Macronutrients in Human Milk and Early Childhood Growth—Is Protein the Main Driver?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Sample Collection
2.2. Macronutrient Measurements
2.3. Calculation of Total Energy, Protein:energy Ratio and Estimated Macronutrient Intake
2.4. Statistical Analysis
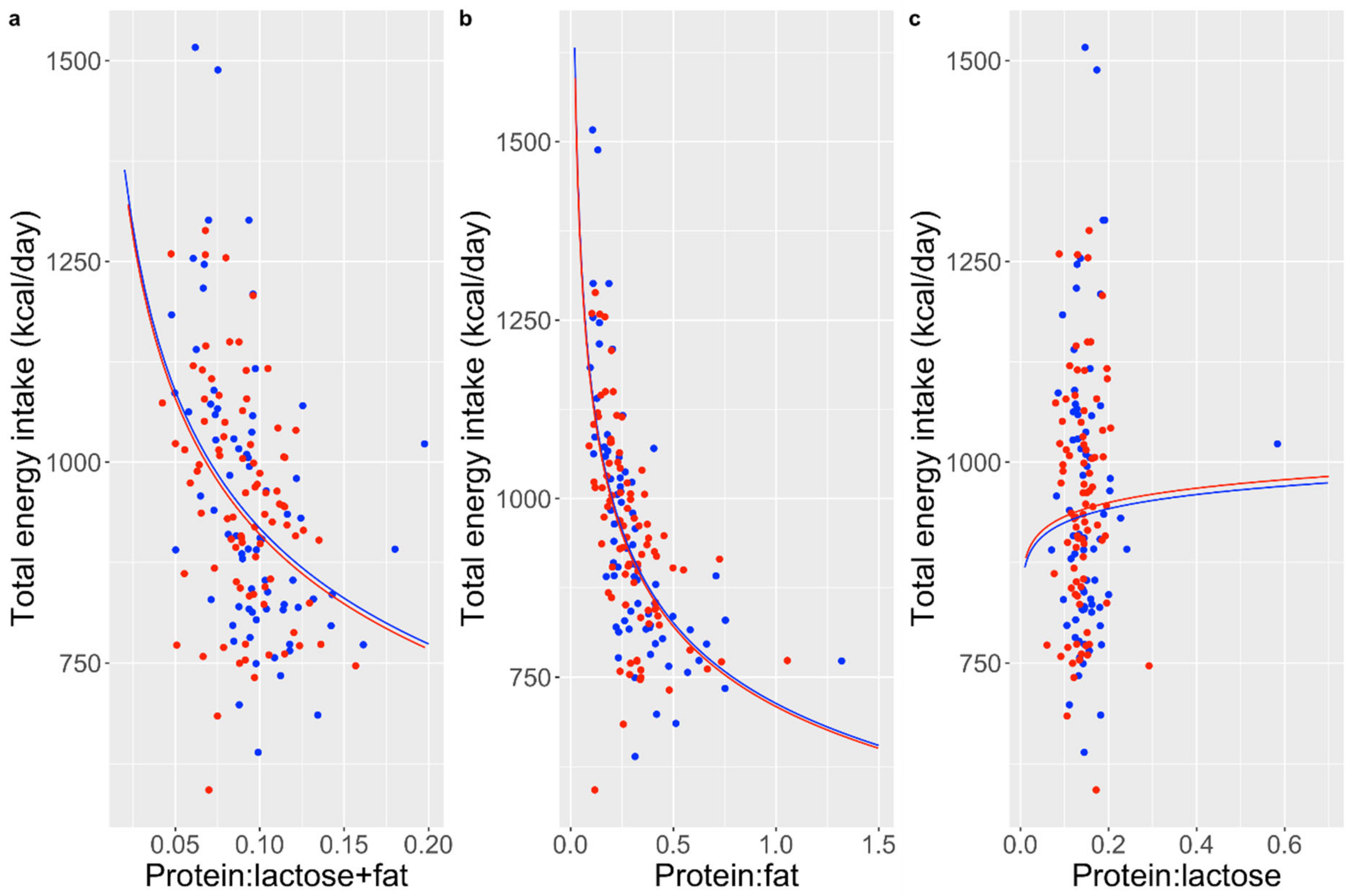
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Anthropometrics from 3 Months to 2.5 Years b | Birth z Scores | Estimate | SE | p-Value |
|---|---|---|---|---|
| BMIAZ | BMIAZ | 0.1590 a | 0.055 | 0.004 |
| ΔBMIAZ | BMIAZ | −0.016 | 0.040 | 0.682 |
| HCAZ | HCAZ | 0.205 | 0.035 | <0.001 |
| ΔHCAZ | HCAZ | −0.010 | 0.023 | 0.668 |
| LAZ | LAZ | 0.192 | 0.057 | 0.001 |
| ΔLAZ | LAZ | −0.048 | 0.038 | 0.215 |
| WAZ | WAZ | 0.354 | 0.061 | <0.001 |
| ΔWAZ | WAZ | 0.931 | 0.271 | 0.001 |
References
- Joglekar, C.V.; Fall, C.H.D.; Deshpande, V.U.; Joshi, N.; Bhalerao, A.; Solat, V.; Deokar, T.M.; Chougule, S.D.; Leary, S.D.; Osmond, C.; et al. Newborn Size, Infant and Childhood Growth, and Body Composition and Cardiovascular Disease Risk Factors at the Age of 6 Years: The Pune Maternal Nutrition Study. Int. J. Obes. 2007, 31, 1534–1544. [Google Scholar] [CrossRef]
- Marinkovic, T.; Toemen, L.; Kruithof, C.J.; Reiss, I.; Van Osch-Gevers, L.; Hofman, A.; Franco, O.H.; Jaddoe, V.W.V. Early Infant Growth Velocity Patterns and Cardiovascular and Metabolic Outcomes in Childhood. J. Pediatr. 2017, 186, 57–63.e4. [Google Scholar] [CrossRef]
- Mook-Kanamori, D.O.; Durmuş, B.; Sovio, U.; Hofman, A.; Raat, H.; Steegers, E.A.P.; Jarvelin, M.-R.; Jaddoe, V.W.V. Fetal and Infant Growth and the Risk of Obesity during Early Childhood: The Generation R Study. Eur. J. Endocrinol. 2011, 165, 623–630. [Google Scholar] [CrossRef]
- Mosca, F.; Giannì, M.L. Human Milk: Composition and Health Benefits. Pediatr. Med. Chir. 2017, 39, 155. [Google Scholar] [CrossRef]
- Andreas, N.J.; Kampmann, B.; Mehring Le-Doare, K. Human Breast Milk: A Review on Its Composition and Bioactivity. Early Hum. Dev. 2015, 91, 629–635. [Google Scholar] [CrossRef]
- Perrella, S.; Gridneva, Z.; Lai, C.T.; Stinson, L.; George, A.; Bilston-John, S.; Geddes, D. Human Milk Composition Promotes Optimal Infant Growth, Development and Health. Semin. Perinatol. 2021, 45, 151380. [Google Scholar] [CrossRef]
- Norrish, I.; Sindi, A.; Sakalidis, V.S.; Lai, C.T.; McEachran, J.L.; Tint, M.T.; Perrella, S.L.; Nicol, M.P.; Gridneva, Z.; Geddes, D.T. Relationships between the Intakes of Human Milk Components and Body Composition of Breastfed Infants: A Systematic Review. Nutrients 2023, 15, 2370. [Google Scholar] [CrossRef]
- Gridneva, Z.; Rea, A.; Tie, W.J.; Lai, C.T.; Kugananthan, S.; Ward, L.C.; Murray, K.; Hartmann, P.E.; Geddes, D.T. Carbohydrates in Human Milk and Body Composition of Term Infants during the First 12 Months of Lactation. Nutrients 2019, 11, 1472. [Google Scholar] [CrossRef]
- Lönnerdal, B.; Forsum, E.; Hambraeus, L. A Longitudinal Study of the Protein, Nitrogen, and Lactose Contents of Human Milk from Swedish Well-Nourished Mothers. Am. J. Clin. Nutr. 1976, 29, 1127–1133. [Google Scholar] [CrossRef]
- Butte, N.F.; Garza, C.; Smith, E.O.; Nichols, B.L. Human Milk Intake and Growth in Exclusively Breast-Fed Infants. J. Pediatr. 1984, 104, 187–195. [Google Scholar] [CrossRef]
- Butte, N.F.; Wong, W.W.; Hopkinson, J.M.; Smith, E.O.; Ellis, K.J. Infant Feeding Mode Affects Early Growth and Body Composition. Pediatrics 2000, 106, 1355–1366. [Google Scholar] [CrossRef]
- Ballard, O.; Morrow, A.L. Human Milk Composition. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef]
- Fay, S.H.; Finlayson, G.S.; King, N.A. Diet-Induced Obesity: When Does Consumption Become Overconsumption? Curr. Obes. Rep. 2013, 2, 104–106. [Google Scholar] [CrossRef]
- Simpson, S.J.; Batley, R.; Raubenheimer, D. Geometric Analysis of Macronutrient Intake in Humans: The Power of Protein? Appetite 2003, 41, 123–140. [Google Scholar] [CrossRef]
- Simpson, S.J.; Raubenheimer, D. Obesity: The Protein Leverage Hypothesis. Obes. Rev. 2005, 6, 133–142. [Google Scholar] [CrossRef]
- Hill, C.M.; Morrison, C.D. The Protein Leverage Hypothesis: A 2019 Update for Obesity. Obesity 2019, 27, 1221. [Google Scholar] [CrossRef]
- Theall, C.L.; Wurtman, J.J.; Wurtman, R.J. Self-Selection and Regulation of Protein:Carbohydrate Ratio in Foods Adult Rats Eat. J. Nutr. 1984, 114, 711–718. [Google Scholar] [CrossRef]
- Tews, J.K.; Repa, J.J.; Harper, A.E. Protein Selection by Rats Adapted to High or Moderately Low Levels of Dietary Protein. Physiol. Behav. 1992, 51, 699–712. [Google Scholar] [CrossRef]
- Embleton, N.D. Optimal Protein and Energy Intakes in Preterm Infants. Early Hum. Dev. 2007, 83, 831–837. [Google Scholar] [CrossRef]
- Bhatia, J. Human Milk and the Premature Infant. Ann. Nutr. Metab. 2013, 62, 8–14. [Google Scholar] [CrossRef]
- Uruakpa, F.O.; Ismond, M.A.H.; Akobundu, E.N.T. Colostrum and Its Benefits: A Review. Nutr. Res. 2002, 22, 755–767. [Google Scholar] [CrossRef]
- Gidrewicz, D.A.; Fenton, T.R. A Systematic Review and Meta-Analysis of the Nutrient Content of Preterm and Term Breast Milk. BMC Pediatr. 2014, 14, 216. [Google Scholar] [CrossRef]
- Meldrum, S.J.; D’Vaz, N.; Dunstan, J.; Mori, T.A.; Prescott, S.L. The Infant Fish Oil Supplementation Study (IFOS): Design and Research Protocol of a Double-Blind, Randomised Controlled N−3 LCPUFA Intervention Trial in Term Infants. Contemp. Clin. Trials 2011, 32, 771–778. [Google Scholar] [CrossRef]
- D’Vaz, N.; Meldrum, S.J.; Dunstan, J.A.; Martino, D.; McCarthy, S.; Metcalfe, J.; Tulic, M.K.; Mori, T.A.; Prescott, S.L. Postnatal Fish Oil Supplementation in High-Risk Infants to Prevent Allergy: Randomized Controlled Trial. Pediatrics 2012, 130, 674–682. [Google Scholar] [CrossRef]
- D’vaz, N.; Amarasekera, M.; Dunstan, J.; Meldrum, S.; Lee-Pullen, T.; Metcalfe, J.; Holt, B.; Serralha, M.; Tulic, M.; Mori, T.; et al. Basic and Clinical Immunology—3020. Fish Oil Supplementation in Early Infancy Modulates Developing Infant Immune Responses but Not Clinical Allergy. World Allergy Organ. J. 2013, 6, P196. [Google Scholar] [CrossRef]
- Meldrum, S.J.; Heaton, A.E.; Foster, J.K.; Prescott, S.L.; Simmer, K. Do Infants of Breast-Feeding Mothers Benefit from Additional Long-Chain PUFA from Fish Oil? A 6-Year Follow-Up. Br. J. Nutr. 2020, 124, 701–708. [Google Scholar] [CrossRef]
- Wang, C.D.; Chu, P.S.; Mellen, B.G.; Shenai, J.P. Creamatocrit and the Nutrient Composition of Human Milk. J. Perinatol. 1999, 19, 343–346. [Google Scholar] [CrossRef]
- Lucas, A.; Gibbs, J.A.; Lyster, R.L.; Baum, J.D. Creamatocrit: Simple Clinical Technique for Estimating Fat Concentration and Energy Value of Human Milk. BMJ 1978, 1, 1018–1020. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Atwood, C.S.; Hartmann, P.E. Collection of Fore and Hind Milk from the Sow and the Changes in Milk Composition during Suckling. J. Dairy Res. 1992, 59, 287–298. [Google Scholar] [CrossRef]
- Arthur, P.G.; Smith, M.; Hartmann, P.E. Milk Lactose, Citrate, and Glucose as Markers of Lactogenesis in Normal and Diabetic Women. J. Pediatr. Gastroenterol. Nutr. 1989, 9, 488–496. [Google Scholar] [CrossRef]
- Perrin, M.T.; Belfort, M.B.; Hagadorn, J.I.; McGrath, J.M.; Taylor, S.N.; Tosi, L.M.; Brownell, E.A. The Nutritional Composition and Energy Content of Donor Human Milk: A Systematic Review. Adv. Nutr. 2020, 11, 960–970. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Nutrient Requirements and Dietary Intakes of Infants and Young Children in the European Union. EFSA J. 2013, 11, 3408. [Google Scholar] [CrossRef]
- Suwaydi, M.A.; Lai, C.T.; Gridneva, Z.; Perrella, S.L.; Wlodek, M.E.; Geddes, D.T. Sampling Procedures for Estimating the Infant Intake of Human Milk Leptin, Adiponectin, Insulin, Glucose, and Total Lipid. Nutrients 2024, 16, 331. [Google Scholar] [CrossRef]
- WHO. WHO Child Growth Standards: Methods and Development; World Health Organization: Geneva, Switzerland, 2007; ISBN 92-4-068719-X. [Google Scholar]
- Beck, C.; Koplin, J.; Dharmage, S.; Wake, M.; Gurrin, L.; McWilliam, V.; Tang, M.; Sun, C.; Foskey, R.; Allen, K.J.; et al. Persistent Food Allergy and Food Allergy Coexistent with Eczema Is Associated with Reduced Growth in the First 4 Years of Life. J. Allergy Clin. Immunol. Pract. 2016, 4, 248–256.e3. [Google Scholar] [CrossRef]
- Massarano, A.A.; Hollis, S.; Devlin, J.; David, T.J. Growth in Atopic Eczema. Arch. Dis. Child. 1993, 68, 677–679. [Google Scholar] [CrossRef]
- Raubenheimer, D.; Simpson, S.J. Protein Leverage: Theoretical Foundations and Ten Points of Clarification. Obesity 2019, 27, 1225–1238. [Google Scholar] [CrossRef]
- Kon, I.Y.; Shilina, N.M.; Gmoshinskaya, M.V.; Ivanushkina, T.A. The Study of Breast Milk IGF-1, Leptin, Ghrelin and Adiponectin Levels as Possible Reasons of High Weight Gain in Breast-Fed Infants. Ann. Nutr. Metab. 2014, 65, 317–323. [Google Scholar] [CrossRef]
- Cheema, A.S.; Stinson, L.F.; Rea, A.; Lai, C.T.; Payne, M.S.; Murray, K.; Geddes, D.T.; Gridneva, Z. Human Milk Lactose, Insulin, and Glucose Relative to Infant Body Composition during Exclusive Breastfeeding. Nutrients 2021, 13, 3724. [Google Scholar] [CrossRef]
- Gosby, A.K.; Conigrave, A.D.; Lau, N.S.; Iglesias, M.A.; Hall, R.M.; Jebb, S.A.; Brand-Miller, J.; Caterson, I.D.; Raubenheimer, D.; Simpson, S.J. Testing Protein Leverage in Lean Humans: A Randomised Controlled Experimental Study. PLoS ONE 2011, 6, e25929. [Google Scholar] [CrossRef]
- Martens, E.A.; Lemmens, S.G.; Westerterp-Plantenga, M.S. Protein Leverage Affects Energy Intake of High-Protein Diets in Humans. Am. J. Clin. Nutr. 2013, 97, 86–93. [Google Scholar] [CrossRef]
- Saner, C.; Senior, A.M.; Zhang, H.; Eloranta, A.-M.; Magnussen, C.G.; Sabin, M.A.; Juonala, M.; Janner, M.; Burgner, D.P.; Schwab, U.; et al. Evidence for Protein Leverage in a General Population Sample of Children and Adolescents. Eur. J. Clin. Nutr. 2023, 77, 652–659. [Google Scholar] [CrossRef]
- Olga, L.; Vervoort, J.; Van Diepen, J.A.; Gross, G.; Petry, C.J.; Prentice, P.M.; Chichlowski, M.; Van Tol, E.A.F.; Hughes, I.A.; Dunger, D.B.; et al. Associations between Breast Milk Intake Volume, Macronutrient Intake and Infant Growth in a Longitudinal Birth Cohort: The Cambridge Baby Growth and Breastfeeding Study (CBGS-BF). Br. J. Nutr. 2023, 130, 56–64. [Google Scholar] [CrossRef]
- Fomon, S.J.; Nelson, S.E. Body Composition of the Male and Female Reference Infants. Annu. Rev. Nutr. 2002, 22, 1–17. [Google Scholar] [CrossRef]
- Salli, K.; Anglenius, H.; Hirvonen, J.; Hibberd, A.A.; Ahonen, I.; Saarinen, M.T.; Tiihonen, K.; Maukonen, J.; Ouwehand, A.C. The Effect of 2′-Fucosyllactose on Simulated Infant Gut Microbiome and Metabolites; a Pilot Study in Comparison to GOS and Lactose. Sci. Rep. 2019, 9, 13232. [Google Scholar] [CrossRef]
- Firrman, J.; Liu, L.; Mahalak, K.; Hu, W.; Bittinger, K.; Moustafa, A.; Jones, S.M.; Narrowe, A.; Tomasula, P. An in Vitro Analysis of How Lactose Modifies the Gut Microbiota Structure and Function of Adults in a Donor-Independent Manner. Front. Nutr. 2023, 9, 1040744. [Google Scholar] [CrossRef]
- Coppa, G.V.; Gabrielli, O.; Pierani, P.; Catassi, C.; Carlucci, A.; Giorgi, P.L. Changes in Carbohydrate Composition in Human Milk Over 4 Months of Lactation. Pediatrics 1993, 91, 637–641. [Google Scholar] [CrossRef]
- Brockway, M.; Daniel, A.I.; Reyes, S.M.; Granger, M.; McDermid, J.M.; Chan, D.; Refvik, R.; Sidhu, K.K.; Musse, S.; Patel, P.P.; et al. Human Milk Macronutrients and Child Growth and Body Composition in the First Two Years: A Systematic Review. Adv. Nutr. 2024, 15, 100149. [Google Scholar] [CrossRef]
- Kobayashi, K.; Kuki, C.; Oyama, S.; Kumura, H. Pro-Inflammatory Cytokine TNF-α Is a Key Inhibitory Factor for Lactose Synthesis Pathway in Lactating Mammary Epithelial Cells. Exp. Cell Res. 2016, 340, 295–304. [Google Scholar] [CrossRef]
- Agostoni, C.; Marangoni, F.; Grandi, F.; Lammardo, A.M.; Giovannini, M.; Riva, E.; Galli, C. Earlier Smoking Habits Are Associated with Higher Serum Lipids and Lower Milk Fat and Polyunsaturated Fatty Acid Content in the First 6 Months of Lactation. Eur. J. Clin. Nutr. 2003, 57, 1466–1472. [Google Scholar] [CrossRef]
- Bachour, P.; Yafawi, R.; Jaber, F.; Choueiri, E.; Abdel-Razzak, Z. Effects of Smoking, Mother’s Age, Body Mass Index, and Parity Number on Lipid, Protein, and Secretory Immunoglobulin A Concentrations of Human Milk. Breastfeed. Med. 2012, 7, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Hascoët, J.-M.; Chauvin, M.; Pierret, C.; Skweres, S.; Van Egroo, L.-D.; Rougé, C.; Franck, P. Impact of Maternal Nutrition and Perinatal Factors on Breast Milk Composition after Premature Delivery. Nutrients 2019, 11, 366. [Google Scholar] [CrossRef] [PubMed]
- Burianova, I.; Bronsky, J.; Pavlikova, M.; Janota, J.; Maly, J. Maternal Body Mass Index, Parity and Smoking Are Associated with Human Milk Macronutrient Content after Preterm Delivery. Early Hum. Dev. 2019, 137, 104832. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | |
|---|---|
| Maternal age (years) (n = 161) | 33.2 ± 4.1 a |
| Parity (n = 161) | 1.8 ± 0.9 |
| Ethnicity (n = 157) | |
| Caucasian | 146 (93.0%) |
| Asian | 7 (4.5%) |
| Other | 4 (2.5%) |
| Birth gestation (weeks) (n = 150) | 39.2 ± 1.2 |
| Maternal atopy (n = 161) | 152 (94.4%) |
| Age breastfeeding stopped (months) (n = 151) | 11.9 ± 5.4 |
| Age solid foods commenced (months) (n = 154) | 5.4 ± 0.7 |
| Infant eczema diagnosed by age of 1 year (n = 147) | 93 (63.3%) |
| Parameters | Birth | 3 Months | 6 Months | 1 Year | 2.5 Years |
|---|---|---|---|---|---|
| Weight (kg) | 3.47 ± 0.42 a | 6.25 ± 0.75 | 7.78 ± 0.88 | 9.91 ± 1.12 | 14.1 ± 1.68 |
| WAZ | 0.058 ± 0.13 | 0.027 ± 0.11 | 0.026 ± 0.11 | 0.071 ± 0.11 | 0.088 ± 0.13 |
| Length/height (cm) | 50.6 ± 2.28 | 61.0 ± 2.25 | 66.8 ± 2.19 | 76.0 ± 3.26 | 92.7 ± 4.02 |
| LAZ | 0.022 ± 0.046 | 0.007 ± 0.036 | 0.003 ± 0.031 | 0.016 ± 0.043 | 0.015 ± 0.044 |
| HC (cm) | 34.9 ± 1.94 | 41.1 ± 1.47 | 43.8 ± 1.41 | 46.8 ± 1.48 | 50.1 ± 1.58 |
| HCAZ | 0.021 ± 0.057 | 0.029 ± 0.033 | 0.025 ± 0.029 | 0.031 ± 0.028 | 0.036 ± 0.030 |
| BMI (kg/m2) | 13.6 ± 1.43 | 16.8 ± 1.54 | 17.4 ± 1.47 | 17.2 ± 1.35 | 16.4 ± 1.38 |
| BMIAZ | 0.016 ± 0.110 | 0.014 ± 0.089 | 0.018 ± 0.084 | 0.038 ± 0.078 | 0.050 ± 0.087 |
| Macronutrients | Concentration (g or kcal/L) | Intake (g or kcal/Day) |
|---|---|---|
| Protein | 11.0 ± 2.55 a | 8.77 ± 2.04 |
| Fat | 43.6 ± 20.3 | 34.9 ± 16.2 |
| Lactose | 76.9 ± 9.50 | 61.5 ± 7.60 |
| Energy | 744 ± 184 | 595 ± 147 |
| Protein:energy ratio | - | 6.16% ± 1.81% |
| Fat:energy ratio | - | 50.3% ± 11.8% |
| Lactose:energy ratio | - | 43.5% ± 10.6% |
| Macronutrients | Predictors | Estimate | SE | Predictor p-Value b | ANOVA p-Value c |
|---|---|---|---|---|---|
| Energy | Not exposed to passive smoke | 75.276 a | 32.522 | 0.022 | - |
| Fat | Not exposed to passive smoke | 8.035 | 3.536 | 0.025 | - |
| Fat:energy ratio | Birth season summer | 0.060 | 0.030 | 0.046 | 0.177 |
| Lactose | Birth season summer | −5.400 | 2.506 | 0.033 | 0.029 |
| Maternal atopy | −8.851 | 4.468 | 0.050 * | - | |
| Lactose:energy ratio | Not exposed to passive smoke | −0.037 | 0.018 | 0.046 | - |
| Protein:energy ratio | Birth season autumn | −0.010 | 0.005 | 0.031 | 0.084 |
| Birth season summer | −0.010 | 0.005 | 0.038 | 0.084 |
| Infant Growth Parameters | Time | Macronutrients | Estimate | SE | Predictor p-Value d | ANOVA p-Value e |
|---|---|---|---|---|---|---|
| ΔBMIAZ | 6 months to 1 year b | Protein:energy ratio | 0.021 a | 0.009 | 0.023 | 0.047 |
| 1 year to 2.5 years b | Lactose intake | −0.027 | 0.012 | 0.025 | 0.051 | |
| 1 year to 2.5 years b | Energy intake | −0.021 | 0.010 | 0.031 | 0.054 | |
| 1 year to 2.5 years b | Fat intake | −0.020 | 0.010 | 0.043 | 0.096 | |
| 6 months to 1 year b | Protein intake | 0.019 | 0.010 | 0.046 | 0.094 | |
| HCAZ | 1 year c | Fat energy ratio | 0.005 | 0.002 | 0.027 | 0.178 |
| 1 year c | Lactose energy ratio | −0.005 | 0.002 | 0.028 | 0.182 | |
| 1 year c | Energy intake | 0.005 | 0.002 | 0.030 | 0.186 | |
| LAZ | 2.5 years c | Protein intake | 0.007 | 0.003 | 0.032 | 0.192 |
| 2.5 years c | Protein:energy ratio | 0.006 | 0.003 | 0.045 | 0.212 | |
| WAZ | 2.5 years c | Protein:energy ratio | 0.021 | 0.009 | 0.015 | 0.022 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Palmer, D.J.; Lai, C.T.; Prescott, S.L.; D’Vaz, N.; Vlaskovsky, P.; Stinson, L.F.; Gridneva, Z.; Geddes, D.T. Macronutrients in Human Milk and Early Childhood Growth—Is Protein the Main Driver? Nutrients 2024, 16, 3514. https://doi.org/10.3390/nu16203514
Ma J, Palmer DJ, Lai CT, Prescott SL, D’Vaz N, Vlaskovsky P, Stinson LF, Gridneva Z, Geddes DT. Macronutrients in Human Milk and Early Childhood Growth—Is Protein the Main Driver? Nutrients. 2024; 16(20):3514. https://doi.org/10.3390/nu16203514
Chicago/Turabian StyleMa, Jie, Debra J. Palmer, Ching Tat Lai, Susan L. Prescott, Nina D’Vaz, Philip Vlaskovsky, Lisa F. Stinson, Zoya Gridneva, and Donna T. Geddes. 2024. "Macronutrients in Human Milk and Early Childhood Growth—Is Protein the Main Driver?" Nutrients 16, no. 20: 3514. https://doi.org/10.3390/nu16203514
APA StyleMa, J., Palmer, D. J., Lai, C. T., Prescott, S. L., D’Vaz, N., Vlaskovsky, P., Stinson, L. F., Gridneva, Z., & Geddes, D. T. (2024). Macronutrients in Human Milk and Early Childhood Growth—Is Protein the Main Driver? Nutrients, 16(20), 3514. https://doi.org/10.3390/nu16203514






