Super Bolus—A Remedy for a High Glycemic Index Meal in Children with Type 1 Diabetes on Insulin Pump Therapy?—A Randomized, Double-Blind, Controlled Trial
Abstract
1. Introduction
- − 50% increase in prandial bolus dose,
- − removal of the basal insulin for 2 h post meal.
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Annan, S.F.; Higgins, L.A.; Jelleryd, E.; Hannon, T.; Rose, S.; Salis, S.; Baptista, J.; Chinchilla, P.; Marcovecchio, M.L. ISPAD Clinical Practice Consensus Guidelines 2022: Nutritional management in children and adolescents with diabetes. Pediatr. Diabetes 2022, 23, 1297–1321. [Google Scholar] [CrossRef] [PubMed]
- Araszkiewicz, A.; Bandurska-Stankiewicz, E.; Borys, S.; Budzyński, A.; Cyganek, K.; Cypryk, K.; Czech, A.; Czupryniak, L.; Drzewoski, J.; Dzida, G. Zalecenia kliniczne dotyczące postępowania u osób z cukrzycą 2023-Stanowisko Polskiego Towarzystwa Diabetologicznego. Curr. Top. Diabetes 2023, 3, 1–147. [Google Scholar] [CrossRef]
- Quarta, A.; Guarino, M.; Tripodi, R.; Giannini, C.; Chiarelli, F.; Blasetti, A. Diet and Glycemic Index in Children with Type 1 Diabetes. Nutrients 2023, 15, 3507. [Google Scholar] [CrossRef] [PubMed]
- Chiavaroli, L.; Lee, D.; Ahmed, A.; Cheung, A.; Khan, T.A.; Blanco, S.; Mirrahimi, A.; Jenkins, D.J.; Livesey, G.; Wolever, T.M.; et al. Effect of low glycaemic index or load dietary patterns on glycaemic control and cardiometabolic risk factors in diabetes: Systematic review and meta-analysis of randomised controlled trials. BMJ 2021, 374, n1651. [Google Scholar] [CrossRef] [PubMed]
- Bell, K.J.; Smart, C.E.; Steil, G.M.; Brand-Miller, J.C.; King, B.; Wolpert, H.A. Impact of fat, protein, and glycemic index on postprandial glucose control in type 1diabetes: Implications for intensive diabetes management in the continuous glucose monitoring era. Diabetes Care 2015, 38, 1008–1015. [Google Scholar] [CrossRef]
- Johansen, M.D.; Gjerløv, I.; Christiansen, J.S.; Hejlesen, O.K. Interindividual and intraindividual variations in postprandial glycemia peak time complicate precise recommendations for self-monitoring of glucose in persons with type 1 diabetes mellitus. J. Diabetes Sci. Technol. 2012, 6, 356–361. [Google Scholar] [CrossRef]
- Bell, K.J.; King, B.R.; Shafat, A.; Smart, C.E. The relationship between carbohydrate and the mealtime insulin dose in type 1 diabetes. J. Diabetes Complicat. 2015, 29, 1323–1329. [Google Scholar] [CrossRef]
- Parillo, M.; Annuzzi, G.; Rivellese, A.A.; Bozzetto, L.; Alessandrini, R.; Riccardi, G.; Capaldo, B. Effects of meals with different glycaemic index on postprandial blood glucose response in patients with Type 1 diabetes treated with continuous subcutaneous insulin infusion. Diabet. Med. 2011, 28, 227–229. [Google Scholar] [CrossRef]
- Faber, E.M.; van Kampen, P.M.; Clement-de Boers, A.; Houdijk, E.C.A.M.; van der Kaay, D.C.M. The influence of food order on postprandial glucose levels in children with type 1 diabetes. Pediatr. Diabetes 2018, 19, 809–815. [Google Scholar] [CrossRef]
- Dżygało, K.; Szypowska, A. Impact of insulins glulisine and aspart on postprandial glycemia after a high-glycemic index meal in children with type 1 diabetes. Eur. J. Endocrinol. 2014, 170, 539–545. [Google Scholar] [CrossRef]
- Fath, M.; Danne, T.; Biester, T.; Erichsen, L.; Kordonouri, O.; Haahr, H. Faster-acting insulin aspart provides faster onset and greater early exposure vs insulin aspart in children and adolescents with type 1 diabetes mellitus. Pediatr. Diabetes 2017, 18, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Haahr, H.; Heise, T. Fast-Acting Insulin Aspart: A Review of its Pharmacokinetic and Pharmacodynamic Properties and the Clinical Consequences. Clin. Pharmacokinet. 2020, 59, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Cutruzzolà, A.; Parise, M.; Fiorentino, R.; Romano, A.; Molinaro, V.; Gnasso, A.; Di Molfetta, S.; Irace, C. The Effect of Two Different Insulin Formulations on Postprandial Hyperglycemia after High and Low Glycemic-Index Meal in Type 1 Diabetes. Nutrients 2022, 14, 3316. [Google Scholar] [CrossRef] [PubMed]
- Marlena Błazik, D.G. Improving the Estimation of Meal-Time Insulin Dose Based On the Glycaemic Load of a Meal in Children with Type 1 Diabetes on Insulin Pump Therapy: A Randomized Study. J. Diabetes Metab. 2014, 05, 1–5. [Google Scholar] [CrossRef]
- John Pemberton. Information Leaflet on Super Bolus. NHS Birmingham Children’s Hospital NHS Foundation Trust, Version 1.0, Produced June 2018, Review Date June 2021, 4–6. Available online: https://childrenwithdiabetes.com/wp-content/uploads/Superbolus-for-insulin-pumps.pdf (accessed on 1 June 2021).
- Alberta Diabetes Link. Types of Boluses for Pump Therapy. 2018, pp. 1–3. Available online: https://albertadiabeteslink.ca/process.php?docname=Types-of-Boluses-and-Practical-Applications-2018.pdf (accessed on 1 January 2018).
- Rosales, N.; De Battista, H.; Vehí, J.; Garelli, F. Open-loop glucose control: Automatic IOB-based super-bolus feature for commercial insulin pumps. Comput. Methods Programs Biomed. 2018, 159, 145–158. [Google Scholar] [CrossRef]
- Bondia, J.; Dassau, E.; Zisser, H.; Calm, R.; Vehí, J.; Jovanovič, L.; Doyle, F.J. Coordinated basal-bolus infusion for tighter postprandial glucose control in insulin pump therapy. J. Diabetes Sci. Technol. 2009, 3, 89–97. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G. MDC 2010 SU guidelines for reporting, parallel group randomised trials. BMJ 2010, 340, 698–702. [Google Scholar] [CrossRef]
- Kowalczyk, E.; Dżygało, K.; Szypowska, A. Super Bolus: A remedy for a high glycemic index meal in children with type 1 diabetes on insulin pump therapy?—Study protocol for a randomized controlled trial. Trials 2022, 23, 240. [Google Scholar] [CrossRef]
- DiMeglio, L.A.; Acerini, C.L.; Codner, E.; Craig, M.E.; Hofer, S.E.; Pillay, K.; Maahs, D.M. ISPAD Clinical Practice Consensus Guidelines 2018: Glycemic control targets and glucose monitoring for children, adolescents, and young adults with diabetes. Pediatr. Diabetes 2018, 19, 105–114. [Google Scholar] [CrossRef]
- Walsh, J.; Ruth, R. Pumping Insulin: Everything You Need for Success on a Smart Insulin Pump, 4th ed.; Torrey Pines Press: San Diego, CA, USA, 2006. [Google Scholar]
- Walsh, J.; Ruth, R. Ways to Improve Consistency, Ease of Use, Safety, and Medical Outcomes with Insulin Pumps. 2021. Available online: https://www.diabetesnet.com/diabetes_presentations/FuturePumpFeatures-0809.ppt (accessed on 8 September 2021).
- Adolfsson, P.; Taplin, C.E.; Zaharieva, D.P.; Pemberton, J.; Davis, E.A.; Riddell, M.C.; McGavock, J.; Moser, O.; Szadkowska, A.; Lopez, P.; et al. ISPAD Clinical Practice Consensus Guidelines 2022: Exercise in children and adolescents with diabetes. Pediatr. Diabetes 2022, 23, 1341–1372. [Google Scholar] [CrossRef]
- Vetrani, C.; Calabrese, I.; Cavagnuolo, L.; Pacella, D.; Napolano, E.; Di Rienzo, S.; Riccardi, G.; Rivellese, A.A.; Annuzzi, G.; Bozzetto, L. Dietary determinants of postprandial blood glucose control in adults with type 1 diabetes on a hybrid closed-loop system. Diabetologia 2022, 65, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Gitsi, E.; Livadas, S.; Angelopoulos, N.; Paparodis, R.D.; Raftopoulou, M.; Argyrakopoulou, G. A Nutritional Approach to Optimizing Pump Therapy in Type 1 Diabetes Mellitus. Nutrients 2023, 15, 4897. [Google Scholar] [CrossRef] [PubMed]

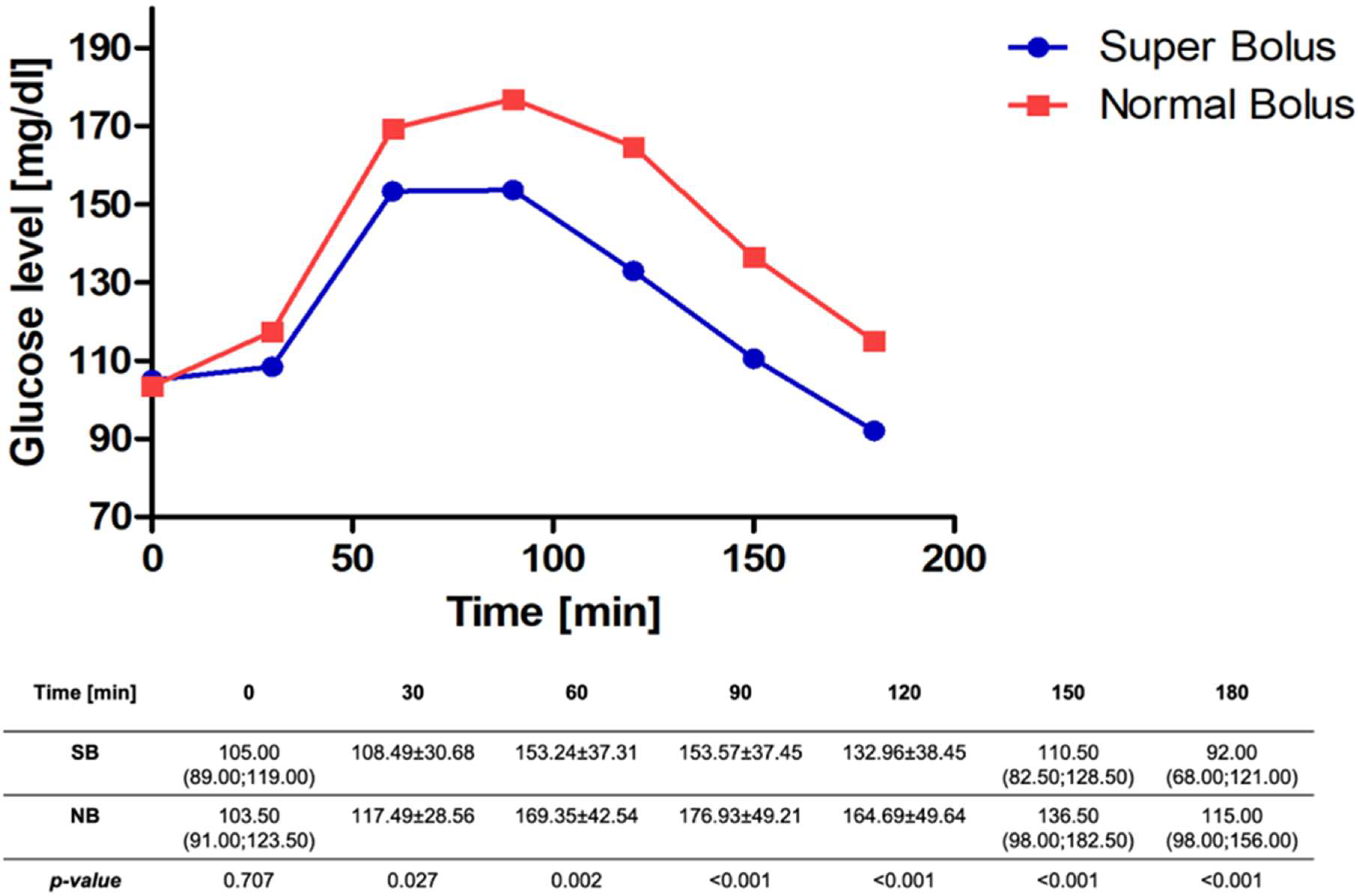
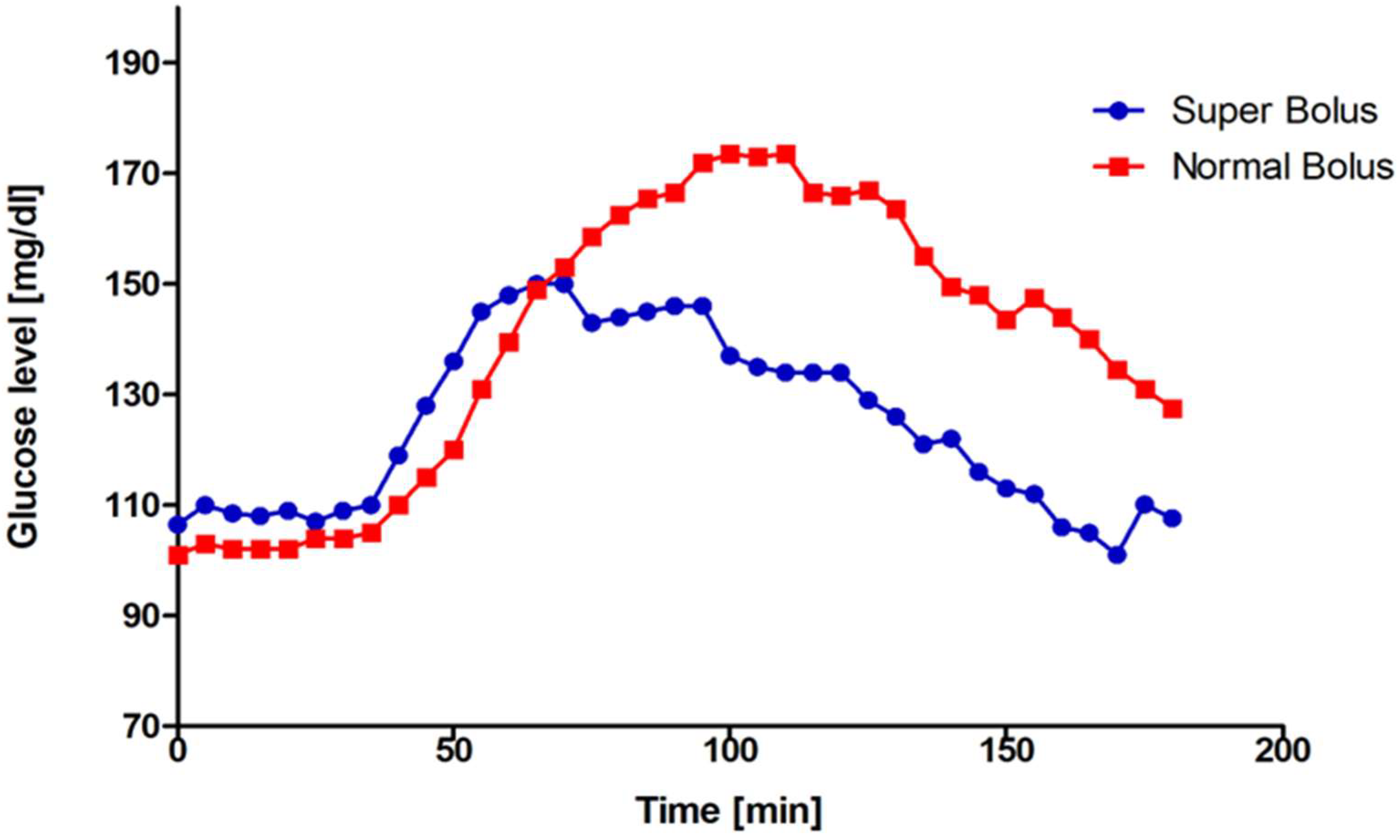


| Characteristic | N | n (%)/Mean ± SD/Median (Q1;Q3) |
|---|---|---|
| Sex, n (%) | 72 | |
| Female | 37 (51.39) | |
| Male | 35 (48.61) | |
| Age (years)1 | 72 | 14.97 (12.96;16.30) |
| Duration of disease (years)1 | 72 | 5.91 (2.79;9.44) |
| BMI (kg/m2)1 | 72 | 20.77 (18.63;22.91) |
| BMI Z-score1 | 72 | −0.07 (−0.71;0.57) |
| TDD/kg (u/kg)2 | 72 | 0.82 ± 0.25 |
| Base/kg (u/kg)2 | 72 | 0.34 ± 0.12 |
| HbA1c (%)2 | 72 | 8.35 (7.45;9.30) |
| Breakfast’s ICR (u)2 | 72 | 1.61 ± 0.56 |
| ISF (mg/dL)1 | 72 | 37.66 (29.75;50.28) |
| Insulin type, n (%) | 72 | |
| Novo Rapid®, Novo Nordisk | 29 (40.28) | |
| Humalog®, Eli Lilly | 13 (18.06) | |
| Apidra®, Sanofi | 10 (13.89) | |
| Liprolog®, Eli Lilly | 19 (26.39) | |
| Lispro®, Sanofi | 1 (1.39) | |
| Infusion set type, n (%) | 72 | |
| Teflon cannula | 39 (54.93) | |
| Metal cannula | 32 (45.07) | |
| Infusion set localization, n (%) | 72 | |
| Abdomen | 19 (26.76) | |
| Arm | 18 (25.35) | |
| Thigh | 23 (32.39) | |
| Buttock | 11 (15.49) |
| Total Number of Episodes | Super Bolus Group | Normal Bolus Group | p-Value |
|---|---|---|---|
| TBR2 (G) | 9 (0.13/pp) | 3 (0.04/pp) | 0.041 |
| TBR2 (CGM) | 4 (0.06/pp) | 0 | 0.072 |
| TBR1 (G) | 33 (0.45/pp) | 14 (0.20/pp) | 0.003 |
| TBR1 (CGM) | 25 (0.36/pp) | 15 (0.21/pp) | 0.029 |
| TAR1 (G) | 45 (0.63/pp) | 99 (1.38/pp) | <0.0001 |
| TAR1 (CGM) | 22 (0.32/pp) | 38 (0.54/pp) | 0.001 |
| TAR2 (G) | 1 (0.01/pp) | 15 (0.21/pp) | 0.022 |
| TAR2 (CGM) | 3 (0.04/pp) | 6 (0.09/pp) | 0.299 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalczyk-Korcz, E.; Dymińska, M.; Szypowska, A. Super Bolus—A Remedy for a High Glycemic Index Meal in Children with Type 1 Diabetes on Insulin Pump Therapy?—A Randomized, Double-Blind, Controlled Trial. Nutrients 2024, 16, 263. https://doi.org/10.3390/nu16020263
Kowalczyk-Korcz E, Dymińska M, Szypowska A. Super Bolus—A Remedy for a High Glycemic Index Meal in Children with Type 1 Diabetes on Insulin Pump Therapy?—A Randomized, Double-Blind, Controlled Trial. Nutrients. 2024; 16(2):263. https://doi.org/10.3390/nu16020263
Chicago/Turabian StyleKowalczyk-Korcz, Emilia, Magdalena Dymińska, and Agnieszka Szypowska. 2024. "Super Bolus—A Remedy for a High Glycemic Index Meal in Children with Type 1 Diabetes on Insulin Pump Therapy?—A Randomized, Double-Blind, Controlled Trial" Nutrients 16, no. 2: 263. https://doi.org/10.3390/nu16020263
APA StyleKowalczyk-Korcz, E., Dymińska, M., & Szypowska, A. (2024). Super Bolus—A Remedy for a High Glycemic Index Meal in Children with Type 1 Diabetes on Insulin Pump Therapy?—A Randomized, Double-Blind, Controlled Trial. Nutrients, 16(2), 263. https://doi.org/10.3390/nu16020263






