Phenome-Wide Analysis of Coffee Intake on Health over 20 Years of Follow-Up Among Adults in Hong Kong Osteoporosis Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Habitual Coffee Intake
2.3. Health Outcomes
2.4. Statistical Analysis
3. Results
3.1. Sample Characteristics
3.2. Phenome-Wide Association Analysis
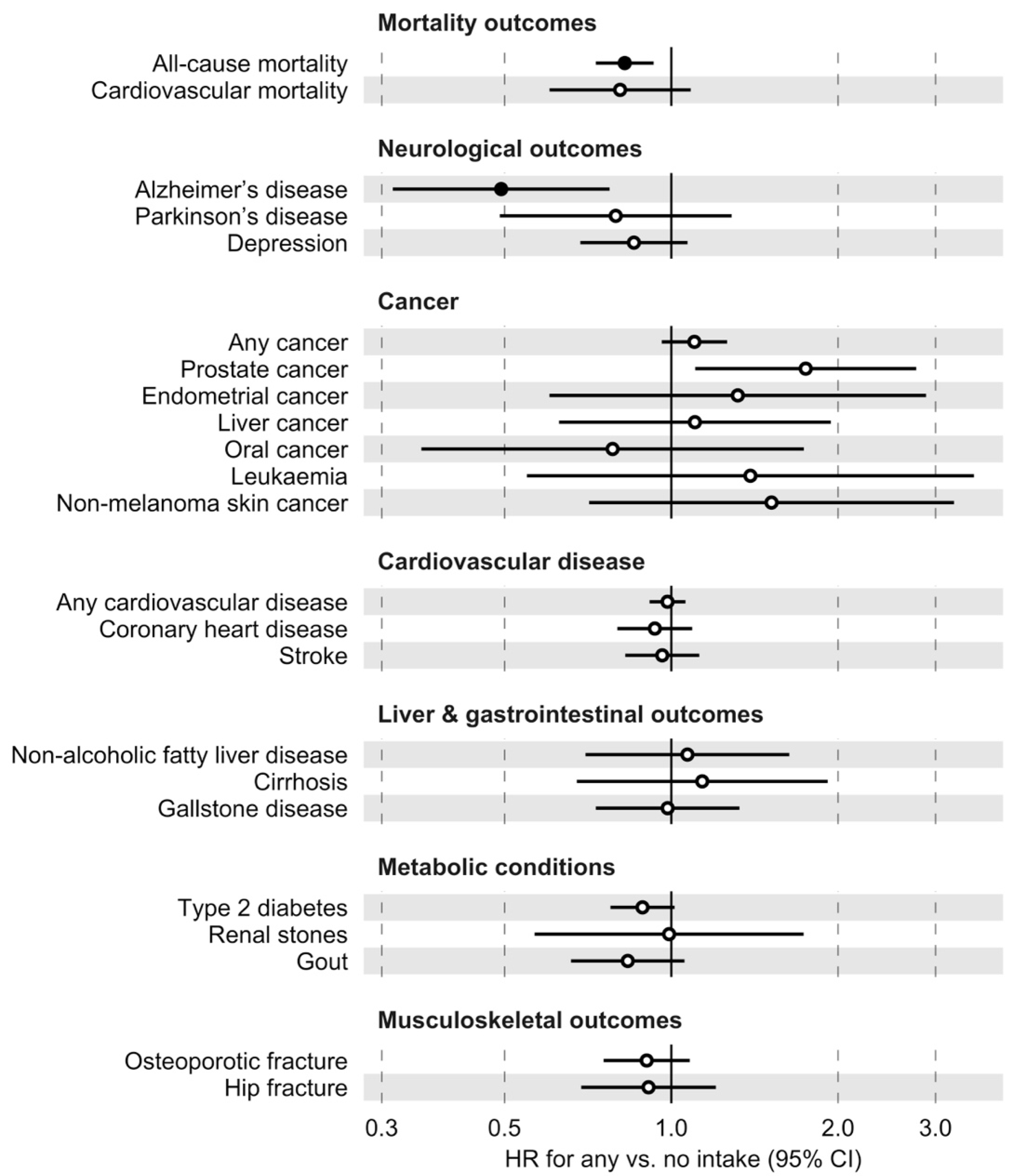
3.3. Selected Disease and Mortality Outcomes
3.4. Sensitivity Analysis
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Dam, R.M.; Hu, F.B.; Willett, W.C. Coffee, Caffeine, and Health. N. Engl. J. Med. 2020, 383, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Poole, R.; Kennedy, O.J.; Roderick, P.; Fallowfield, J.A.; Hayes, P.C.; Parkes, J. Coffee Consumption and Health: Umbrella Review of Meta-Analyses of Multiple Health Outcomes. BMJ 2017, 359, j5024. [Google Scholar] [CrossRef] [PubMed]
- Jee, S.H.; He, J.; Whelton, P.K.; Suh, I.; Klag, M.J. The Effect of Chronic Coffee Drinking on Blood Pressure: A Meta-Analysis of Controlled Clinical Trials. Hypertension 1999, 33, 647–652. [Google Scholar] [CrossRef]
- Hallström, H.; Byberg, L.; Glynn, A.; Lemming, E.W.; Wolk, A.; Michaëlsson, K. Long-Term Coffee Consumption in Relation to Fracture Risk and Bone Mineral Density in Women. Am. J. Epidemiol. 2013, 178, 898–909. [Google Scholar] [CrossRef]
- Liu, C.; Wang, L.; Zhang, C.; Hu, Z.; Tang, J.; Xue, J.; Lu, W. Caffeine Intake and Anxiety: A Meta-Analysis. Front. Psychol. 2024, 15, 1270246. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, I.A.; Clifford, M.N.; Lean, M.E.J.; Ashihara, H.; Crozier, A. Coffee: Biochemistry and Potential Impact on Health. Food Funct. 2014, 5, 1695–1717. [Google Scholar] [CrossRef]
- Rojas-González, A.; Figueroa-Hernández, C.Y.; González-Rios, O.; Suárez-Quiroz, M.L.; González-Amaro, R.M.; Hernández-Estrada, Z.J.; Rayas-Duarte, P. Coffee Chlorogenic Acids Incorporation for Bioactivity Enhancement of Foods: A Review. Molecules 2022, 27, 3400. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Wang, C.; Xu, J.; Wang, S. Cafestol and Kahweol: A Review on Their Bioactivities and Pharmacological Properties. Int. J. Mol. Sci. 2019, 20, 4238. [Google Scholar] [CrossRef]
- Ding, M.; Bhupathiraju, S.N.; Satija, A.; van Dam, R.M.; Hu, F.B. Long-Term Coffee Consumption and Risk of Cardiovascular Disease. Circulation 2014, 129, 643–659. [Google Scholar] [CrossRef]
- Chieng, D.; Canovas, R.; Segan, L.; Sugumar, H.; Voskoboinik, A.; Prabhu, S.; Ling, L.-H.; Lee, G.; Morton, J.B.; Kaye, D.M.; et al. The Impact of Coffee Subtypes on Incident Cardiovascular Disease, Arrhythmias, and Mortality: Long-Term Outcomes from the UK Biobank. Eur. J. Prev. Cardiol. 2022, 29, 2240–2249. [Google Scholar] [CrossRef]
- van Dam, R.M.; Hu, F.B. Coffee Consumption and Risk of Type 2 Diabetes: A Systematic Review. JAMA 2005, 294, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Orsini, N. Coffee Consumption and Risk of Stroke: A Dose-Response Meta-Analysis of Prospective Studies. Am. J. Epidemiol. 2011, 174, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.; Hong, C.-T.; Bai, C.-H. Coffee Consumption and the Risk of Cerebrovascular Disease: A Meta-Analysis of Prospective Cohort Studies. BMC Neurol. 2021, 21, 380. [Google Scholar] [CrossRef] [PubMed]
- Di Pietrantonio, D.; Pace Palitti, V.; Cichelli, A.; Tacconelli, S. Protective Effect of Caffeine and Chlorogenic Acids of Coffee in Liver Disease. Foods 2024, 13, 2280. [Google Scholar] [CrossRef] [PubMed]
- Torabynasab, K.; Shahinfar, H.; Payandeh, N.; Jazayeri, S. Association between Dietary Caffeine, Coffee, and Tea Consumption and Depressive Symptoms in Adults: A Systematic Review and Dose-Response Meta-Analysis of Observational Studies. Front. Nutr. 2023, 10, 1051444. [Google Scholar] [CrossRef]
- Hong, C.T.; Chan, L.; Bai, C.-H. The Effect of Caffeine on the Risk and Progression of Parkinson’s Disease: A Meta-Analysis. Nutrients 2020, 12, 1860. [Google Scholar] [CrossRef]
- Chen, J.Q.A.; Scheltens, P.; Groot, C.; Ossenkoppele, R. Associations Between Caffeine Consumption, Cognitive Decline, and Dementia: A Systematic Review. J. Alzheimers Dis. 2020, 78, 1519–1546. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, H.; Li, S.; Li, W.; Wang, Y. Consumption of Coffee and Tea and Risk of Developing Stroke, Dementia, and Poststroke Dementia: A Cohort Study in the UK Biobank. PLoS Med. 2021, 18, e1003830. [Google Scholar] [CrossRef]
- Zhao, L.-G.; Li, Z.-Y.; Feng, G.-S.; Ji, X.-W.; Tan, Y.-T.; Li, H.-L.; Gunter, M.J.; Xiang, Y.-B. Coffee Drinking and Cancer Risk: An Umbrella Review of Meta-Analyses of Observational Studies. BMC Cancer 2020, 20, 101. [Google Scholar] [CrossRef]
- Gunter, M.J.; Murphy, N.; Cross, A.J.; Dossus, L.; Dartois, L.; Fagherazzi, G.; Kaaks, R.; Kühn, T.; Boeing, H.; Aleksandrova, K.; et al. Coffee Drinking and Mortality in 10 European Countries. Ann. Intern. Med. 2017, 167, 236–247. [Google Scholar] [CrossRef]
- Simon, J.; Fung, K.; Raisi-Estabragh, Z.; Aung, N.; Khanji, M.Y.; Kolossváry, M.; Merkely, B.; Munroe, P.B.; Harvey, N.C.; Piechnik, S.K.; et al. Light to Moderate Coffee Consumption Is Associated with Lower Risk of Death: A UK Biobank Study. Eur. J. Prev. Cardiol. 2022, 29, 982–991. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Lee, J.E.; Loftfield, E.; Shu, X.-O.; Abe, S.K.; Rahman, M.S.; Saito, E.; Islam, M.R.; Tsugane, S.; Sawada, N.; et al. Coffee and Tea Consumption and Mortality from All Causes, Cardiovascular Disease and Cancer: A Pooled Analysis of Prospective Studies from the Asia Cohort Consortium. Int. J. Epidemiol. 2022, 51, 626–640. [Google Scholar] [CrossRef]
- Zeng, X.; Su, Y.; Tan, A.; Zou, L.; Zha, W.; Yi, S.; Lv, Y.; Kwok, T. The Association of Coffee Consumption with the Risk of Osteoporosis and Fractures: A Systematic Review and Meta-Analysis. Osteoporos. Int. 2022, 33, 1871–1893. [Google Scholar] [CrossRef]
- Li, J.; Zhao, H.; Song, J.-M.; Zhang, J.; Tang, Y.-L.; Xin, C.-M. A Meta-Analysis of Risk of Pregnancy Loss and Caffeine and Coffee Consumption during Pregnancy. Int. J. Gynecol. Obstet. 2015, 130, 116–122. [Google Scholar] [CrossRef]
- Greenwood, D.C.; Thatcher, N.J.; Ye, J.; Garrard, L.; Keogh, G.; King, L.G.; Cade, J.E. Caffeine Intake during Pregnancy and Adverse Birth Outcomes: A Systematic Review and Dose–Response Meta-Analysis. Eur. J. Epidemiol. 2014, 29, 725–734. [Google Scholar] [CrossRef]
- Treur, J.L.; Taylor, A.E.; Ware, J.J.; McMahon, G.; Hottenga, J.-J.; Baselmans, B.M.L.; Willemsen, G.; Boomsma, D.I.; Munafò, M.R.; Vink, J.M. Associations between Smoking and Caffeine Consumption in Two European Cohorts. Addiction 2016, 111, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- Treloar, H.R.; Piasecki, T.M.; McCarthy, D.E.; Baker, T.B. Relations Among Caffeine Consumption, Smoking, Smoking Urge, and Subjective Smoking Reinforcement in Daily Life. J. Caffeine Res. 2014, 4, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.-L.; Tan, K.C.B.; Kung, A.W.C. Cohort Profile: The Hong Kong Osteoporosis Study and the Follow-up Study. Int. J. Epidemiol. 2018, 47, 397–398f. [Google Scholar] [CrossRef]
- Hong Kong Hospital Authority. Hospital Authority Statistical Report (2016–2017); Hong Kong Hospital Authority: Hong Kong, China, 2018. [Google Scholar]
- Chau, Y.-P.; Au, P.C.M.; Li, G.H.Y.; Sing, C.-W.; Cheng, V.K.F.; Tan, K.C.B.; Kung, A.W.C.; Cheung, C.-L. Serum Metabolome of Coffee Consumption and Its Association with Bone Mineral Density: The Hong Kong Osteoporosis Study. J. Clin. Endocrinol. Metab. 2020, 105, e619–e627. [Google Scholar] [CrossRef]
- Denny, J.C.; Bastarache, L.; Ritchie, M.D.; Carroll, R.J.; Zink, R.; Mosley, J.D.; Field, J.R.; Pulley, J.M.; Ramirez, A.H.; Bowton, E.; et al. Systematic Comparison of Phenome-Wide Association Study of Electronic Medical Record Data and Genome-Wide Association Study Data. Nat. Biotechnol. 2013, 31, 1102–1111. [Google Scholar] [CrossRef]
- Wei, W.-Q.; Bastarache, L.A.; Carroll, R.J.; Marlo, J.E.; Osterman, T.J.; Gamazon, E.R.; Cox, N.J.; Roden, D.M.; Denny, J.C. Evaluating Phecodes, Clinical Classification Software, and ICD-9-CM Codes for Phenome-Wide Association Studies in the Electronic Health Record. PLoS ONE 2017, 12, e0175508. [Google Scholar] [CrossRef] [PubMed]
- Carroll, R.J.; Bastarache, L.; Denny, J.C. R PheWAS: Data Analysis and Plotting Tools for Phenome-Wide Association Studies in the R Environment. Bioinformatics 2014, 30, 2375–2376. [Google Scholar] [CrossRef] [PubMed]
- Miniño, A.M.; Klein, R.J. Mortality from Major Cardiovascular Diseases: United States. 2007. Available online: https://www.cdc.gov/nchs/data/hestat/cardio2007/cardio2007.htm (accessed on 10 September 2024).
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Mostofsky, E.; Johansen, M.B.; Lundbye-Christensen, S.; Tjønneland, A.; Mittleman, M.A.; Overvad, K. Risk of Atrial Fibrillation Associated with Coffee Intake: Findings from the Danish Diet, Cancer, and Health Study. Eur. J. Prev. Cardiol. 2016, 23, 922–930. [Google Scholar] [CrossRef]
- Bodar, V.; Chen, J.; Gaziano, J.M.; Albert, C.; Djoussé, L. Coffee Consumption and Risk of Atrial Fibrillation in the Physicians’ Health Study. J. Am. Heart Assoc. 2019, 8, e011346. [Google Scholar] [CrossRef]
- Surma, S.; Sahebkar, A.; Banach, M. Coffee or Tea: Anti-Inflammatory Properties in the Context of Atherosclerotic Cardiovascular Disease Prevention. Pharmacol. Res. 2023, 187, 106596. [Google Scholar] [CrossRef]
- Violi, F.; Pastori, D.; Pignatelli, P.; Loffredo, L. Antioxidants for Prevention of Atrial Fibrillation: A Potentially Useful Future Therapeutic Approach? A Review of the Literature and Meta-Analysis. EP Eur. 2014, 16, 1107–1116. [Google Scholar] [CrossRef]
- Ikram, M.; Park, T.J.; Ali, T.; Kim, M.O. Antioxidant and Neuroprotective Effects of Caffeine against Alzheimer’s and Parkinson’s Disease: Insight into the Role of Nrf-2 and A2AR Signaling. Antioxidants 2020, 9, 902. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Woolf, B.; Gill, D. Appraisal of the Causal Effect of Plasma Caffeine on Adiposity, Type 2 Diabetes, and Cardiovascular Disease: Two Sample Mendelian Randomisation Study. BMJ Med. 2023, 2, e000335. [Google Scholar] [CrossRef]
- Zagkos, L.; Cronjé, H.T.; Woolf, B.; de La Harpe, R.; Burgess, S.; Mantzoros, C.S.; Elliott, P.; Yuan, S.; Larsson, S.C.; Tzoulaki, I.; et al. Genetic Investigation into the Broad Health Implications of Caffeine: Evidence from Phenome-Wide, Proteome-Wide and Metabolome-Wide Mendelian Randomization. BMC Med. 2024, 22, 81. [Google Scholar] [CrossRef]
- Alfaro, T.M.; Monteiro, R.A.; Cunha, R.A.; Cordeiro, C.R. Chronic Coffee Consumption and Respiratory Disease: A Systematic Review. Clin. Respir. J. 2018, 12, 1283–1294. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Yang, H.; Ma, Y.; Zhou, L.; Lin, J.; Hou, Y.; Yu, B.; Wang, Y. Association of Coffee and Tea Consumption with Cardiovascular Disease, Chronic Respiratory Disease, and Their Comorbidity. Mol. Nutr. Food Res. 2022, 66, 2200419. [Google Scholar] [CrossRef] [PubMed]
- Welsh, E.J.; Bara, A.; Barley, E.; Cates, C.J. Caffeine for Asthma. Cochrane Database Syst. Rev. 2010, 2010, CD001112. [Google Scholar] [CrossRef]
- Lam, J.M.Y.; Siu, W.S.; Lam, T.S.; Cheung, N.K.; Graham, C.A.; Rainer, T.H. The Epidemiology of Patients with Dizziness in an Emergency Department. Hong Kong J. Emerg. Med. 2006, 13, 133–139. [Google Scholar] [CrossRef]
- Onakpoya, I.J.; Spencer, E.A.; Thompson, M.J.; Heneghan, C.J. The Effect of Chlorogenic Acid on Blood Pressure: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. J. Hum. Hypertens. 2015, 29, 77–81. [Google Scholar] [CrossRef]
- Grosso, G.; Micek, A.; Godos, J.; Pajak, A.; Sciacca, S.; Bes-Rastrollo, M.; Galvano, F.; Martinez-Gonzalez, M.A. Long-Term Coffee Consumption Is Associated with Decreased Incidence of New-Onset Hypertension: A Dose–Response Meta-Analysis. Nutrients 2017, 9, 890. [Google Scholar] [CrossRef]
- Langan, S.M.; Irvine, A.D.; Weidinger, S. Atopic Dermatitis. Lancet 2020, 396, 345–360. [Google Scholar] [CrossRef]
- Park, S.-Y.; Freedman, N.D.; Haiman, C.A.; Le Marchand, L.; Wilkens, L.R.; Setiawan, V.W. Association of Coffee Consumption with Total and Cause-Specific Mortality Among Nonwhite Populations. Ann. Intern. Med. 2017, 167, 228–235. [Google Scholar] [CrossRef]
- Shao, C.; Tang, H.; Wang, X.; He, J. Coffee Consumption and Stroke Risk: Evidence from a Systematic Review and Meta-Analysis of More than 2.4 Million Men and Women. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2021, 30, 105452. [Google Scholar] [CrossRef] [PubMed]
- Mostofsky, E.; Rice, M.S.; Levitan, E.B.; Mittleman, M.A. Habitual Coffee Consumption and Risk of Heart Failure: A Dose-Response Meta-Analysis. Circ. Heart Fail. 2012, 5, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Hu, G.; Caballero, B.; Appel, L.; Chen, L. Habitual Coffee Consumption and Risk of Hypertension: A Systematic Review and Meta-Analysis of Prospective Observational Studies. Am. J. Clin. Nutr. 2011, 93, 1212–1219. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, M.C.; Byrne, E.M.; Esko, T.; Nalls, M.A.; Ganna, A.; Paynter, N.; Monda, K.L.; Amin, N.; Fischer, K.; Renstrom, F.; et al. Genome-Wide Meta-Analysis Identifies Six Novel Loci Associated with Habitual Coffee Consumption. Mol. Psychiatry 2015, 20, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Sing, C.-W.; Woo, Y.-C.; Lee, A.C.H.; Lam, J.K.Y.; Chu, J.K.P.; Wong, I.C.K.; Cheung, C.-L. Validity of Major Osteoporotic Fracture Diagnosis Codes in the Clinical Data Analysis and Reporting System in Hong Kong. Pharmacoepidemiol. Drug Saf. 2017, 26, 973–976. [Google Scholar] [CrossRef] [PubMed]
- Kwok, W.C.; Tam, T.C.C.; Sing, C.W.; Chan, E.W.Y.; Cheung, C.-L. Validation of Diagnostic Coding for Asthma in an Electronic Health Record System in Hong Kong. J. Asthma Allergy 2023, 16, 315–321. [Google Scholar] [CrossRef]
- Tverdal, A.; Selmer, R.; Cohen, J.M.; Thelle, D.S. Coffee Consumption and Mortality from Cardiovascular Diseases and Total Mortality: Does the Brewing Method Matter? Eur. J. Prev. Cardiol. 2020, 27, 1986–1993. [Google Scholar] [CrossRef]

| Characteristic | Overall (n = 7420) | Women (n = 5355) | Men (n = 2065) |
|---|---|---|---|
| Age, mean (SD) | 53.2 (16.8) | 51.2 (16.3) | 58.2 (16.9) |
| Body mass index, mean (SD) | 22.8 (3.6) | 22.6 (3.7) | 23.3 (3.3) |
| Ever-smoker, n (%) | 916 (12.3) | 250 (4.7) | 666 (32.3) |
| Ever-alcohol drinker, n (%) | 849 (11.4) | 302 (5.6) | 547 (26.5) |
| Education level, n (%) | |||
| Primary or below | 2414 (32.5) | 1899 (35.5) | 515 (24.9) |
| Secondary | 2983 (40.2) | 2122 (39.6) | 861 (41.7) |
| College or university | 2023 (27.3) | 1334 (24.9) | 689 (33.4) |
| Coffee intake, cup/day, mean (SD) | 0.21 (0.47) | 0.18 (0.42) | 0.29 (0.57) |
| Non-coffee drinker, n (%) | 4006 (54.0) | 2978 (55.6) | 1028 (49.8) |
| ≤1 cup of coffee per day, n (%) | 3215 (43.3) | 2283 (42.6) | 932 (45.1) |
| >1 cup of coffee per day, n (%) | 199 (2.7) | 94 (1.8) | 105 (5.1) |
| Died during follow-up, n (%) | 1558 (21.0) | 964 (18.0) | 594 (28.8) |
| Phecode | Description | N a | Incident Cases | HR (95% CI) b | p-Value |
|---|---|---|---|---|---|
| Mental disorders | |||||
| 290 | Delirium dementia and amnestic and other cognitive disorders | 6936 | 255 | 0.56 (0.40, 0.78) | 5.8 × 10−4 |
| 290.1 | Dementias | 6933 | 250 | 0.57 (0.41, 0.80) | 1.1 × 10−3 |
| Sense organs | |||||
| 386.9 | Dizziness and giddiness (light-headedness and vertigo) | 7212 | 943 | 0.77 (0.66, 0.89) | 3.2 × 10−4 |
| Circulatory system | |||||
| 427.2 | Atrial fibrillation and flutter | 6816 | 481 | 0.67 (0.54, 0.83) | 2.9 × 10−4 |
| 427.21 | Atrial fibrillation | 6803 | 467 | 0.67 (0.54, 0.83) | 3.4 × 10−4 |
| Respiratory | |||||
| 465 | Acute upper respiratory infections of multiple or unspecified sites | 7284 | 588 | 0.68 (0.57, 0.81) | 2.3 × 10−5 |
| 512 | Other symptoms of respiratory system | 7388 | 533 | 0.68 (0.56, 0.83) | 1.2 × 10−4 |
| 512.2 | Painful respiration | 7027 | 152 | 0.43 (0.29, 0.63) | 2.2 × 10−5 |
| Symptoms | |||||
| 783 | Fever of unknown origin | 7398 | 776 | 0.73 (0.62, 0.86) | 1.5 × 10−4 |
| Dermatologic | |||||
| 939 | Atopic/contact dermatitis due to other or unspecified origins | 7261 | 167 | 0.53 (0.38, 0.75) | 3.5 × 10−4 |
| Subgroup | N | Deaths | HR (95% CI) a | p-Value |
|---|---|---|---|---|
| Full sample | 7420 | 1558 | 0.82 (0.73, 0.93) | 0.002 |
| Women | 5355 | 964 | 0.82 (0.70, 0.97) | 0.019 |
| Men | 2065 | 594 | 0.81 (0.68, 0.98) | 0.026 |
| Age < 60 years | 4498 | 184 | 0.95 (0.70, 1.29) | 0.74 |
| Age ≥ 60 years | 2922 | 1374 | 0.80 (0.70, 0.91) | 7.5 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mak, J.K.L.; Chau, Y.-P.; Tan, K.C.-B.; Kung, A.W.-C.; Cheung, C.-L. Phenome-Wide Analysis of Coffee Intake on Health over 20 Years of Follow-Up Among Adults in Hong Kong Osteoporosis Study. Nutrients 2024, 16, 3536. https://doi.org/10.3390/nu16203536
Mak JKL, Chau Y-P, Tan KC-B, Kung AW-C, Cheung C-L. Phenome-Wide Analysis of Coffee Intake on Health over 20 Years of Follow-Up Among Adults in Hong Kong Osteoporosis Study. Nutrients. 2024; 16(20):3536. https://doi.org/10.3390/nu16203536
Chicago/Turabian StyleMak, Jonathan K. L., Yin-Pan Chau, Kathryn Choon-Beng Tan, Annie Wai-Chee Kung, and Ching-Lung Cheung. 2024. "Phenome-Wide Analysis of Coffee Intake on Health over 20 Years of Follow-Up Among Adults in Hong Kong Osteoporosis Study" Nutrients 16, no. 20: 3536. https://doi.org/10.3390/nu16203536
APA StyleMak, J. K. L., Chau, Y.-P., Tan, K. C.-B., Kung, A. W.-C., & Cheung, C.-L. (2024). Phenome-Wide Analysis of Coffee Intake on Health over 20 Years of Follow-Up Among Adults in Hong Kong Osteoporosis Study. Nutrients, 16(20), 3536. https://doi.org/10.3390/nu16203536






