Development of a Diabetes Dietary Quality Index: Reproducibility and Associations with Measures of Insulin Resistance, Beta Cell Function, and Hyperglycemia
Abstract
1. Introduction
2. Materials and Methods
2.1. Questionnaire
2.2. Intake Assessment
2.3. Population
2.4. Screening Visit
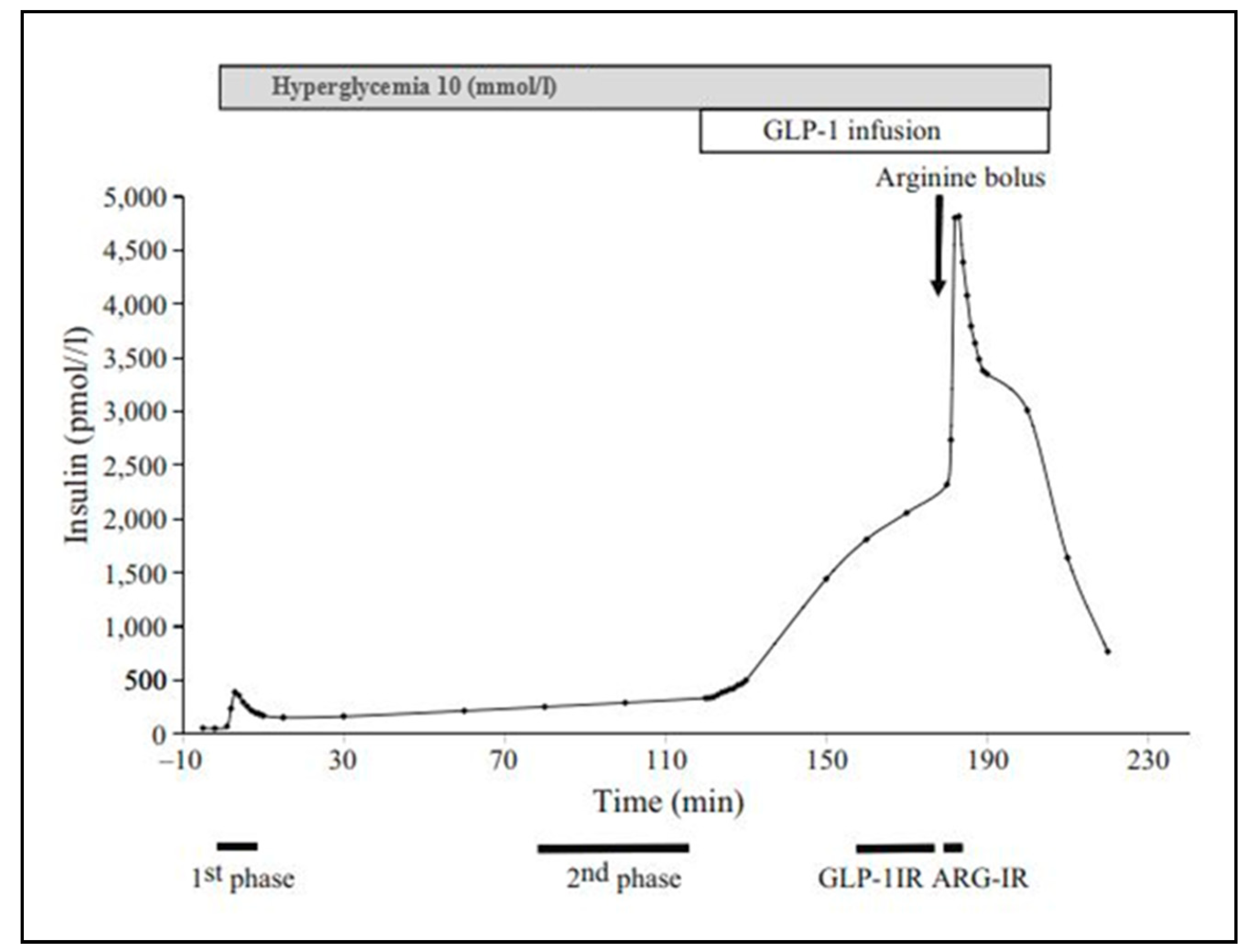
2.4.1. Mixed Meal Test
2.4.2. Clamp
2.4.3. Laboratory Analysis
2.5. Insulin Sensitivity and Beta Cell Function Parameters During MMT
2.6. Insulin Sensitivity and Beta Cell Function Parameters During Clamp
2.7. Scoring
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lindström, J.; Peltonen, M.; Eriksson, J.G.; Ilanne-Parikka, P.; Aunola, S.; Keinänen-Kiukaanniemi, S.; Uusitupa, M.; Tuomilehto, J. Improved Lifestyle and Decreased Diabetes Risk Over 13 years: Long-Term Follow-Up of the Randomised Finnish Diabetes Prevention Study (DPS). Diabetologia 2013, 56, 284–293. [Google Scholar] [CrossRef]
- Knowler, W.C.; Fowler, S.E.; Hamman, R.F.; Christophi, C.A.; Hoffman, H.J.; Brenneman, A.T.; Brown-Friday, J.O.; Goldberg, R.; Venditti, E.; Nathan, D.M. 10-Year Follow-Up of Diabetes Incidence and Weight Loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009, 374, 1677–1686. [Google Scholar] [PubMed]
- Sumamo Schellenberg, E.; Dryden, D.M.; Vandermeer, B.; Ha, C.; Korownyk, C. Lifestyle Interventions for Patients with and at Risk for Type 2 Diabetes: A Systematic Review and Meta-Analysis. Ann. Intern. Med. 2013, 159, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Ley, S.H.; Hamdy, O.; Mohan, V.; Hu, F.B. Prevention and Management of Type 2 Diabetes: Dietary Components and Nutritional Strategies. Lancet 2014, 383, 1999–2007. [Google Scholar] [CrossRef] [PubMed]
- England, C.Y.; Andrews, R.C.; Jago, R.; Thompson, J.L. A Systematic Review of Brief Dietary Questionnaires Suitable for Clinical use in the Prevention and Management of Obesity, Cardiovascular Disease and Type 2 Diabetes. Eur. J. Clin. Nutr. 2015, 69, 977–1003. [Google Scholar] [CrossRef] [PubMed]
- England, C.Y.; Thompson, J.L.; Jago, R.; Cooper, A.R.; Andrews, R.C. Development of a Brief, Reliable and Valid Diet Assessment Tool for Impaired Glucose Tolerance and Diabetes: The UK Diabetes and Diet Questionnaire. Public Health Nutr. 2017, 20, 191–199. [Google Scholar] [CrossRef]
- Van Dam, R.M.; Rimm, E.B.; Willett, W.C.; Stampfer, M.J.; Hu, F.B. Dietary Patterns and Risk for Type 2 Diabetes Mellitus in U.S. Men. Ann. Intern. Med. 2002, 136, 201–209. [Google Scholar] [CrossRef]
- Keijzers, G.B.; De Galan, B.E.; Tack, C.J.; Smits, P. Caffeine can Decrease Insulin Sensitivity in Humans. Diabetes Care 2002, 25, 364–369. [Google Scholar] [CrossRef]
- van Dam, R.M. Coffee Consumption and the Decreased Risk of Diabetes Mellitus Type 2. Ned. Tijdschr. Voor Geneeskd. 2006, 150, 1821–1825. [Google Scholar]
- Salazar-Martinez, E.; Willett, W.C.; Ascherio, A.; Manson, J.E.; Leitzmann, M.F.; Stampfer, M.J.; Hu, F.B. Coffee Consumption and Risk for Type 2 Diabetes Mellitus. Ann. Intern. Med. 2004, 140, 1–8. [Google Scholar] [CrossRef]
- Greenberg, J.A.; Axen, K.V.; Schnoll, R.; Boozer, C.N. Coffee, Tea and Diabetes: The Role of Weight Loss and Caffeine. Int. J. Obes. 2005, 29, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Van Dam, R.M.; Willett, W.C.; Manson, J.E.; Hu, F.B. Coffee, Caffeine, and Risk of Type 2 Diabetes: A Prospective Cohort Study in Younger and Middle-Aged U.S. Women. Diabetes Care 2006, 29, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Kiechl, S.; Willeit, J.; Poewe, W.; Egger, G.; Oberhollenzer, F.; Muggeo, M.; Bonora, E. Insulin Sensitivity and Regular Alcohol Consumption: Large, Prospective, Cross Sectional Population Study (Bruneck Study). BMJ 1996, 313, 1040–1044. [Google Scholar] [CrossRef] [PubMed]
- Koppes, L.L.J.; Dekker, J.M.; Hendriks, H.F.J.; Bouter, L.M.; Heine, R.J. Moderate Alcohol Consumption Lowers the Risk of Type 2 Diabetes: A Meta-Analysis of Prospective Observational Studies. Diabetes Care 2005, 28, 719–725. [Google Scholar] [CrossRef]
- Howard, A.A.; Arnsten, J.H.; Gourevitch, M.N. Effect of Alcohol Consumption on Diabetes Mellitus: A Systematic Review. Ann. Intern. Med. 2004, 140, 211–219. [Google Scholar] [CrossRef]
- Weickert, M.O.; Möhlig, M.; Schöfl, C.; Arafat, A.M.; Otto, B.; Viehoff, H.; Koebnick, C.; Kohl, A.; Spranger, J.; Pfeiffer, A.F.H. Cereal Fiber Improves Whole-Body Insulin Sensitivity in Overweight and Obese Women. Diabetes Care 2006, 29, 775–780. [Google Scholar] [CrossRef]
- Meyer, K.A.; Kushi, L.H.; Jacobs, D.R.; Folsom, A.R. Dietary Fat and Incidence of Type 2 Diabetes in Older Iowa Women. Diabetes Care 2001, 24, 1528–1535. [Google Scholar] [CrossRef]
- Delarue, J.; Lefoll, C.; Corporeau, C.; Lucas, D. N-3 Long Chain Polyunsaturated Fatty Acids: A Nutritional Tool to Prevent Insulin Resistance Associated to Type 2 Diabetes and Obesity? Reprod. Nutr. Dev. 2004, 44, 289–299. [Google Scholar] [CrossRef]
- Van Dam, R.M.; Willett, W.C.; Rimm, E.B.; Stampfer, M.J.; Hu, F.B. Dietary Fat and Meat Intake in Relation to Risk of Type 2 Diabetes in Men. Diabetes Care 2002, 25, 417–424. [Google Scholar] [CrossRef]
- Fung, T.T.; Schulze, M.; Manson, J.E.; Willett, W.C.; Hu, F.B. Dietary Patterns, Meat Intake, and the Risk of Type 2 Diabetes in Women. Arch. Intern. Med. 2004, 164, 2235–2240. [Google Scholar] [CrossRef]
- Rifas-Shiman, S.L.; Willett, W.C.; Lobb, R.; Kotch, J.; Dart, C.; Gillman, M.W. PrimeScreen, a Brief Dietary Screening Tool: Reproducibility and Comparability with both a Longer Food Frequency Questionnaire and Biomarkers. Public Health Nutr. 2001, 4, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Ocke, M.C.; Bas Bueno-de-Mesquita, H.; Goddijn, H.E.; Jansen, A.; Pols, M.A.; Van Staveren, W.A.; Kromhout, D. The Dutch EPIC Food Frequency Questionnaire. I. Description of the Questionnaire, and Relative Validity and Reproducibility for Food Groups. Int. J. Epidemiol. 1997, 26, S37–S48. [Google Scholar] [CrossRef] [PubMed]
- Feunekes, G.; Van Staveren, W.; De Vries, J.; Burema, J.; Hautvast, J. Relative and Biomarker-Based Validity of a Food-Frequency Questionnaire Estimating Intake of Fats and Cholesterol. Am. J. Clin. Nutr. 1993, 58, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Stichting Nederlands Voedingsstoffenbestand. NEVO-Tabel: Nederlands Voedingsstoffenbestand; Voedingscentrum: Den Haag, The Netherlands, 2006. [Google Scholar]
- Simonis-Bik, A.M.C.; Boomsma, D.I.; Dekker, J.M.; Diamant, M.; de Geus, E.J.C.; ’t Hart, L.M.; Heine, R.J.; Kramer, M.H.H.; Maassen, J.A.; Mari, A.; et al. The Heritability of Beta Cell Function Parameters in a Mixed Meal Test Design. Diabetologia 2011, 54, 1043–1051. [Google Scholar] [CrossRef][Green Version]
- Bik, A.M.C. Genetic Influences on Β-Cell Function: A Dutch Twin-Family Study. 2010, pp. 31–43. Available online: http://www.tweelingenregister.org/fileadmin/user_upload/publicaties/verslaggeving/proefschriften/Simonis-Bik_2010.pdf (accessed on 23 January 2020).
- Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycemia. Report of a WHO 2006; WHO: Geneva, Switzerland, 2006; ISBN 9241594934. Available online: https://iris.who.int/bitstream/handle/10665/43588/9241594934_eng.pdf?sequence=1 (accessed on 23 November 2010).
- Mari, A.; Ferrannini, E. Β-Cell Function Assessment from Modelling of Oral Tests: An Effective Approach. Diabetes Obes. Metab. 2008, 10, 77–87. [Google Scholar] [CrossRef]
- Mari, A.; Pacini, G.; Brazzale, A.R.; Ahren, B. Comparative Evaluation of Simple Insulin Sensitivity Methods Based on the Oral Glucose Tolerance Test. Diabetologia 2005, 48, 748–751. [Google Scholar] [CrossRef]
- van Lee, L.; Geelen, A.; van Huysduynen, E.J.C.H.; de Vries, J.H.M.; van’t Veer, P.; Feskens, E.J.M. The Dutch Healthy Diet Index (DHD-Index): An Instrument to Measure Adherence to the Dutch Guidelines for a Healthy Diet. Nutr. J. 2012, 11, 49. [Google Scholar] [CrossRef]
- van Dieren, S.; Uiterwaal, C.S.P.M.; van der Schouw, Y.T.; van der A, D.L.; Boer, J.M.A.; Spijkerman, A.; Grobbee, D.E.; Beulens, J.W.J. Coffee and Tea Consumption and Risk of Type 2 Diabetes. Diabetologia 2009, 52, 2561–2569. [Google Scholar] [CrossRef]
- Bendinelli, B.; Palli, D.; Masala, G.; Sharp, S.J.; Schulze, M.B.; Guevara, M.; van der A, D.L.; Sera, F.; Amiano, P.; Balkau, B.; et al. Association between Dietary Meat Consumption and Incident Type 2 Diabetes: The EPIC-InterAct Study. Diabetologia 2013, 56, 47–59. [Google Scholar]
- de Vet, H.C.W.; Terwee, C.B.; Knol, D.L.; Bouter, L.M. When to use Agreement Versus Reliability Measures. J. Clin. Epidemiol. 2006, 59, 1033–1039. [Google Scholar] [CrossRef]
- Willett, W.C. Nutritional Epidemiology, 2nd ed.; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Thompson, F.E.; Midthune, D.; Williams, G.C.; Yaroch, A.L.; Hurley, T.G.; Resnicow, K.; Hebert, J.R.; Toobert, D.J.; Greene, G.W.; Peterson, K.; et al. Evaluation of a Short Dietary Assessment Instrument for Percentage Energy from Fat in an Intervention Study. J. Nutr. 2008, 13 8, 193S–199S. [Google Scholar] [CrossRef]
- Block, G.; Gillespie, C.; Rosenbaum, E.H.; Jenson, C. A Rapid Food Screener to Assess Fat and Fruit and Vegetable Intake. Am. J. Prev. Med. 2000, 18, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.; Eissenstat, B.; Jaax, S.; Srinath, U.; Scott, L.; Rader, J.; Pearson, T. Validation for MEDFICTS, a Dietary Assessment Instrument for Evaluating Adherence to Total and Saturated Fat Recommendations of the National Cholesterol Education Program Step 1 and Step 2 Diets. J. Am. Diet. Assoc. 2001, 101, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Kraschnewski, J.L.; Gold, A.D.; Gizlice, Z.; Johnston, L.F.; Garcia, B.A.; Samuel-Hodge, C.D.; Keyserling, T.C. Development and Evaluation of a Brief Questionnaire to Assess Dietary Fat Quality in Low-Income Overweight Women in the Southern United States. J. Nutr. Educ. Behav. 2013, 45, 355–361. [Google Scholar] [CrossRef]
- Thompson, F.E.; Kipnis, V.; Subar, A.F.; Krebs-Smith, S.M.; Kahle, L.L.; Midthune, D.; Potischman, N.; Schatzkin, A. Evaluation of 2 Brief Instruments and a Food-Frequency Questionnaire to Estimate Daily Number of Servings of Fruit and Vegetables. Am. J. Clin. Nutr. 2000, 71, 1503–1510. [Google Scholar] [CrossRef]
- Traynor, M.M.; Holowaty, P.H.; Reid, D.J.; Gray-Donald, K. Vegetable and Fruit Food Frequency Questionnaire Serves as a Proxy for Quantified Intake. Can. J. Public Health 2006, 97, 286–290. [Google Scholar] [CrossRef]
- Van Assema, P.; Brug, J.; Ronda, G.; Steenhuis, I.; Oenema, A. A Short Dutch Questionnaire to Measure Fruit and Vegetable Intake: Relative Validity among Adults and Adolescents. Nutr. Health 2002, 16, 85–106. [Google Scholar] [CrossRef]
- Ling, A.M.C.; Horwath, C.; Parnell, W. Validation of a Short Food Frequency Questionnaire to Assess Consumption of Cereal Foods, Fruit and Vegetables in Chinese Singaporeans. Eur. J. Clin. Nutr. 1998, 52, 557–564. [Google Scholar] [CrossRef]
- Landais, E.; Gartner, A.; Bour, A.; McCullough, F.; Delpeuch, F.; Holdsworth, M. Reproducibility and Relative Validity of a Brief Quantitative Food Frequency Questionnaire for Assessing Fruit and Vegetable Intakes in North-African Women. J. Hum. Nutr. Diet. 2014, 27, 152–159. [Google Scholar] [CrossRef]
- Schröder, H.; Fitó, M.; Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventós, R.; Ros, E.; Salaverría, I.; Fiol, M.; et al. A Short Screener is Valid for Assessing Mediterranean Diet Adherence among Older Spanish Men and Women. J. Nutr. 2011, 141, 1140–1145. [Google Scholar] [CrossRef]
- Crozier, S.R.; Inskip, H.M.; Barker, M.E.; Lawrence, W.T.; Cooper, C.; Robinson, S.M. Development of a 20-Item Food Frequency Questionnaire to Assess a ‘prudent’ Dietary Pattern among Young Women in Southampton. Eur. J. Clin. 2010, 64, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Lin, K.; Chen, H.; Wu, Y.; Chang, C.; Shin, S.; Hung, H.; Lee, C.; Huang, Y.; Hsu, C. Validity of a Short Food Frequency Questionnaire Assessing Macronutrient and Fiber Intakes in Patients of Han Chinese Descent with Type 2 Diabetes. Int. J. Environ. Res. Public Health 2018, 15, 1142. [Google Scholar] [CrossRef] [PubMed]
- Akohoue, S.A.; Wallston, K.A.; Schlundt, D.G.; Rothman, R.L. Psychometric Evaluation of the Short Version of the Personal Diabetes Questionnaire to Assess Dietary Behaviors and Exercise in Patients with Type 2 Diabetes. Eat. Behav. Int. J. 2017, 26, 182–188. [Google Scholar] [CrossRef]
- van Lee, L.; Feskens, E.J.M.; Hooft van Huysduynen, E.J.C.; de Vries, J.H.M.; van ‘t Veer, P.; Geelen, A. The Dutch Healthy Diet Index as Assessed by 24 h Recalls and FFQ: Associations with Biomarkers from a Cross-Sectional Study. J. Nutr. Sci. 2013, 2, e40. [Google Scholar] [CrossRef]
- Fung, T.T.; McCullough, M.; van Dam, R.M.; Hu, F.B. Prospective Study of overall Diet Quality and Risk of Type 2 Diabetes in Women. Diabetes Care 2007, 30, 1753–1757. [Google Scholar] [CrossRef]
- McCullough, M.L.; Feskanich, D.; Stampfer, M.J.; Giovannucci, E.L.; Rimm, E.B.; Hu, F.B.; Spiegelman, D.; Hunter, D.J.; Colditz, G.A.; Willett, W.C. Diet Quality and Major Chronic Disease Risk in Men and Women: Moving Toward Improved Dietary Guidance. Am. J. Clin. Nutr. 2002, 76, 1261–1271. [Google Scholar] [CrossRef]
- Fogli-Cawley, J.J.; Dwyer, J.T.; Saltzman, E.; McCullough, M.L.; Troy, L.M.; Meigs, J.B.; Jacques, P.F. 2005 Dietary Guidelines for Americans and Risk of the Metabolic Syndrome. Am. J. Clin. Nutr. 2007, 86, 1193–1201. [Google Scholar] [CrossRef]
- Flock, M.R.; Kris-Etherton, P.M. Dietary Guidelines for Americans 2010: Implications for Cardiovascular Disease. Curr. Atheroscler. Rep. 2011, 13, 499–507. [Google Scholar] [CrossRef]
- García-Fernández, E.; Rico-Cabanas, L.; Rosgaard, N.; Estruch, R.; Bach-Faig, A. Mediterranean Diet and Cardiodiabesity: A Review. Nutrients 2014, 6, 3474–3500. [Google Scholar] [CrossRef]
- Shah, B.S.; Freeland-Graves, J.H.; Cahill, J.M.; Lu, H.; Graves, G.R. Diet Quality as Measured by the Healthy Eating Index and the Association with Lipid Profile in Low-Income Women in Early Postpartum. J. Am. Diet. Assoc. 2010, 110, 274–279. [Google Scholar] [CrossRef]
- Diabetes Prevention Program Research Group. HbA1c as a Predictor of Diabetes and as an Outcome in the Diabetes Prevention Program: A Randomized Clinical Trial. Diabetes Care 2015, 38, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Rijkelijkhuizen, J.M.; Girman, C.J.; Mari, A.; Alssema, M.; Rhodes, T.; Nijpels, G.; Kostense, P.J.; Stein, P.P.; Eekhoff, E.M.; Heine, R.J.; et al. Classical and Model-Based Estimates of Beta-Cell Function during a Mixed Meal vs. an OGTT in a Population-Based Cohort. Diabetes Res. Clin. Pract. 2009, 83, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Bacha, F.; Gungor, N.; Arslanian, S.A. Measures of Β-Cell Function during Oral Glucose Tolerance Test and Liquid Mixed Meal and the Hyperglycemic Clamp. J. Pediatr. 2008, 152, 618–621. [Google Scholar] [CrossRef]
- Muscelli, E.; Mari, A.; Natali, A.; Astiarraga, B.D.; Camastra, S.; Frascerra, S.; Holst, J.J.; Ferrannini, E. Impact of Incretin Hormones on Beta-Cell Function in Subjects with Normal or Impaired Glucose Tolerance. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E1144–E1150. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Ionut, V.; Mooradian, V.; Stefanovski, D.; Bergman, R.N. Portal Glucose Infusion-Glucose Clamp Measures Hepatic Influence on Postprandial Systemic Glucose Appearance as Well as Whole Body Glucose Disposal. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E346–E353. [Google Scholar] [CrossRef][Green Version]
- Heise, T.; Zijlstra, E.; Nosek, L.; Heckermann, S.; Plum-Mörschel, L.; Forst, T. Euglycaemic Glucose Clamp: What it can and Cannot do, and how to do It. Diabetes Obes. Metab. 2016, 18, 962–972. [Google Scholar] [CrossRef]
- Wang, M.; Yu, M.; Fang, L.; Hu, R. Association between Sugar-sweetened Beverages and Type 2 Diabetes: A Meta-analysis. J. Cutan. Immunol. Allergy 2015, 6, 360–366. [Google Scholar] [CrossRef]
- Hanssen, N.M.J.; Kraakman, M.J.; Flynn, M.C.; Nagareddy, P.R.; Schalkwijk, C.G.; Murphy, A.J. Postprandial Glucose Spikes, an Important Contributor to Cardiovascular Disease in Diabetes? Front. Cardiovasc. Med. 2020, 7, 570553. [Google Scholar] [CrossRef]

| Dutch Guidelines and Risk Factors | Minimum Score 0 | Maximum Score 10 | |
|---|---|---|---|
| Vegetable (daily) | 150 to 200 g of vegetables | 0 g | ≥200 g |
| Fruit (daily) | 200 g of fruit (2 pieces) | 0 g | ≥200 g |
| Fiber (daily) (whole grains) | 30 to 40 g a day of dietary fiber | 0 g | ≥40 g |
| Fish (daily)—omega-3-fatty-acids * | Two portions of fish a week, at least one of which should be oily fish. At least 450 mg omega-3-fatty-acids a day | 0 mg | ≥two portions oily fish or more frequent consumption of other fish |
| Alcohol (daily) | If alcohol is consumed at all, male intake should be limited to two Dutch standard units (20 g ethanol) a day and female intake to one. | Male: ≥60 g Female: ≥40 g | Male: ≤20 g Female: ≤10 g |
| Coffee (daily) ** | Consumption of at least three cups per day may lower the risk of type 2 diabetes | 0 cups | ≥3 cups |
| Tea (daily) ** | Consumption of at least three cups per day may lower the risk of type 2 diabetes | 0 cups | ≥3 cups |
| Red meat (daily) *** | There is a positive association between the consumption of red meat of a least 19 g a day and the incidence of type 2 diabetes | ≥70 g | ≤26 g |
| All Subjects N = 176 | |
|---|---|
| Demographics | |
| Age (years), median (IQR) | 31 (27–35) |
| Male (%) | 42.6 |
| Characteristics | |
| Body mass index (kg/m2), median (IQR) | 23.3 (21.6–25.6) |
| Engaging in sports (%) | 75 |
| Current smoking (%) | 32.4 |
| Glucose metabolism variables | |
| Fasting glucose (mmol/L), median (IQR) | 4.3 (4.1–4.6) |
| OGTT glucose at t120 (mmol/L), median (IQR) | 5.4 (4.6–6.1) ^ |
| Meal glucose at t120 (mmol/L), median (IQR) | 5.3 (5.0–5.9) |
| IFG or IGT (%) | 6.3 ^^ |
| HbA1c (%), median (IQR) | 5.3 (5.1–5.4) |
| Cardiovascular variables | |
| Total cholesterol (mmol/L), median (IQR) | 4.2 (3.6–4.7) |
| LDL cholesterol (mmol/L), median (IQR) | 2.2 (1.7–2.9) |
| HDL cholesterol (mmol/L), median (IQR) | 1.42 (1.2–1.7) |
| Fasting triglycerides (mmol/L), median (IQR) | 0.8 (0.6–1.0) |
| SBP (mmHg), median (IQR) | 120.0 (112.5–128.3) |
| DBP (mmHg), median (IQR) | 68.5 (112.5–128.3) |
| ALAT (U/l), median (IQR) | 19.0 (15.0–27.0) |
| Diet | |
| Dutch Dietary Quality index score (max 80), median (IQR) | 48.5 (42.2–54.4) |
| Foods and Food Components | Mean Intake (sd) | Mean Difference | p Value | ICC (95% cIs) |
|---|---|---|---|---|
| Coffee FFQ1 Coffee FFQ2 (cups (125 g) per day) | 3.75 (3.96) 3.60 (3.45) | 0.15 | 0.74 | 0.84 (0.77–0.89) |
| Tea FFQ1 Tea FFQ2 (cups (125 g) per day) | 3.86 (3.80) 3.85 (3.97) | 0.012 | 0.64 | 0.83 (0.75–0.88) |
| Alcohol FFQ1 Alcohol FFQ2 (g/w) | 79.12 (80.15) 77.14 (91.27) | 1.98 | 0.10 | 0.86 (0.75–0.91) |
| Vegetables FFQ1 Vegetables FFQ2 (g/d) | 248.83 (102.20) 247.27 (95.46) | 1.56 | 0.24 | 0.65 (0.48–0.77) |
| Fruit FFQ1 Fruit FFQ2 (g/d) | 99.17 (70.96) 97.87 (71.25) | 1.30 | 0.46 | 0.81 (0.74–0.87) |
| Fish FFQ1 Fish FFQ2 (Total amount) (g/w) Fish (fat) FFQ1 Fish (fat) FFQ2 (g/w) | 71.06 (97.16) 84.60 (112.46) 21.18 (30.50) 23.63 (37.41) | −13.54 −2.45 | 0.96 0.39 | 0.55 (0.36–0.69) 0.75 (0.55–0.85) |
| Cereal fiber FFQ1 Cereal fiber FFQ2 (g/d) | 12.15 (6.47) 12.62 (7.06) | −0.46 | 0.47 | 0.86 (0.79–0.90) |
| Red meat FFQ1 Red meat FFQ2 (g/d) | 49.34 (31.55) 49,10 (28.43) | 0.24 | 0.95 | 0.67 (0.54–0.77) |
| Snacks FFQ1 Snacks FFQ2 (frequency/d) | 1.30 (0.85) 1.14 (0.77) | 0.16 | 0.40 | 0.65 (0.51–0.76) |
| DDQ-Index (10 Points Increment) | |
|---|---|
| OUTCOME | |
| Fasting glucose (mmol/L) | 0.02 (0.04) |
| OGTT glucose at t120 (mmol/L) | −0.06 (0.10) |
| HbA1c (%) | −0.07 (0.02) * |
| SBP (mmHg) | −0.64 (0.95) |
| DBP (mmHg) | −0.65 (0.73) |
| Total cholesterol (mmol/L) | −0.20 (0.07) * |
| HDL cholesterol (mmol/L) | 0.02 (0.03) |
| LDL cholesterol (mmol/L) (calculated) | −0.19 (0.07) * |
| Fasting triglycerides (mmol/L) (ln-transformed) | −0.05 (0.04) |
| ALAT (ln-transformed) | 0.09 (0.05) |
| Metabolic Parameters | DDQ-Index (10-Point Increment) |
|---|---|
| Meal (classical) | |
| Meal glucose at t120 (mmol/L) | −0.02 (0.006) * |
| Fasting insulin (pmol/L) | −0.40 (0.16) * |
| Serum insulin at t120 (pmol/L) | −3.93 (1.40) * |
| Serum insulin IAUC (0–240) (pmol·h/L) | −4.06 (3.52) |
| Glucose iAUC (0–240) (mmol·h/L) | −1.91 (0.97) * |
| Insulinogenic index | 0.00 (0.08) |
| AUCinsulin/AUCglucose ratio (pmol/mmol) | −0.21 (0.17) |
| iAUCinsulin/iAUCglucose ratio (pmol/mmol) | 2.37 (2.08) |
| Insulin resistance (IR HOMA) | −0.01 (0.01) * |
| Ln β-cell function (HOMA) | −0.01 (0.00) |
| Meal (model-based) | |
| β-cell glucose sensitivity (pmol min−1 m−2 [mmol/L]−1) | 0.14 (0.54) |
| Rate sensitivity (pmol min−1 m−2 [mmol/L]−1) | −0.86 (6.73) |
| Potentiation factor ratio (220–240)/(0–20) | <0.005 |
| Fasting ISR (pmol min−1 m−2) | −0.17 (0.17) |
| Integral of insulin secretion (nmol/m2) | −0.05 (0.16) |
| OGIS180 (mL min−1 m−2) | 0.89 (0.39) * |
| Clamp | |
| Insulin sensitivity index (μmol min−1 kg−1 [pmol/L]−1) | <0.005 |
| Insulin level at t0 of the clamp (pmol/L) | −0.07 (0.24) |
| iAUC of glucose-stimulated insulin from t1-t10 of clamp (pmol/L) | 3.85 (14.09) |
| Second phase iAUC of glucose-stimulated insulin (pmol/L) | −14.20 (77.89) |
| GLP-1-stimulated iAUC of insulin (pmol/L) | 9.44 (282.11) |
| Arginine-stimulated iAUC (pmol/L) | 12.93 (31.31) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zelis, M.; Simonis, A.M.C.; van Dam, R.M.; Boomsma, D.I.; van Lee, L.; Kramer, M.H.H.; Serné, E.H.; van Raalte, D.H.; Mari, A.; de Geus, E.J.C.; et al. Development of a Diabetes Dietary Quality Index: Reproducibility and Associations with Measures of Insulin Resistance, Beta Cell Function, and Hyperglycemia. Nutrients 2024, 16, 3512. https://doi.org/10.3390/nu16203512
Zelis M, Simonis AMC, van Dam RM, Boomsma DI, van Lee L, Kramer MHH, Serné EH, van Raalte DH, Mari A, de Geus EJC, et al. Development of a Diabetes Dietary Quality Index: Reproducibility and Associations with Measures of Insulin Resistance, Beta Cell Function, and Hyperglycemia. Nutrients. 2024; 16(20):3512. https://doi.org/10.3390/nu16203512
Chicago/Turabian StyleZelis, Maartje, Annemarie M. C. Simonis, Rob M. van Dam, Dorret I. Boomsma, Linde van Lee, Mark H. H. Kramer, Erik H. Serné, Daniel H. van Raalte, Andrea Mari, Eco J. C. de Geus, and et al. 2024. "Development of a Diabetes Dietary Quality Index: Reproducibility and Associations with Measures of Insulin Resistance, Beta Cell Function, and Hyperglycemia" Nutrients 16, no. 20: 3512. https://doi.org/10.3390/nu16203512
APA StyleZelis, M., Simonis, A. M. C., van Dam, R. M., Boomsma, D. I., van Lee, L., Kramer, M. H. H., Serné, E. H., van Raalte, D. H., Mari, A., de Geus, E. J. C., & Eekhoff, E. M. W. (2024). Development of a Diabetes Dietary Quality Index: Reproducibility and Associations with Measures of Insulin Resistance, Beta Cell Function, and Hyperglycemia. Nutrients, 16(20), 3512. https://doi.org/10.3390/nu16203512






