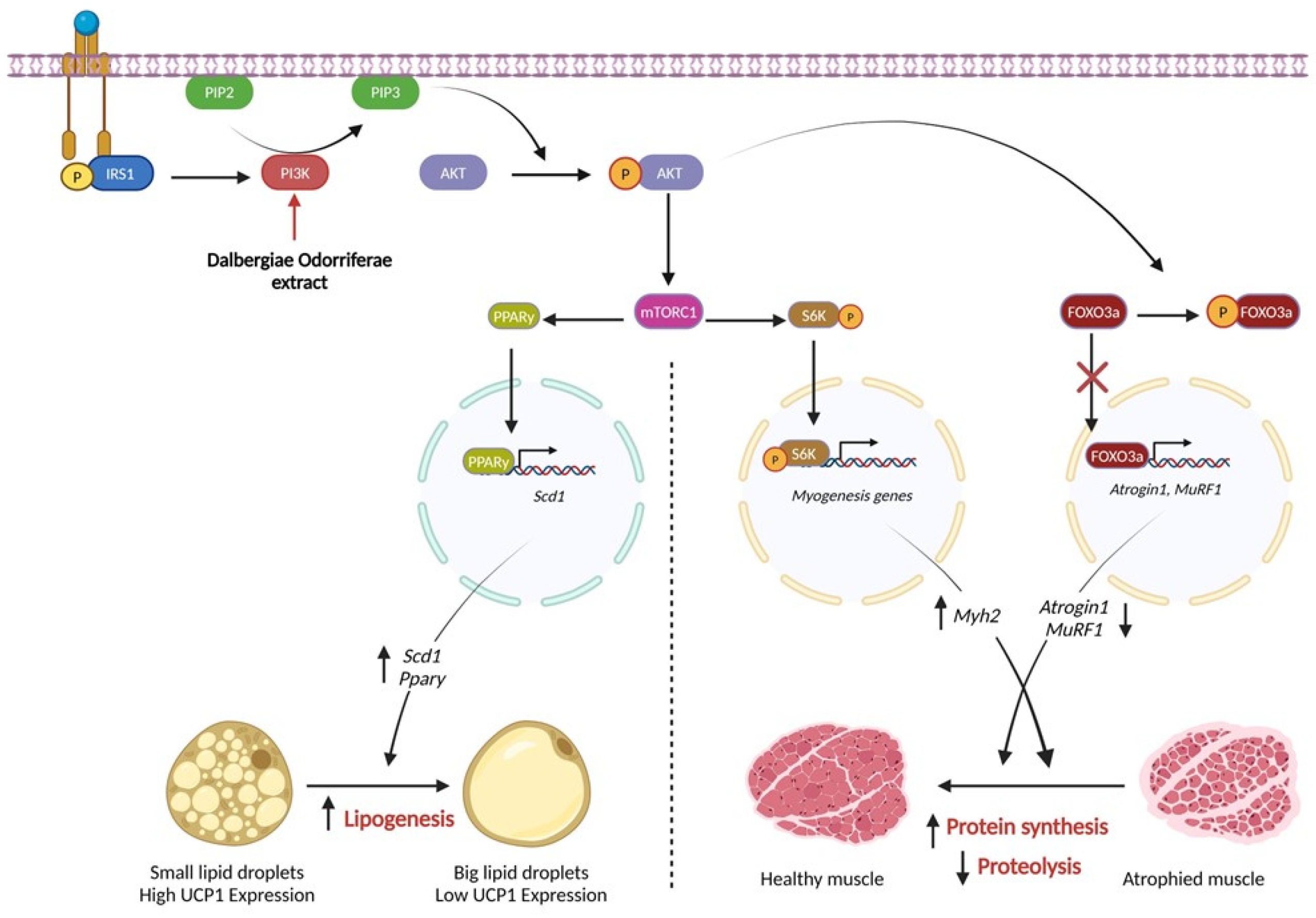
3.6. DOE Improves Muscle Wasting and Lipolysis by Promoting the PI3K/AKT Signaling Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M.; Anthony, T.G.; Ayres, J.S.; Biffi, G.; Brown, J.C.; Caan, B.J.; Feliciano, E.M.C.; Coll, A.P.; Dunne, R.F.; Goncalves, M.D.; et al. Cachexia: A systemic consequence of progressive, unresolved disease. Cell 2023, 186, 1824–1845. [Google Scholar] [CrossRef] [PubMed]
- McGovern, J.; Dolan, R.D.; Skipworth, R.J.; Laird, B.J.; McMillan, D.C. Cancer cachexia: A nutritional or a systemic inflammatory syndrome? Br. J. Cancer 2022, 127, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Im, M.; Gu, M.J.; Ma, J.Y. Citrus unshiu peel extract alleviates cancer-induced weight loss in mice bearing CT-26 adenocarcinoma. Sci. Rep. 2016, 6, 24214. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.F.; Rohm, M.; Herzig, S.; Diaz, M.B. Cancer Cachexia: More Than Skeletal Muscle Wasting. Trends Cancer 2018, 4, 849–860. [Google Scholar] [CrossRef]
- Kadakia, K.C.; Hamilton-Reeves, J.M.; Baracos, V.E. Current Therapeutic Targets in Cancer Cachexia: A Pathophysiologic Approach. Am. Soc. Clin. Oncol. Educ. Book 2023, 43, e389942. [Google Scholar] [CrossRef]
- Tisdale, M.J. Cachexia in cancer patients. Nat. Rev. Cancer 2002, 2, 862–871. [Google Scholar] [CrossRef]
- Porporato, P.E. Understanding cachexia as a cancer metabolism syndrome. Oncogenesis 2016, 5, e200. [Google Scholar] [CrossRef]
- Siddiqui, J.A.; Pothuraju, R.; Jain, M.; Batra, S.K.; Nasser, M.W. Advances in cancer cachexia: Intersection between affected organs, mediators, and pharmacological interventions. Biochim. Biophys. Acta (BBA)—Rev. Cancer 2020, 1873, 188359. [Google Scholar] [CrossRef]
- Johns, N.; Stephens, N.A.; Fearon, K.C. Muscle wasting in cancer. Int. J. Biochem. Cell Biol. 2013, 45, 2215–2229. [Google Scholar] [CrossRef]
- Dahlman, I.; Mejhert, N.; Linder, K.; Agustsson, T.; Mutch, D.M.; Kulyte, A.; Isaksson, B.; Permert, J.; Petrovic, N.; Nedergaard, J.; et al. Adipose tissue pathways involved in weight loss of cancer cachexia. Br. J. Cancer 2010, 102, 1541–1548. [Google Scholar] [CrossRef] [PubMed]
- Geremia, A.; Sartori, R.; Baraldo, M.; Nogara, L.; Balmaceda, V.; Dumitras, G.A.; Ciciliot, S.; Scalabrin, M.; Nolte, H.; Blaauw, B. Activation of Akt-mTORC1 signalling reverts cancer-dependent muscle wasting. J. Cachexia Sarcopenia Muscle 2022, 13, 648–661. [Google Scholar] [CrossRef] [PubMed]
- Sartori, R.; Romanello, V.; Sandri, M. Mechanisms of muscle atrophy and hypertrophy: Implications in health and disease. Nat. Commun. 2021, 12, 330. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Huang, J.; Wu, H.; Wang, Y.; Du, Z.; Ling, Y.; Wang, W.; Wu, Q.; Gao, W. Molecular mechanisms of cancer cachexia-induced muscle atrophy (Review). Mol. Med. Rep. 2020, 22, 4967–4980. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, L.; Wan, L.; Huo, Y.; Huang, J.; Li, J.; Lu, J.; Xin, B.; Yang, Q.; Guo, C. Matrine improves skeletal muscle atrophy by inhibiting E3 ubiquitin ligases and activating the Akt/mTOR/FoxO3α signaling pathway in C2C12 myotubes and mice. Oncol. Rep. 2019, 42, 479–494. [Google Scholar] [CrossRef]
- Glass, D.J. Signaling pathways perturbing muscle mass. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 225–229. [Google Scholar] [CrossRef]
- Choi, S.M.; Tucker, D.F.; Gross, D.N.; Easton, R.M.; DiPilato, L.M.; Dean, A.S.; Monks, B.R.; Birnbaum, M.J. Insulin regulates adipocyte lipolysis via an akt-independent signaling pathway. Mol. Cell. Biol. 2010, 30, 5009–5020. [Google Scholar] [CrossRef]
- Fearon, K.C.; Glass, D.J.; Guttridge, D.C. Cancer Cachexia: Mediators, Signaling, and Metabolic Pathways. Cell Metab. 2012, 16, 153–166. [Google Scholar] [CrossRef]
- The, S.N. A Review on the Medicinal Plant Dalbergia odorifera Species: Phytochemistry and Biological Activity. Evidence-Based Complement. Altern. Med. 2017, 2017, 7142370. [Google Scholar]
- Zhao, X.; Wang, C.; Meng, H.; Yu, Z.; Yang, M.; Wei, J. Dalbergia odorifera: A review of its traditional uses, phytochemistry, pharmacology, and quality control. J. Ethnopharmacol. 2020, 248, 112328. [Google Scholar] [CrossRef]
- McColl, R.; Nkosi, M.; Snyman, C.; Niesler, C. Analysis and Quantification of in Vitro Myoblast Fusion using the LADD Multiple Stain. BioTechniques 2016, 61, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Setiawan, T.; Sari, I.N.; Wijaya, Y.T.; Julianto, N.M.; Muhammad, J.A.; Lee, H.; Chae, J.H.; Kwon, H.Y. Cancer cachexia: Molecular mechanisms and treatment strategies. J. Hematol. Oncol. 2023, 16, 54. [Google Scholar] [CrossRef] [PubMed]
- Webster, J.M.; Kempen, L.J.A.P.; Hardy, R.S.; Langen, R.C.J. Inflammation and Skeletal Muscle Wasting During Cachexia. Front. Physiol. 2020, 11, 597675. [Google Scholar] [CrossRef] [PubMed]
- Katakami, N.; Uchino, J.; Yokoyama, T.; Naito, T.; Kondo, M.; Yamada, K.; Kitajima, H.; Yoshimori, K.; Sato, K.; Saito, H.; et al. Anamorelin (ONO-7643) for the treatment of patients with non–small cell lung cancer and cachexia: Results from a randomized, double-blind, placebo-controlled, multicenter study of Japanese patients (ONO-7643-04). Cancer 2018, 124, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.M.; Friend, J.; Allen, S. Therapeutic potential of anamorelin, a novel, oral ghrelin mimetic, in patients with cancer-related cachexia: A multicenter, randomized, double-blind, crossover, pilot study. Support. Care Cancer 2013, 21, 129–137. [Google Scholar] [CrossRef]
- Hanada, K.; Fukasawa, K.; Hinata, H.; Imai, S.; Takayama, K.; Hirai, H.; Ohfusa, R.; Hayashi, Y.; Itoh, F. Combination therapy with anamorelin and a myostatin inhibitor is advantageous for cancer cachexia in a mouse model. Cancer Sci. 2022, 113, 3547–3557. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Arai, H.; Inui, A. The regulatory approval of anamorelin for treatment of cachexia in patients with non-small cell lung cancer, gastric cancer, pancreatic cancer, and colorectal cancer in Japan: Facts and numbers. J. Cachexia Sarcopenia Muscle 2021, 12, 14–16. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, X.; Yang, Q.; Guo, C. Cucurbitacin IIb attenuates cancer cachexia induced skeletal muscle atrophy by regulating the IL-6/STAT3/FoxO signaling pathway. Phytother. Res. 2023, 37, 3380–3393. [Google Scholar] [CrossRef]
- Liu, L.; Hu, R.; You, H.; Li, J.; Liu, Y.; Li, Q.; Wu, X.; Huang, J.; Cai, X.; Wang, M.; et al. Formononetin ameliorates muscle atrophy by regulating myostatin-mediated PI3K/Akt/FoxO3a pathway and satellite cell function in chronic kidney disease. J. Cell. Mol. Med. 2021, 25, 1493–1506. [Google Scholar] [CrossRef]
- Stitt, T.N.; Drujan, D.; Clarke, B.A.; Panaro, F.; Timofeyva, Y.; Kline, W.O.; Gonzalez, M.; Yancopoulos, G.D.; Glass, D.J. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol. Cell 2004, 14, 395–403. [Google Scholar] [CrossRef]
- Savova, M.S.; Mihaylova, L.V.; Tews, D.; Wabitsch, M.; Georgiev, M.I. Targeting PI3K/AKT signaling pathway in obesity. Biomed. Pharmacother. 2023, 159, 114244. [Google Scholar] [CrossRef] [PubMed]
- Vaitkus, J.A.; Celi, F.S. The role of adipose tissue in cancer-associated cachexia. Exp. Biol. Med. 2017, 242, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Yecies, J.L.; Zhang, H.H.; Menon, S.; Liu, S.; Yecies, D.; Lipovsky, A.I.; Gorgun, C.; Kwiatkowski, D.J.; Hotamisligil, G.S.; Lee, C.-H.; et al. Akt stimulates hepatic srebp1c and lipogenesis through parallel mtorc1-dependent and independent pathways. Cell Metab. 2011, 14, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Cheng, X.; Feng, X.; Zhao, K.; Zhang, M.; Yao, R.; Chen, Y.; Wang, Y.; Hao, H.; Wang, Z. The mTORC1/4EBP1/PPARγ Axis Mediates Insulin-Induced Lipogenesis by Regulating Lipogenic Gene Expression in Bovine Mammary Epithelial Cells. J. Agric. Food Chem. 2019, 67, 6007–6018. [Google Scholar] [CrossRef] [PubMed]
- Bing, C.; Russell, S.; Becket, E.; Pope, M.; Tisdale, M.J.; Trayhurn, P.; Jenkins, J.R. Adipose atrophy in cancer cachexia: Morphologic and molecular analysis of adipose tissue in tumour-bearing mice. Br. J. Cancer 2006, 95, 1028–1037. [Google Scholar] [CrossRef]
- Daas, S.I.; Rizeq, B.R.; Nasrallah, G.K. Adipose tissue dysfunction in cancer cachexia. J. Cell. Physiol. 2019, 234, 13–22. [Google Scholar] [CrossRef]
- Song, G.; Park, W.Y.; Jiao, W.; Park, J.Y.; Jung, S.J.; Ma, S.; Lee, J.; Lee, K.Y.; Choe, S.-K.; Park, J.; et al. Moderating AKT signaling with baicalein protects against weight loss by preventing muscle atrophy in a cachexia model caused by CT26 colon cancer. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2024, 1871, 119670. [Google Scholar] [CrossRef]
- Arneson-Wissink, P.C.; Ducharme, A.M.; Doles, J.D. A novel transplantable model of lung cancer-associated tissue loss and disrupted muscle regeneration. Skelet. Muscle 2020, 10, 6. [Google Scholar] [CrossRef]


| Primer Name | Primer Sequence (5′-3′) |
|---|---|
| Atrogin1-F | ATGCACACTGGTGCAGAGAG |
| Atrogin1-R | TGTAAGCACACAGGCAGGTC |
| MuRF1-F | CACGAAGACGAGAAGATCAACATC |
| MuRF1-R | AGCCCCAAACACCTTGCA |
| IL6-F | CTTCTTGGGACTGATGCTGGTGAC |
| IL6-R | TCTGTTGGGAGTGGTATCCTCTGTG |
| Irs1-F | TGTCACCCAGTGGTAGTTGCTC |
| Irs1-R | CTCTCAACAGGAGGTTTGGCATG |
| Myh2-F | AGGCGGCTGAGGAGCACGTA |
| Myh2-R | GCGGCACAAGCAGCGTTGG |
| Igf-F | GGACCAGAGCCCTTTGCGG |
| Igf-R | GGCTGCTTTTGTAGGCTTCAGTGG |
| Gapdh-F | AACATCATCCCTGCTTCCACT |
| Gapdh-R | GGCAGGTCAGATCCACAAC |
| Ucp1-F | AAGCTGTGCGATGTCCATGT |
| Ucp1-R | AAGCCACAAACCCTTTGAAAA |
| Irs1-F | TGTCACCCAGTGGTAGTTGCTC |
| Irs1-R | CTCTCAACAGGAGGTTTGGCATG |
| Pparγ-F | CAAGAATACCAAAGTGCGATCAA |
| Pparγ-R | GAGCTGGGTCTTTTCAGAATAATAAG |
| Scd1-F | CTGACCTGAAAGCCGAGAAG |
| Scd1-R | GCGTTGAGCACCAGAGTGTA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, P.T.; Park, E.; Luong, Q.X.T.; Hakim, M.D.; Hoang, P.T.; Vo, T.T.B.; Kawalin, K.; Kang, H.; Lee, T.-K.; Lee, S. Amelioration of Cancer Cachexia by Dalbergia odorifera Extract Through AKT Signaling Pathway Regulation. Nutrients 2024, 16, 3671. https://doi.org/10.3390/nu16213671
Ho PT, Park E, Luong QXT, Hakim MD, Hoang PT, Vo TTB, Kawalin K, Kang H, Lee T-K, Lee S. Amelioration of Cancer Cachexia by Dalbergia odorifera Extract Through AKT Signaling Pathway Regulation. Nutrients. 2024; 16(21):3671. https://doi.org/10.3390/nu16213671
Chicago/Turabian StyleHo, Phuong T., Eulyong Park, Quynh Xuan Thi Luong, Meutia Diva Hakim, Phuong T. Hoang, Thuy T. B. Vo, Kantawong Kawalin, Hee Kang, Taek-Kyun Lee, and Sukchan Lee. 2024. "Amelioration of Cancer Cachexia by Dalbergia odorifera Extract Through AKT Signaling Pathway Regulation" Nutrients 16, no. 21: 3671. https://doi.org/10.3390/nu16213671
APA StyleHo, P. T., Park, E., Luong, Q. X. T., Hakim, M. D., Hoang, P. T., Vo, T. T. B., Kawalin, K., Kang, H., Lee, T.-K., & Lee, S. (2024). Amelioration of Cancer Cachexia by Dalbergia odorifera Extract Through AKT Signaling Pathway Regulation. Nutrients, 16(21), 3671. https://doi.org/10.3390/nu16213671







