Metataxonomics and Metabolomics Profiles in Metabolic Dysfunction-Associated Fatty Liver Disease Patients on a “Navelina” Orange-Enriched Diet
Highlights
- The cohort of patients with Metabolic Dysfunction-Associated Fatty Liver Disease, following a dietary regimen enriched with ‘Navelina’ oranges, exhibited changes in both metataxonomics and metabolomics profiles.
- The use of a multi-omics approach enabled the identification of a set of taxa and VOCs that may serve as markers for dysbiosis and the comorbidities associated with the disease burden of MAFLD.
- The findings of this study underscore how dietary interventions and lifestyle modifications can assist in the management of MAFLD and its related comorbidities.
- The changes in gut microbiota observed after orange consumption suggest that the microbiota could become a therapeutic target, potentially through the use of pro-biotic therapies or tailored interventions to modulate the microbiota in patients with MAFLD.
- Volatile compounds (VOCs) detected in fecal and urinary analyses could be explored as novel diagnostic and prognostic biomarkers to monitor the progression of MAFLD and the effectiveness of dietary interventions.
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Recruitment
2.2. Study Design
2.3. DNA Fecal Extraction and Pooled Library Preparation
2.4. DNA Amplification and Sequencing
2.5. Bioinformatics Analyses, Filtering, and Annotation
2.6. Statistical Analyses
2.7. Fecal and Urinary Metabolomics
3. Results
3.1. Metataxonomics Diversity Metric Estimate
3.2. Clustering Analyses on 16S Taxa at the Genus Levels
3.3. Pairwise Group Comparison Genus Annotation
3.4. Pathway Prediction from 16S Matrix
3.5. Fecal and Urinary Metabolomics VOC Profiles
3.6. Statistical Correlation of Clinical, Metataxonomics, and Metabolomics Variables
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wong, V.W.-S.; Wong, G.L.-H.; Woo, J.; Abrigo, J.M.; Chan, C.K.-M.; Shu, S.S.-T.; Leung, J.K.-Y.; Chim, A.M.-L.; Kong, A.P.-S.; Lui, G.C.-Y.; et al. Impact of the New Definition of Metabolic Associated Fatty Liver Disease on the Epidemiology of the Disease. Clin. Gastroenterol. Hepatol. 2021, 19, 2161–2171.e5. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wai-Sun Wong, V.; Dufour, J.-F.; Schattenberg, J.M.; et al. A New Definition for Metabolic Dysfunction-Associated Fatty Liver Disease: An International Expert Consensus Statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Boccatonda, A.; Andreetto, L.; D’Ardes, D.; Cocco, G.; Rossi, I.; Vicari, S.; Schiavone, C.; Cipollone, F.; Guagnano, M.T. From NAFLD to MAFLD: Definition, Pathophysiological Basis and Cardiovascular Implications. Biomedicines 2023, 11, 883. [Google Scholar] [CrossRef] [PubMed]
- Lippolis, T.; Cofano, M.; Caponio, G.R.; De Nunzio, V.; Notarnicola, M. Bioaccessibility and Bioavailability of Diet Polyphenols and Their Modulation of Gut Microbiota. Int. J. Mol. Sci. 2023, 24, 3813. [Google Scholar] [CrossRef]
- Waheed Janabi, A.H.; Kamboh, A.A.; Saeed, M.; Xiaoyu, L.; BiBi, J.; Majeed, F.; Naveed, M.; Mughal, M.J.; Korejo, N.A.; Kamboh, R.; et al. Flavonoid-Rich Foods (FRF): A Promising Nutraceutical Approach against Lifespan-Shortening Diseases. Iran. J. Basic. Med. Sci. 2020, 23, 140–153. [Google Scholar] [CrossRef]
- Caponio, G.; Cofano, M.; Lippolis, T.; Gigante, I.; De Nunzio, V.; Difonzo, G.; Noviello, M.; Tarricone, L.; Gambacorta, G.; Giannelli, G.; et al. Anti-Proliferative and Pro-Apoptotic Effects of Digested Aglianico Grape Pomace Extract in Human Colorectal Cancer Cells. Molecules 2022, 27, 6791. [Google Scholar] [CrossRef]
- Assini, J.M.; Mulvihill, E.E.; Huff, M.W. Citrus Flavonoids and Lipid Metabolism. Curr. Opin. Lipidol. 2013, 24, 34–40. [Google Scholar] [CrossRef]
- Verny, M.-A.; Milenkovic, D.; Macian, N.; Pereira, B.; Evrard, R.; Gilcher, C.; Steingass, C.B.; Mosoni, P.; Gladine, C.; Monfoulet, L.-E.; et al. Evaluating the Role of Orange Juice, HESPERidin in Vascular HEALTH Benefits (HESPER-HEALTH Study): Protocol for a Randomised Controlled Trial. BMJ Open 2021, 11, e053321. [Google Scholar] [CrossRef]
- Peterson, J.J.; Dwyer, J.T.; Beecher, G.R.; Bhagwat, S.A.; Gebhardt, S.E.; Haytowitz, D.B.; Holden, J.M. Flavanones in Oranges, Tangerines (Mandarins), Tangors, and Tangelos: A Compilation and Review of the Data from the Analytical Literature. J. Food Compos. Anal. 2006, 19, S66–S73. [Google Scholar] [CrossRef]
- Nie, T.; Wang, X.; Li, A.; Shan, A.; Ma, J. The Promotion of Fatty Acid β-Oxidation by Hesperidin via Activating SIRT1/PGC1α to Improve NAFLD Induced by a High-Fat Diet. Food Funct. 2024, 15, 372–386. [Google Scholar] [CrossRef]
- Rizza, S.; Muniyappa, R.; Iantorno, M.; Kim, J.; Chen, H.; Pullikotil, P.; Senese, N.; Tesauro, M.; Lauro, D.; Cardillo, C.; et al. Citrus Polyphenol Hesperidin Stimulates Production of Nitric Oxide in Endothelial Cells While Improving Endothelial Function and Reducing Inflammatory Markers in Patients with Metabolic Syndrome. J. Clin. Endocrinol. Metab. 2011, 96, E782–E792. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, K.G.; Yoshinaga, M.Y.; Glezer, I.; de Britto Chaves-Filho, A.; de Santana, A.A.; Kovacs, C.; Magnoni, C.D.; Lajolo, F.M.; Miyamoto, S.; Hassimotto, N.M.A. Orange Juice Intake by Obese and Insulin-Resistant Subjects Lowers Specific Plasma Triglycerides: A Randomized Clinical Trial. Clin. Nutr. ESPEN 2022, 51, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.J.; Mendis, B.; Macdonald, I.A. Orange Juice Consumption and Its Effect on Blood Lipid Profile and Indices of the Metabolic Syndrome; a Randomised, Controlled Trial in an at-Risk Population. Food Funct. 2016, 7, 1884–1891. [Google Scholar] [CrossRef] [PubMed]
- Yoshitomi, R.; Yamamoto, M.; Kumazoe, M.; Fujimura, Y.; Yonekura, M.; Shimamoto, Y.; Nakasone, A.; Kondo, S.; Hattori, H.; Haseda, A.; et al. The Combined Effect of Green Tea and α-Glucosyl Hesperidin in Preventing Obesity: A Randomized Placebo-Controlled Clinical Trial. Sci. Rep. 2021, 11, 19067. [Google Scholar] [CrossRef]
- Grande, F.; Occhiuzzi, M.A.; Perri, M.R.; Ioele, G.; Rizzuti, B.; Statti, G.; Garofalo, A. Polyphenols from Citrus Tacle® Extract Endowed with HMGCR Inhibitory Activity: An Antihypercholesterolemia Natural Remedy. Molecules 2021, 26, 5718. [Google Scholar] [CrossRef]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W. Obesity Is Associated with Macrophage Accumulation in Adipose Tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef]
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A.; et al. Chronic Inflammation in Fat Plays a Crucial Role in the Development of Obesity-Related Insulin Resistance. J. Clin. Investig. 2003, 112, 1821–1830. [Google Scholar] [CrossRef]
- Subramanian, S.; Han, C.Y.; Chiba, T.; McMillen, T.S.; Wang, S.A.; Haw, A.; Kirk, E.A.; O’Brien, K.D.; Chait, A. Dietary Cholesterol Worsens Adipose Tissue Macrophage Accumulation and Atherosclerosis in Obese LDL Receptor–Deficient Mice. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 685–691. [Google Scholar] [CrossRef]
- Subramanian, S.; Goodspeed, L.; Wang, S.; Kim, J.; Zeng, L.; Ioannou, G.N.; Haigh, W.G.; Yeh, M.M.; Kowdley, K.V.; O’Brien, K.D.; et al. Dietary Cholesterol Exacerbates Hepatic Steatosis and Inflammation in Obese LDL Receptor-Deficient Mice. J. Lipid Res. 2011, 52, 1626–1635. [Google Scholar] [CrossRef]
- Kumar Sharma, A.; Bharti, S.; Ojha, S.; Bhatia, J.; Kumar, N.; Ray, R.; Kumari, S.; Singh Arya, D. Up-Regulation of PPARγ, Heat Shock Protein-27 and -72 by Naringin Attenuates Insulin Resistance, β-Cell Dysfunction, Hepatic Steatosis and Kidney Damage in a Rat Model of Type 2 Diabetes. Br. J. Nutr. 2011, 106, 1713–1723. [Google Scholar] [CrossRef]
- Li, J.-J.; Jiang, H.-C.; Wang, A.; Bu, F.-T.; Jia, P.-C.; Zhu, S.; Zhu, L.; Huang, C.; Li, J. Hesperetin Derivative-16 Attenuates CCl4-Induced Inflammation and Liver Fibrosis by Activating AMPK/SIRT3 Pathway. Eur. J. Pharmacol. 2022, 915, 174530. [Google Scholar] [CrossRef] [PubMed]
- Martin, M. Cutadapt Removes Adapter Sequences from High-Throughput Sequencing Reads. EMBnet J. 2011, 17, 10. [Google Scholar] [CrossRef]
- Maestri, M.; Santopaolo, F.; Pompili, M.; Gasbarrini, A.; Ponziani, F.R. Gut Microbiota Modulation in Patients with Non-Alcoholic Fatty Liver Disease: Effects of Current Treatments and Future Strategies. Front. Nutr. 2023, 10, 1110536. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, F.; Disciglio, V.; Franco, I.; Sorino, P.; Bonfiglio, C.; Bianco, A.; Campanella, A.; Lippolis, T.; Pesole, P.; Polignano, M.; et al. A Low Glycemic Index Mediterranean Diet Combined with Aerobic Physical Activity Rearranges the Gut Microbiota Signature in NAFLD Patients. Nutrients 2022, 14, 1773. [Google Scholar] [CrossRef]
- Mirabelli, M.; Chiefari, E.; Arcidiacono, B.; Corigliano, D.M.; Brunetti, F.S.; Maggisano, V.; Russo, D.; Foti, D.P.; Brunetti, A. Mediterranean Diet Nutrients to Turn the Tide against Insulin Resistance and Related Diseases. Nutrients 2020, 12, 1066. [Google Scholar] [CrossRef]
- Han, J.M.; Jo, A.N.; Lee, S.M.; Bae, H.S.; Jun, D.W.; Cho, Y.K.; Suk, K.T.; Yoon, J.H.; Ahn, S.B.; Cho, Y.J.; et al. Associations between Intakes of Individual Nutrients or Whole Food Groups and Non-alcoholic Fatty Liver Disease among Korean Adults. J. Gastroenterol. Hepatol. 2014, 29, 1265–1272. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Ye, M.; Zhang, S.; Zhang, Q.; Meng, G.; Liu, L.; Wu, H.; Gu, Y.; Wang, Y.; et al. Does a High Intake of Green Leafy Vegetables Protect from NAFLD? Evidence from a Large Population Study. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1691–1701. [Google Scholar] [CrossRef]
- Notarnicola, M.; Tutino, V.; De Nunzio, V.; Cisternino, A.M.; Cofano, M.; Donghia, R.; Giannuzzi, V.; Zappimbulso, M.; Milella, R.A.; Giannelli, G.; et al. Daily Orange Consumption Reduces Hepatic Steatosis Prevalence in Patients with Metabolic Dysfunction-Associated Steatotic Liver Disease: Exploratory Outcomes of a Randomized Clinical Trial. Nutrients 2024, 16, 3191. [Google Scholar] [CrossRef]
- Dridi, B.; Henry, M.; El Khéchine, A.; Raoult, D.; Drancourt, M. High Prevalence of Methanobrevibacter Smithii and Methanosphaera Stadtmanae Detected in the Human Gut Using an Improved DNA Detection Protocol. PLoS ONE 2009, 4, e7063. [Google Scholar] [CrossRef]
- Bang, C.; Weidenbach, K.; Gutsmann, T.; Heine, H.; Schmitz, R.A. The Intestinal Archaea Methanosphaera Stadtmanae and Methanobrevibacter Smithii Activate Human Dendritic Cells. PLoS ONE 2014, 9, e99411. [Google Scholar] [CrossRef]
- Blais Lecours, P.; Marsolais, D.; Cormier, Y.; Berberi, M.; Haché, C.; Bourdages, R.; Duchaine, C. Increased Prevalence of Methanosphaera Stadtmanae in Inflammatory Bowel Diseases. PLoS ONE 2014, 9, e87734. [Google Scholar] [CrossRef] [PubMed]
- Barnett, D.J.M.; Mommers, M.; Penders, J.; Arts, I.C.W.; Thijs, C. Intestinal Archaea Inversely Associated with Childhood Asthma. J. Allergy Clin. Immunol. 2019, 143, 2305–2307. [Google Scholar] [CrossRef] [PubMed]
- Kawada, Y.; Goshima, T.; Sawamura, R.; Yokoyama, S.; Yanase, E.; Niwa, T.; Ebihara, A.; Inagaki, M.; Yamaguchi, K.; Kuwata, K.; et al. Daidzein Reductase of Eggerthella Sp. YY7918, Its Octameric Subunit Structure Containing FMN/FAD/4Fe-4S, and Its Enantioselective Production of R-Dihydroisoflavones. J. Biosci. Bioeng. 2018, 126, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-B.; Gupta, H.; Min, B.-H.; Ganesan, R.; Sharma, S.P.; Won, S.-M.; Jeong, J.-J.; Cha, M.-G.; Kwon, G.-H.; Jeong, M.-K.; et al. A Consortium of Hordeum Vulgare and Gut Microbiota against Non-Alcoholic Fatty Liver Disease via Data-Driven Analysis. Artif. Cells Nanomed. Biotechnol. 2024, 52, 250–260. [Google Scholar] [CrossRef]
- Liu, Y.; Jin, D.; He, T.; Liao, X.; Shao, L.; Shi, L.; Liu, L. Effect of the Combined Intervention of low-FODMAPs Diet and Probiotics on IBS Symptoms in Western China: A Randomized Controlled Trial. Food Sci. Nutr. 2024, 12, 3993–4004. [Google Scholar] [CrossRef]
- Tsai, C.-C.; Chiu, M.-H.; Kek, H.-P.; Yang, M.-C.; Su, Y.-T.; Liu, H.-K.; Wu, M.-S.; Yeh, Y.-T. The Reduced Gut Lachnospira Species Is Linked to Liver Enzyme Elevation and Insulin Resistance in Pediatric Fatty Liver Disease. Int. J. Mol. Sci. 2024, 25, 3640. [Google Scholar] [CrossRef]
- Dai, W.; Cai, D.; Zhou, S.; Li, A.; Xie, J.; Zhang, J. Uncovering a Causal Connection between the Lachnoclostridium Genus in Fecal Microbiota and Non-Alcoholic Fatty Liver Disease: A Two-Sample Mendelian Randomization Analysis. Front. Microbiol. 2023, 14, 1276790. [Google Scholar] [CrossRef]
- Mai, H.; Yang, X.; Xie, Y.; Zhou, J.; Wang, Q.; Wei, Y.; Yang, Y.; Lu, D.; Ye, L.; Cui, P.; et al. The Role of Gut Microbiota in the Occurrence and Progression of Non-Alcoholic Fatty Liver Disease. Front. Microbiol. 2024, 14, 1257903. [Google Scholar] [CrossRef]
- El Hachlafi, N.; Aanniz, T.; El Menyiy, N.; El Baaboua, A.; El Omari, N.; Balahbib, A.; Shariati, M.A.; Zengin, G.; Fikri-Benbrahim, K.; Bouyahya, A. In Vitro and in Vivo Biological Investigations of Camphene and Its Mechanism Insights: A Review. Food Rev. Int. 2023, 39, 1799–1826. [Google Scholar] [CrossRef]
- Couch, R.D.; Dailey, A.; Zaidi, F.; Navarro, K.; Forsyth, C.B.; Mutlu, E.; Engen, P.A.; Keshavarzian, A. Alcohol Induced Alterations to the Human Fecal VOC Metabolome. PLoS ONE 2015, 10, e0119362. [Google Scholar] [CrossRef]
- McGregor, D. Tertiary-Butanol: A Toxicological Review. Crit. Rev. Toxicol. 2010, 40, 697–727. [Google Scholar] [CrossRef] [PubMed]
- Mezmale, L.; Leja, M.; Lescinska, A.M.; Pčolkins, A.; Kononova, E.; Bogdanova, I.; Polaka, I.; Stonans, I.; Kirsners, A.; Ager, C.; et al. Identification of Volatile Markers of Colorectal Cancer from Tumor Tissues Using Volatilomic Approach. Molecules 2023, 28, 5990. [Google Scholar] [CrossRef] [PubMed]
- Thapa, D.; Louis, P.; Losa, R.; Zweifel, B.; Wallace, R.J. Essential Oils Have Different Effects on Human Pathogenic and Commensal Bacteria in Mixed Faecal Fermentations Compared with Pure Cultures. Microbiology 2015, 161, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Fowler, D.; Sizer, J.; Walton, C. Faecal Volatile Biomarkers of Clostridium Difficile Infection. PLoS ONE 2019, 14, e0215256. [Google Scholar] [CrossRef] [PubMed]
- Hershberger, C.E.; Raj, R.; Mariam, A.; Aykun, N.; Allende, D.S.; Brown, M.; Aucejo, F.; Rotroff, D.M. Characterization of Salivary and Plasma Metabolites as Biomarkers for HCC: A Pilot Study. Cancers 2023, 15, 4527. [Google Scholar] [CrossRef]
- Calabrese, F.M.; Celano, G.; Bonfiglio, C.; Campanella, A.; Franco, I.; Annunziato, A.; Giannelli, G.; Osella, A.R.; De Angelis, M. Synergistic Effect of Diet and Physical Activity on a NAFLD Cohort: Metabolomics Profile and Clinical Variable Evaluation. Nutrients 2023, 15, 2457. [Google Scholar] [CrossRef]
- Jia, X.; Cui, H.; Qin, S.; Ren, J.; Zhang, Z.; An, Q.; Zhang, N.; Yang, J.; Yang, Y.; Fan, G.; et al. Characterizing and Decoding the Key Odor Compounds of Spirulina Platensis at Different Processing Stages by Sensomics. Food Chem. 2024, 461, 140944. [Google Scholar] [CrossRef]
- Magpusao, J.; Oey, I.; Kebede, B. Chemical, Rheological, and Volatile Profiling of Microalgae Arthrospira, Isochrysis, Nannochloropsis, and Tetraselmis Species. Food Innov. Adv. 2024, 3, 75–87. [Google Scholar] [CrossRef]
- Li, Z.; Gong, R.; Chu, H.; Zeng, J.; Chen, C.; Xu, S.; Hu, L.; Gao, W.; Zhang, L.; Yuan, H.; et al. A Universal Plasma Metabolites-Derived Signature Predicts Cardiovascular Disease Risk in MAFLD. Atherosclerosis 2024, 392, 117526. [Google Scholar] [CrossRef]
- Panyod, S.; Wu, W.-K.; Peng, S.-Y.; Tseng, Y.-J.; Hsieh, Y.-C.; Chen, R.-A.; Huang, H.-S.; Chen, Y.-H.; Chuang, H.-L.; Hsu, C.-C.; et al. Ginger Essential Oil and Citral Ameliorate Atherosclerosis Via Modulating TMAO and Gut Microbiota in ApoE−/− Mice Fed on Gubra Amylin NASH Diet with ʟ-Carnitine. SSRN Electron. J. 2022, 7, 19. [Google Scholar] [CrossRef]
- Zhao, Q.; Wu, Z.E.; Li, B.; Li, F. Recent Advances in Metabolism and Toxicity of Tyrosine Kinase Inhibitors. Pharmacol. Ther. 2022, 237, 108256. [Google Scholar] [CrossRef] [PubMed]
- Tomita, K.; Teratani, T.; Yokoyama, H.; Suzuki, T.; Irie, R.; Ebinuma, H.; Saito, H.; Hokari, R.; Miura, S.; Hibi, T. Plasma Free Myristic Acid Proportion Is a Predictor of Nonalcoholic Steatohepatitis. Dig. Dis. Sci. 2011, 56, 3045–3052. [Google Scholar] [CrossRef] [PubMed]
- López-Bautista, F.; Barbero-Becerra, V.J.; Ríos, M.Y.; Ramírez-Cisneros, M.Á.; Sánchez-Pérez, C.A.; Ramos-Ostos, M.H.; Uribe, M.; Chávez-Tapia, N.C.; Juárez-Hernández, E. Dietary Consumption and Serum Pattern of Bioactive Fatty Acids in NAFLD Patients. Ann. Hepatol. 2020, 19, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Tamilmani, P.; Sathibabu Uddandrao, V.V.; Chandrasekaran, P.; Saravanan, G.; Brahma Naidu, P.; Sengottuvelu, S.; Vadivukkarasi, S. Linalool Attenuates Lipid Accumulation and Oxidative Stress in Metabolic Dysfunction-Associated Steatotic Liver Disease via Sirt1/Akt/PPRA-α/AMPK and Nrf-2/HO-1 Signaling Pathways. Clin. Res. Hepatol. Gastroenterol. 2023, 47, 102231. [Google Scholar] [CrossRef]
- Periyasamy, T.; Sathibabu Uddandrao, V.V.; Ponnusamy, C.; Ganapathy, S.; Pudhupalayam, S.P.; Singaravel, S.; Ponnusamy, P.; Ramasamy, J.; Aiyasamy, K.; Sasikumar, V. Linalool Mitigated High-Fat Diet–Induced Non-Alcoholic Fatty Liver Disease by Regulating the Intestinal-Hepatic Axis via TGF-β/NF-KB/TLR4/ZO-1 Pathway. Rev. Bras. Farmacogn. 2023, 33, 617–628. [Google Scholar] [CrossRef]
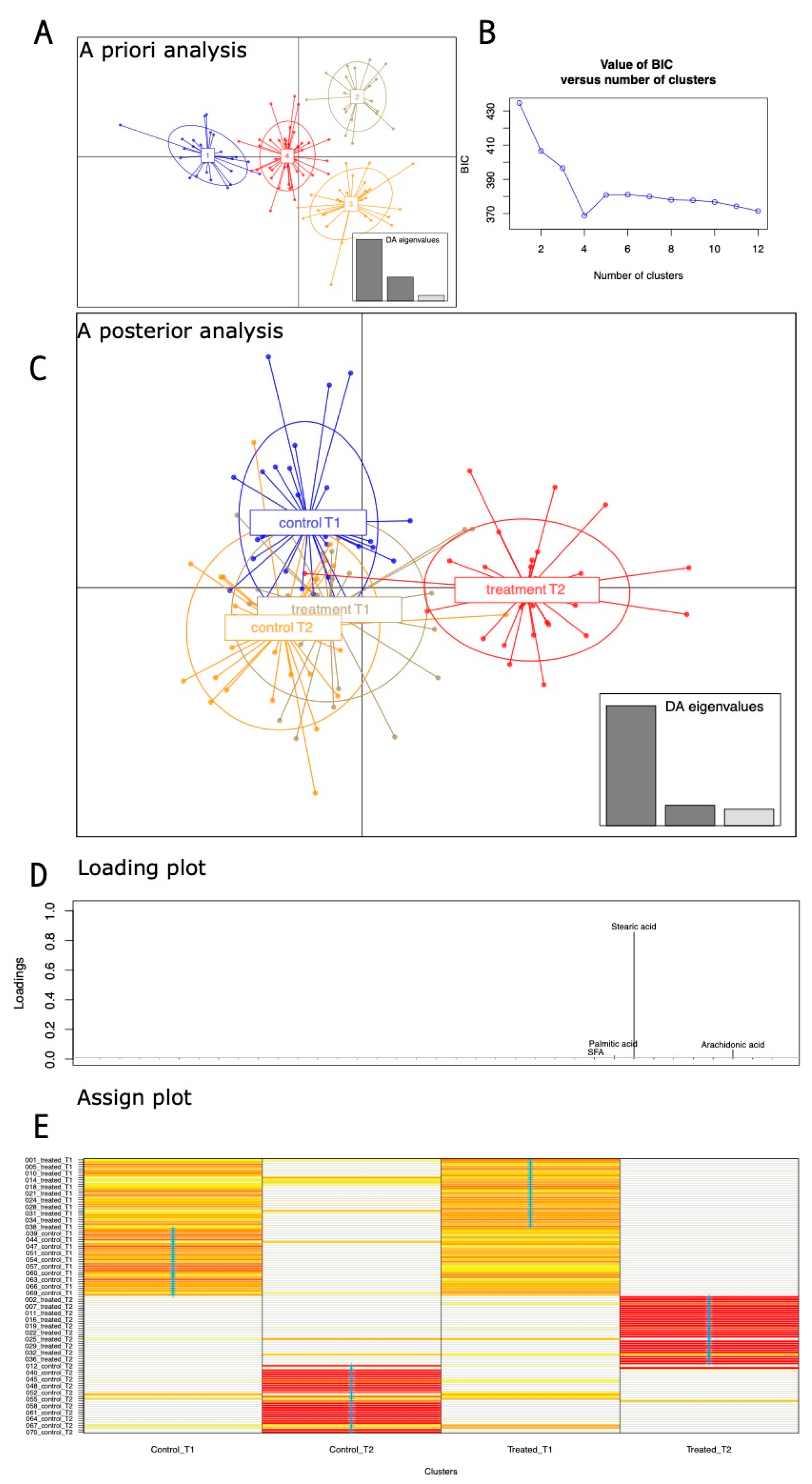
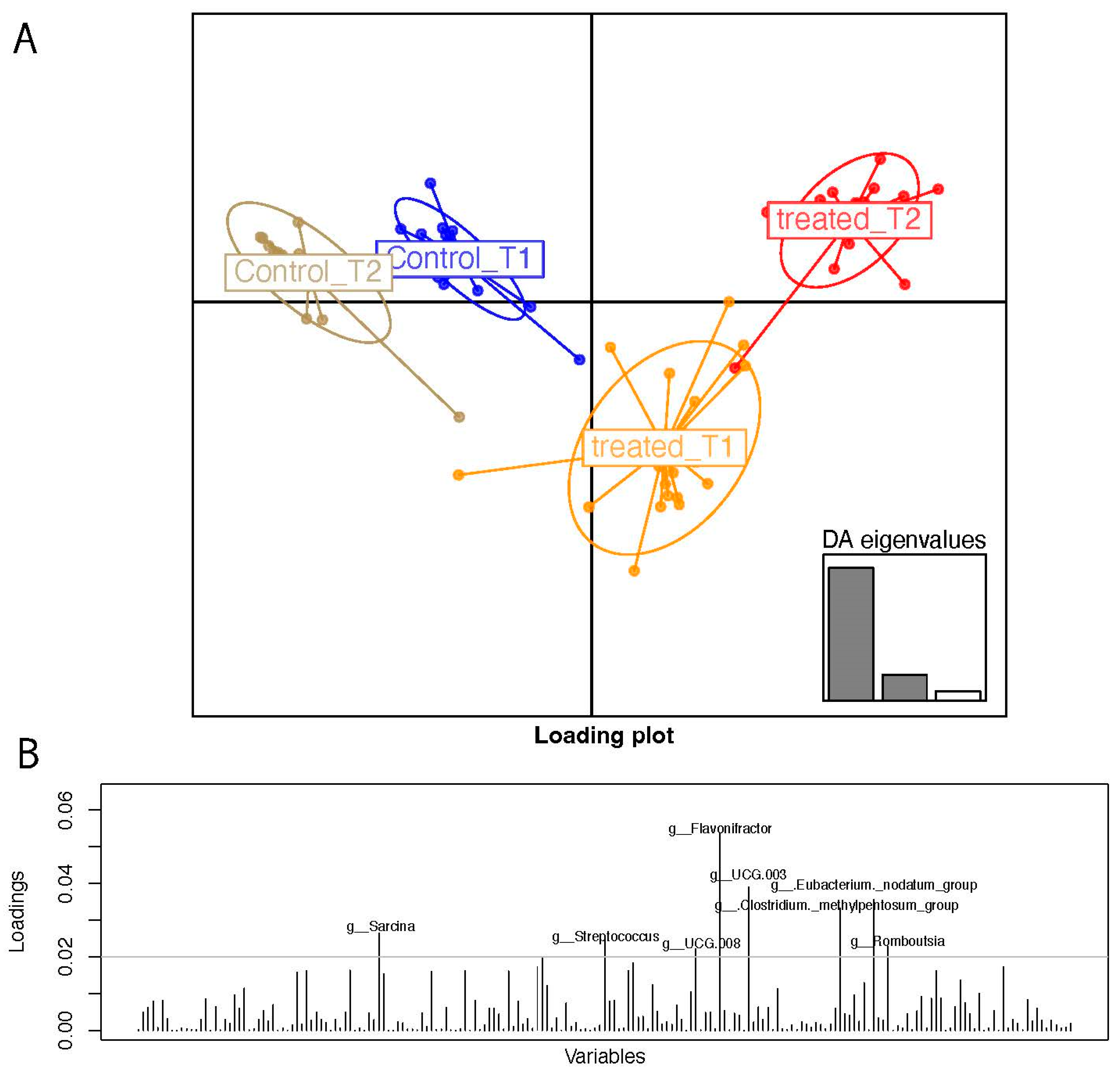
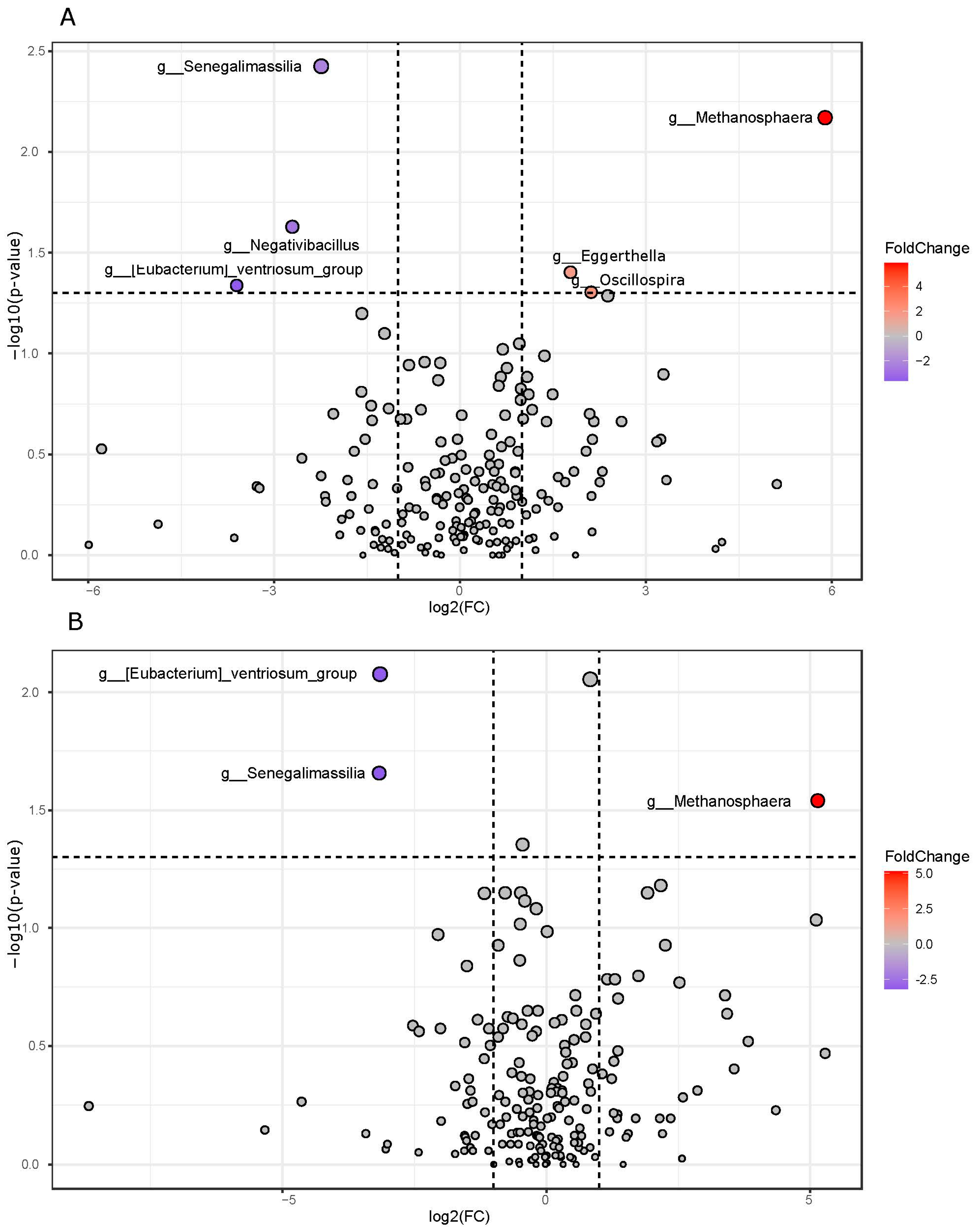

| Factor 1 | Factor 2 | Factor 3 | Uniqueness | |
|---|---|---|---|---|
| Height | −0.064699529 | 0.06826696 | 0.178581556 | 0.959115368 |
| Weight | 0.126617531 | 0.2365219 | 0.125839197 | 0.912321273 |
| BMI | 0.193744351 | 0.14582586 | −0.024936679 | 0.940697259 |
| Proteins | 0.285081134 | 0.23399605 | 0.47936257 | 0.63412446 |
| Lipids | 0.424481027 | 0.07006638 | 0.64060739 | 0.404533298 |
| Calorie | 0.506103266 | 0.35677247 | 0.044906034 | 0.614558334 |
| Proteins | −0.007605841 | 0.04698285 | 0.507746945 | 0.739936533 |
| Lipids | 0.236640486 | −0.1452059 | 0.807417816 | 0.270992434 |
| Carbohydrates | −0.19903003 | 0.11616393 | −0.970562583 | 0.005 |
| Amido | −0.001685977 | −0.0974902 | −0.530899533 | 0.708639495 |
| Sugars | 0.160694805 | 0.4711531 | −0.292088125 | 0.666855951 |
| Total fiber | 0.070447191 | 0.79506517 | −0.18020291 | 0.330408794 |
| Na | −0.023490989 | 0.4842104 | 0.017883766 | 0.764596798 |
| K | 0.113929299 | 0.64016249 | −0.000987283 | 0.577349561 |
| Iron | 0.104782206 | 0.45273011 | 0.104974903 | 0.773027662 |
| Calcium | 0.10083975 | 0.74665784 | −0.128002353 | 0.415941726 |
| Phosphorous | 0.069290061 | 0.77494489 | 0.002742983 | 0.394648949 |
| Thiamine | 0.150254483 | 0.70648018 | 0.163626428 | 0.451516532 |
| Riboflavin | 0.150915412 | 0.8769164 | 0.230477158 | 0.155121734 |
| Niacin | 0.076939901 | 0.6948258 | 0.246204482 | 0.450680114 |
| Vit.A. | 0.054915714 | 0.69734496 | 0.020818834 | 0.510275315 |
| Vit.C | 0.119471353 | 0.43628544 | 0.098776716 | 0.785623631 |
| Vit.E | 0.086494254 | 0.19081292 | 0.527066423 | 0.678332102 |
| Water | 0.074253403 | 0.82138447 | −0.022175257 | 0.319320812 |
| SFA | 0.98497784 | 0.141914 | 0.095471251 | 0.005 |
| Palmitic acid | 0.984976759 | 0.14174306 | 0.095671108 | 0.005 |
| Stearic acid | 0.984794254 | 0.14417705 | 0.093389265 | 0.005 |
| MUFA | 0.984955587 | 0.14201112 | 0.095558933 | 0.005 |
| Palmitoleic acid | 0.984548983 | 0.14297274 | 0.097210616 | 0.005 |
| Oleic acid | 0.984958489 | 0.14199322 | 0.095557386 | 0.005 |
| PUFA | 0.984973149 | 0.14202627 | 0.0953504 | 0.005 |
| Arachidonic acid | 0.984919328 | 0.14188511 | 0.09528714 | 0.005 |
| Eicosapentaeic acid | 0.984934595 | 0.14200122 | 0.095758523 | 0.005 |
| IPAQ | 0.190713702 | −0.0178699 | −0.018727683 | 0.962848331 |
| Group Comparison | URINE VOC | FC | log2 (FC) | P Adjusted | −LOG10 (p) |
| T2 treated vs. T2 control | Dodecane | 0.3338 | −1.583 | 0.036061 | 1.443 |
| T2 treated vs. T1 treated | Camphene | 0.19777 | 2.3381 | 0.010314 | 1.9866 |
| Phenol, 2-methoxy-4-(1-propenyl)- | 3.7734 | 1.9159 | 0.022719 | 1.6436 | |
| 2-Propanol, 2-methyl- | 0.43842 | −1.1896 | 0.022719 | 1.6436 | |
| Group Comparison | FECES VOC | FC | log2 (FC) | P adjusted | −LOG10 (p) |
| T2 treated vs. T2 controls | 1-Hexadecanol | 0.25404 | −1.9769 | 0.062675 | 1.2029 |
| 5-Hepten-2-one, 6-methyl- | 0.33325 | 1.5853 | 0.062675 | 1.2029 | |
| 2,6-Octadienal, 3,7-dimethyl-, (E)- | 0.334 | 1.5821 | 0.062675 | 1.2029 | |
| T2 treated vs. T1 treated | Ethanone, 1-(1-cyclohexen-1-yl)- | 0.11429 | −3.1292 | 0.0017474 | 2.7576 |
| 1H-Indole, 2-methyl- | 0.12487 | −3.0015 | 0.0033397 | 2.4763 | |
| Ketene | 0.25691 | −1.9606 | 0.0054678 | 2.2622 | |
| 5-Hepten-2-one, 6-methyl- | 0.49425 | 1.0167 | 0.0065457 | 2.184 | |
| 2-Propanamine, 2-methyl- | 0.33833 | −1.5635 | 0.016406 | 1.785 | |
| Methyl tetradecanoate | 0.28477 | −1.8121 | 0.025727 | 1.5896 | |
| Acetamide | 0.44592 | −1.1651 | 0.030388 | 1.5173 | |
| Phenol, 2,6-dimethyl- | 0.30373 | −1.7191 | 0.031394 | 1.5032 | |
| Dodecanoic acid, ethyl ester | 0.34307 | −1.5434 | 0.033506 | 1.4749 | |
| Mequinol | 0.45427 | −1.1384 | 0.03692 | 1.4327 | |
| Pyrazine, 2,6-dimethyl- | 0.011799 | −6.4052 | 0.039344 | 1.4051 | |
| Linalool | 0.3135 | 1.6735 | 0.048158 | 1.3173 | |
| Propanal, 2-methyl- | 0.39026 | −1.3575 | 0.048158 | 1.3173 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calabrese, F.M.; Aloisio Caruso, E.; De Nunzio, V.; Celano, G.; Pinto, G.; Cofano, M.; Sallustio, S.; Iacobellis, I.; Apa, C.A.; Santamaria, M.; et al. Metataxonomics and Metabolomics Profiles in Metabolic Dysfunction-Associated Fatty Liver Disease Patients on a “Navelina” Orange-Enriched Diet. Nutrients 2024, 16, 3543. https://doi.org/10.3390/nu16203543
Calabrese FM, Aloisio Caruso E, De Nunzio V, Celano G, Pinto G, Cofano M, Sallustio S, Iacobellis I, Apa CA, Santamaria M, et al. Metataxonomics and Metabolomics Profiles in Metabolic Dysfunction-Associated Fatty Liver Disease Patients on a “Navelina” Orange-Enriched Diet. Nutrients. 2024; 16(20):3543. https://doi.org/10.3390/nu16203543
Chicago/Turabian StyleCalabrese, Francesco Maria, Emanuela Aloisio Caruso, Valentina De Nunzio, Giuseppe Celano, Giuliano Pinto, Miriam Cofano, Stefano Sallustio, Ilaria Iacobellis, Carmen Aurora Apa, Monica Santamaria, and et al. 2024. "Metataxonomics and Metabolomics Profiles in Metabolic Dysfunction-Associated Fatty Liver Disease Patients on a “Navelina” Orange-Enriched Diet" Nutrients 16, no. 20: 3543. https://doi.org/10.3390/nu16203543
APA StyleCalabrese, F. M., Aloisio Caruso, E., De Nunzio, V., Celano, G., Pinto, G., Cofano, M., Sallustio, S., Iacobellis, I., Apa, C. A., Santamaria, M., Calasso, M., Giannelli, G., De Angelis, M., & Notarnicola, M. (2024). Metataxonomics and Metabolomics Profiles in Metabolic Dysfunction-Associated Fatty Liver Disease Patients on a “Navelina” Orange-Enriched Diet. Nutrients, 16(20), 3543. https://doi.org/10.3390/nu16203543






