A Randomized Controlled Trial on the Effects of Leucine-Supplement Combined with Nutritional Counseling on Body Composition in Mix Cancer Older Men
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
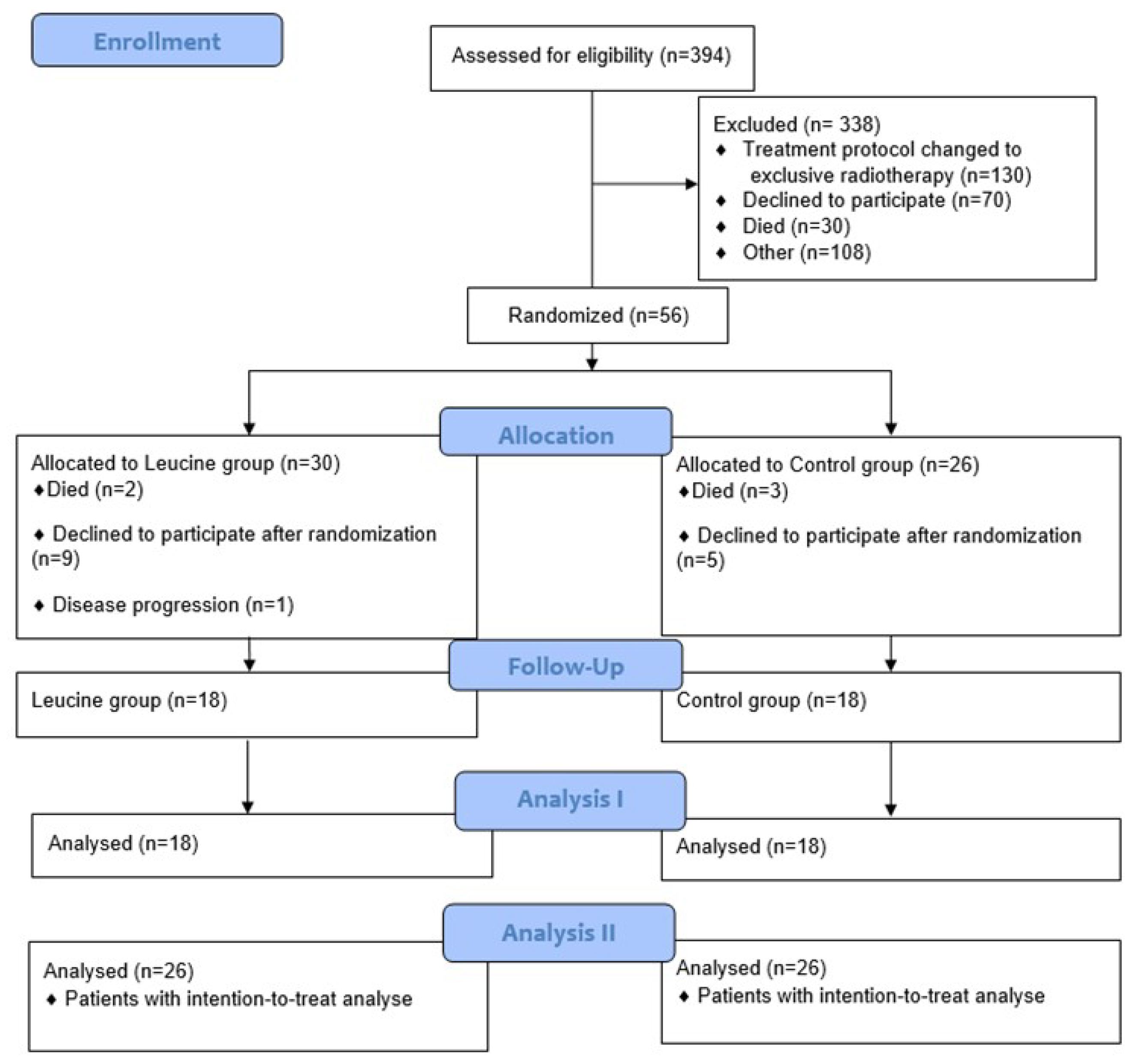
2.2. Patients, Sample Size, and Procedures
2.3. Nutritional Intervention
2.4. Nutritional Assessment
2.4.1. Anthropometry, Body Composition, and Cancer Cachexia Classification
2.4.2. Dietary Intake and Adherence to Supplementation
2.5. Clinical Data
2.6. Statistical Analyses
3. Results
4. Discussion
Limtations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F.; Bsc, M.F.B.; Me, J.F.; Soerjomataram, M.I.; et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, L.; Huang, S.; Hui, Q.; Shi, X.; Zhang, Q. Low muscle mass and Charlson comorbidity index are risk factors for short-term postoperative prognosis of elderly patients with gastrointestinal tumor: A cross-sectional study. BMC Geriatr. 2021, 21, 730. [Google Scholar] [CrossRef]
- Siqueira, J.M.; de Oliveira, I.C.L.; Soares, J.D.P.; Pimentel, G.D. SARC-F has low correlation and reliability with skeletal muscle mass index in older gastrointestinal cancer patients. Clin. Nutr. 2021, 40, 890–894. [Google Scholar] [CrossRef] [PubMed]
- Nipp, R.D.; Fuchs, G.; El-Jawahri, A.; Mario, J.; Troschel, F.M.; Greer, J.A.; Gallagher, E.R.; Jackson, V.A.; Kambadakone, A.; Hong, T.S.; et al. Sarcopenia Is Associated with Quality of Life and Depression in Patients with Advanced Cancer. Oncologist 2018, 23, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Shachar, S.S.; Deal, A.M.; Weinberg, M.; Nyrop, K.A.; Williams, G.R.; Nishijima, T.F.; Benbow, J.M.; Muss, H.B. Skeletal muscle measures as predictors of toxicity, hospitalization, and survival in patients with metastatic breast cancer receiving taxane-based chemotherapy. Clin. Cancer Res. 2017, 23, 658–665. [Google Scholar] [CrossRef]
- Soares, J.D.P.; Gomes, T.L.N.; Siqueira, J.M.; Oliveira, I.C.; Mota, J.F.; Laviano, A.; Pimentel, G.D. Muscle function loss is associated with anxiety in patients with gastrointestinal cancer. Clin. Nutr. ESPEN 2019, 29, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Argilés, J.M.; López-Soriano, F.J.; Stemmler, B.; Busquets, S. Cancer-associated cachexia—Understanding the tumour macroenvironment and microenvironment to improve management. Nat. Rev. Clin. Oncol. 2023, 20, 250–264. [Google Scholar] [CrossRef]
- Soares, J.D.P.; Howell, S.L.; Teixeira, F.J.; Pimentel, G.D. Dietary Amino Acids and Immunonutrition Supplementation in Cancer-Induced Skeletal Muscle Mass Depletion: A Mini-Review. Curr. Pharm. Des. 2020, 26, 970–978. [Google Scholar] [CrossRef]
- Arends, J.; Baracos, V.; Bertz, H.; Bozzetti, F.; Calder, P.C.; Deutz, N.E.P.; Erickson, N.; Laviano, A.; Lisanti, M.P.; Lobo, D.N. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin. Nutr. 2017, 36, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef] [PubMed]
- Muscaritoli, M.; Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN practical guideline: Clinical Nutrition in cancer. Clin. Nutr. 2021, 40, 2898–2913. [Google Scholar] [CrossRef] [PubMed]
- Mertz, K.H.; Reitelseder, S.; Bechshoeft, R.; Bulow, J.; Højfeldt, G.; Jensen, M.; Schacht, S.R.; Lind, M.V.; A Rasmussen, M.; Mikkelsen, U.R.; et al. The effect of daily protein supplementation, with or without resistance training for 1 year, on muscle size, strength, and function in healthy older adults: A randomized controlled trial. Am. J. Clin. Nutr. 2021, 113, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Breen, L.; Stokes, K.A.; Churchward-Venne, T.A.; Moore, D.R.; Baker, S.K.; Smith, K.; Atherton, P.J.; Phillips, S.M. Two weeks of reduced activity decreases leg lean mass and induces ‘anabolic resistance’ of myofibrillar protein synthesis in healthy elderly. J. Clin. Endocrinol. Metab. 2013, 98, 2604–2612. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, U.R.; Couppé, C.; Karlsen, A.; Grosset, J.; Schjerling, P.; Mackey, A.; Klausen, H.; Magnusson, S.; Kjær, M. Life-long endurance exercise in humans: Circulating levels of inflammatory markers and leg muscle size. Mech. Ageing Dev. 2013, 134, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Deutz, N.E.P.; Safar, A.; Schutzler, S.; Memelink, R.; Ferrando, A.; Spencer, H.; van Helvoort, A.; Wolfe, R.R. Muscle protein synthesis in cancer patients can be stimulated with a specially formulated medical food. Clin. Nutr. 2011, 30, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Hopewell, S.; Schulz, K.F.; Montori, V.; Gøtzsche, P.C.; Devereaux, P.J.; Elbourne, D.; Egger, M.; Altman, D.G. CONSORT 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c869. [Google Scholar] [CrossRef]
- Leenders, M.; Verdijk, L.B.; van der Hoeven, L.; van Kranenburg, J.; Hartgens, F.; Wodzig, W.K.W.H.; Saris, W.H.M.; van Loon, L.J.C. Prolonged leucine supplementation does not augment muscle mass or affect glycemic control in elderly type 2 diabetic men. J. Nutr. 2011, 141, 1070–1076. [Google Scholar] [CrossRef]
- Sergi, G.; De Rui, M.; Veronese, N.; Bolzetta, F.; Berton, L.; Carraro, S.; Bano, G.; Coin, A.; Manzato, E.; Perissinotto, E. Assessing appendicular skeletal muscle mass with bioelectrical impedance analysis in free-living Caucasian older adults. Clin. Nutr. 2015, 34, 667–673. [Google Scholar] [CrossRef]
- Oken, M.M.; Creech, R.H.; Torney, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and responsive criteria of the Eastern Cooperative Oncology Group. Am. J. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef]
- White, I.R.; Royston, P.; Wood, A.M. Multiple imputation using chained equations: Issues and guidance for practice. Stat. Med. 2011, 30, 377–399. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.D.P.; Siqueira, J.M.; Oliveira, I.C.L.; Laviano, A.; Pimentel, G.D. A high-protein diet, not isolated BCAA, is associated with skeletal muscle mass index in patients with gastrointestinal cancer. Nutrition 2020, 72, 110698. [Google Scholar] [CrossRef] [PubMed]
- Sharples, A.P.; Hughes, D.C.; Deane, C.S.; Saini, A.; Selman, C.; Stewart, C.E. Longevity and skeletal muscle mass: The role of IGF signalling, the sirtuins, dietary restriction and protein intake. Aging Cell 2015, 14, 511–523. [Google Scholar] [CrossRef]
- Garcia, D.; Shaw, R.J. AMPK: Mechanisms of Cellular Energy Sensing and Restoration of Metabolic Balance. Mol. Cell 2017, 66, 789–800. [Google Scholar] [CrossRef] [PubMed]
- González, A.; Hall, M.N.; Lin, S.C.; Hardie, D.G. AMPK and TOR: The Yin and Yang of Cellular Nutrient Sensing and Growth Control. Cell Metab. 2020, 31, 472–492. [Google Scholar] [CrossRef] [PubMed]
- Mendes, M.C.S.; Pimentel, G.D.; Costa, F.O.; Carvalheira, J.B.C. Molecular and neuroendocrine mechanisms of cancer cachexia. J. Endocrinol. 2015, 226, R29–R43. [Google Scholar] [CrossRef] [PubMed]
- Bennani-Baiti, N.; Davis, M.P. Review article: Cytokines and cancer anorexia cachexia syndrome. Am. J. Hosp. Palliat. Med. 2008, 25, 407–411. [Google Scholar] [CrossRef]
- Cruz, B.; Oliveira, A.; Viana, L.R.; Lopes-Aguiar, L.; Canevarolo, R.; Colombera, M.C.; Valentim, R.R.; Garcia-Fóssa, F.; de Sousa, L.M.; Castelucci, B.G.; et al. Leucine-rich diet modulates the metabolomic and proteomic profile of skeletal muscle during cancer cachexia. Cancers 2020, 12, 1880. [Google Scholar] [CrossRef]
- Beaudry, A.G.; Law, M.L. Leucine Supplementation in Cancer Cachexia: Mechanisms and a Review of the Pre-Clinical Literature. Nutrients 2022, 14, 2824. [Google Scholar] [CrossRef]
- van der Ende, M.; Grefte, S.; Plas, R.; Meijerink, J.; Witkamp, R.F.; Keijer, J.; van Norren, K. Mitochondrial dynamics in cancer-induced cachexia. Biochim. Biophys. Acta-Rev. Cancer 2018, 1870, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Argilés, J.M.; Busquets, S.; Stemmler, B.; López-Soriano, F.J. Cancer cachexia: Understanding the molecular basis. Nat. Rev. Cancer 2014, 14, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Argilés, J.M.; Busquets, S.; Felipe, A.; López-Soriano, F.J. Molecular mechanisms involved in muscle wasting in cancer and ageing: Cachexia versus sarcopenia. Int. J. Biochem. Cell Biol. 2005, 37, 1084–1104. [Google Scholar] [CrossRef] [PubMed]
- Cruz, B.; Oliveira, A.; Ventrucci, G.; Gomes-Marcondes, M.C.C. A leucine-rich diet modulates the mTOR cell signalling pathway in the gastrocnemius muscle under different Walker-256 tumour growth conditions. BMC Cancer 2019, 19, 349. [Google Scholar] [CrossRef]
- Storck, L.J.; Ruehlin, M.; Gaeumann, S.; Gisi, D.; Schmocker, M.; Meffert, P.J.; Imoberdorf, R.; Pless, M.; Ballmer, P.E. Effect of a leucine-rich supplement in combination with nutrition and physical exercise in advanced cancer patients: A randomized controlled intervention trial. Clin. Nutr. 2020, 39, 3637–3644. [Google Scholar] [CrossRef]
| Characteristics | Total (n = 36) | Leucine (n = 18) | Control (n = 18) | p |
|---|---|---|---|---|
| ± SD | ± SD | ± SD | ||
| Age (years) § | 65.11 ± 7.50 | 65.22 ± 8.19 | 65.00 ± 7.23 | 0.786 |
| BMI (kg/m2) § | 22.38 ± 3.98 | 22.34 ± 2.79 | 22.41 ± 3.64 | 0.933 |
| n (%) | n (%) | n (%) | ||
| Site of primary tumor ** | ||||
| Esophagus | 2 | 1 (5.56) | 1 (5.56) | 0.863 |
| Stomach | 9 | 5 (27.78) | 4 (22.22) | |
| Colorectal | 22 | 10 (55.57) | 12 (66.67) | |
| Liver | 1 | 1 (5.56) | 00 (0.00) | |
| Pancreas | 2 | 1 (5.56) | 1 (5.56) | |
| Type of chemotherapy ** | ||||
| Adjuvant | 35 | 17 (94.44) | 18 (100) | 0.310 |
| Neoadjuvant | 1 | 1 (5.56) | 0 (0.00) | |
| Cancer stage ** | ||||
| I | 1 | 1 (5.56) | 0 (0.00) | 0.528 |
| II | 10 | 6 (33.33) | 4 (22.22) | |
| III | 11 | 4 (22.22) | 7 (38.89) | |
| IV | 14 | 7 (38.89) | 7 (38.89) | |
| Cancer cachexia stage ** | ||||
| No cachexia | 7 | 3 (16.67) | 4 (22.22) | 0.210 |
| Pre cachexia | 14 | 7 (38.89) | 7 (38.89) | |
| Cachexia | 15 | 8 (44.44) | 7 (38.89) | |
| Performance status ** | ||||
| 0 | 27 | 14 (77.78) | 13 (72.22) | 0.700 |
| 1 | 9 | 4 (22.22) | 5 (27.78) |
| Variables | Treatment | p * | |||||
|---|---|---|---|---|---|---|---|
| Leucine (n = 18) | p | Control (n = 18) | p | ||||
| Time | Time | ||||||
| T0 | T1 | T0 | T1 | ||||
| Body composition | |||||||
| Weight (kg) | 61.79 ± 9.02 | 64.06 ± 9.45 | 0.01 | 62.29 ± 10.03 | 62.51 ± 10.94 | 0.85 | 0.88 |
| BMI (kg/m2) | 22.16 ± 2.56 | 22.92 ± 2.62 | 0.09 | 22.44 ± 3.97 | 22.33 ± 4.21 | 0.89 | 0.10 |
| FFM (kg) | 48.44 ± 6.00 | 50.09 ± 6.49 | 0.11 | 48.93 ± 5.23 | 49.25 ± 6.46 | 0.93 | 0.39 |
| FM (kg) | 13.43 ± 5.64 | 13.96 ± 5.95 | 0.38 | 13.34 ± 6.62 | 13.65 ± 7.97 | 0.92 | 0.83 |
| Visceral fat (kg) | 2.68 ± 0.86 | 2.85 ± 0.94 | 0.76 | 2.80 ± 1.39 | 2.72 ± 1.46 | 0.98 | 0.48 |
| ASMM (kg) | 7.64 ± 1.24 | 7.81 ± 1.20 | 0.02 | 7.41 ± 1.53 | 7.51 ± 1.68 | 0.64 | 0.48 |
| Dietary intake | |||||||
| Energy (kcal/day) | 1545.85 ± 373.66 | 1419.69 ± 347.34 | 0.23 | 1605.90 ± 289.86 | 1538.47 ± 453.91 | 0.25 | 0.68 |
| Carbohydrate (%) | 54.02 ± 7.55 | 53.83 ± 9.81 | 0.75 | 52.27 ± 7.54 | 53.52 ± 7.55 | 0.51 | 0.74 |
| Protein (%) | 20.02 ± 5.26 | 18.46 ± 4.98 | 0.14 | 19.82 ± 4.41 | 18.60 ± 4.98 | 0.87 | 0.85 |
| Protein (g) | 75.37 ± 21.32 | 88.12 ± 22.16 | 0.98 | 75.37 ± 21.32 | 80.97 ± 22.16 | 0.72 | 0.27 |
| Protein/kg | 1.22 ± 0.30 | 1.37 ± 0.57 | 0.57 | 1.32 ± 0.43 | 1.40 ± 0.40 | 0.42 | 0.78 |
| Leucine (g/day) | 4.32 ± 1.81 | 12.65 ± 2.00 | 0.01 | 4.78 ± 2.35 | 4.03 ± 2.15 | 0.01 | 0.01 |
| Isoleucine (g/day) | 2.48 ± 1.09 | 2.45 ± 1.20 | 0.30 | 2.83 ± 1.77 | 2.34 ± 1.25 | 0.75 | 0.25 |
| Valine (g/day) | 2.80 ± 1.16 | 2.81 ± 1.29 | 0.29 | 3.28 ± 2.23 | 2.63 ± 1.37 | 0.73 | 0.21 |
| Variables | Treatment | p * | |||||
|---|---|---|---|---|---|---|---|
| Leucine (n = 26) | p | Control (n = 26) | p | ||||
| Time | Time | ||||||
| T0 | T1 | T0 | T1 | ||||
| Body composition | |||||||
| Weight (kg) | 63.01 ± 1.90 | 65.27 ± 1.97 | 0.01 | 62.68 ± 2.02 | 62.51 ± 2.24 | 0.74 | 0.25 |
| BMI (kg/m2) | 22.31 ± 2.77 | 23.08 ± 2.87 | 0.01 | 22.41 ± 3.64 | 22.74 ± 3.93 | 0.89 | 0.23 |
| FFM (kg) | 48.56 ± 5.77 | 50.21 ± 6.02 | 0.03 | 49.07 ± 6.59 | 49.33 ± 7.08 | 0.90 | 0.20 |
| FM (kg) | 14.46 ± 7.50 | 14.94 ± 7.41 | 0.27 | 13.59 ± 6.66 | 13.84 ± 7.77 | 0.64 | 0.75 |
| Visceral fat (kg) | 2.90 ± 1.12 | 3.01 ± 1.08 | 0.88 | 2.69 ± 1.31 | 2.62 ± 1.35 | 0.33 | 0.56 |
| ASMM (kg) | 7.70 ± 1.21 | 7.91 ± 1.16 | 0.01 | 7.42 ± 1.46 | 7.62 ± 1.59 | 0.49 | 0.89 |
| Dietary intake | |||||||
| Energy (kcal/day) | 1475.20 ± 422.66 | 1400.32 ± 302.66 | 0.43 | 1475.20 ± 332.14 | 1467.00 ± 406.65 | 0.67 | 0.45 |
| Carbohydrate (%) | 53.73 ± 7.12 | 53.54 ± 9.54 | 0.53 | 52.52 ± 8.31 | 54.11 ± 10.21 | 0.77 | 0.64 |
| Protein (%) | 19.91 ± 4.87 | 18.10 ± 5.59 | 0.08 | 18.95 ± 5.39 | 18.54 ± 4.19 | 0.56 | 0.53 |
| Protein (g) | 72.59 ± 23.15 | 87.08 ± 17.53 | 0.01 | 71.63 ± 23.15 | 86.91 ± 18.82 | 0.44 | 0.15 |
| Protein/kg | 1.15 ± 0.35 | 1.26 ± 0.53 | 0.01 | 1.18 ± 0.45 | 1.36 ± 0.36 | 0.25 | 0.70 |
| Leucine (g/day) | 4.21 ± 1.70 | 12.56 ± 1.56 | 0.01 | 4.22 ± 2.39 | 3.73 ± 1.93 | 0.19 | 0.01 |
| Isoleucine (g/day) | 2.37 ± 1.11 | 2.42 ± 1.00 | 0.48 | 2.51 ± 1.69 | 2.20 ± 1.09 | 0.88 | 0.24 |
| Valine (g/day) | 2.69 ± 1.22 | 2.75 ± 1.08 | 0.43 | 2.93 ± 2.05 | 2.52 ± 1.16 | 0.98 | 0.22 |
| Variables | Treatment | p * | |||||
|---|---|---|---|---|---|---|---|
| Leucine (n = 14) | p | Control (n = 14) | p | ||||
| Time | Time | ||||||
| T0 | T1 | T0 | T1 | ||||
| Body composition | |||||||
| Weight (kg) | 59.71 ± 8.43 | 62.10 ± 9.11 | 0.01 | 60.65 ± 9.97 | 60.97 ± 10.63 | 0.74 | 0.07 |
| BMI (kg/m2) | 21.52 ± 2.26 | 22.35 ± 2.40 | 0.01 | 21.67 ± 3.91 | 21.54 ± 4.00 | 0.79 | 0.06 |
| FFM (kg) | 47.18 ± 5.63 | 49.42 ± 6.83 | 0.01 | 47.90 ± 4.75 | 48.80 ± 6.26 | 0.50 | 0.28 |
| FM (kg) | 12.53 ± 5.65 | 12.68 ± 5.98 | 0.84 | 12.71 ± 7.27 | 12.16 ± 7.78 | 0.38 | 0.39 |
| Visceral fat (kg) | 2.62 ± 0.85 | 2.62 ± 0.87 | 0.63 | 2.53 ± 1.14 | 2.45 ± 1.29 | 0.46 | 0.40 |
| ASMM (kg) | 7.40 ± 1.23 | 7.56 ± 1.17 | 0.03 | 7.16 ± 1.49 | 7.21 ± 1.63 | 0.59 | 0.36 |
| Dietary intake | |||||||
| Energy (kcal/day) | 1515.57 ± 406.21 | 1388.81 ± 365.73 | 0.21 | 1561.57 ± 310.83 | 1498.68 ± 456.22 | 0.67 | 0.69 |
| Carbohydrate (%) | 53.36 ± 7.07 | 53.56 ± 11.79 | 0.94 | 52.63 ± 8.02 | 54.97 ± 8.95 | 0.23 | 0.64 |
| Protein (%) | 20.49 ± 5.19 | 18.61 ± 5.31 | 0.20 | 19.39 ± 4.74 | 17.89 ± 5.30 | 0.16 | 0.82 |
| Protein (g) | 75.90 ± 23.08 | 93.75 ± 23.54 | 0.03 | 78.23 ± 26.28 | 86.56 ± 30.39 | 0.13 | 0.34 |
| Protein/kg | 1.26 ± 0.31 | 1.41 ± 0.42 | 0.12 | 1.31 ± 0.47 | 1.34 ± 0.59 | 0.09 | 0.50 |
| Leucine (g/day) | 4.34 ± 1.80 | 12.45 ± 2.01 | 0.01 | 4.67 ± 2.40 | 3.87 ± 2.35 | 0.15 | 0.01 |
| Isoleucine (g/day) | 2.46 ± 1.05 | 2.33 ± 1.26 | 0.66 | 2.77 ± 1.88 | 2.26 ± 1.37 | 0.20 | 0.53 |
| Valine (g/day) | 2.78 ± 1.15 | 2.66 ± 1.33 | 0.72 | 3.24 ± 2.43 | 2.53 ± 1.50 | 0.20 | 0.43 |
| Variables | Treatment | p * | |||||
|---|---|---|---|---|---|---|---|
| Leucine (n = 19) | p | Control (n = 19) | p | ||||
| Time | Time | ||||||
| T0 | T1 | T0 | T1 | ||||
| Body composition | |||||||
| Weight (kg) | 61.20 ± 8.45 | 63.56 ± 9.00 | 0.01 | 60.72 ± 10.82 | 63.30 ± 11.61 | 0.28 | 0.92 |
| BMI (kg/m2) | 21.80 ± 2.19 | 22.61 ± 2.30 | 0.01 | 21.63 ± 3.72 | 22.26 ± 3.76 | 0.29 | 0.76 |
| FFM (kg) | 47.91 ± 5.43 | 49.78 ± 6.35 | 0.01 | 47.98 ± 6.46 | 48.72 ± 7.21 | 0.44 | 0.23 |
| FM (kg) | 13.28 ± 5.80 | 13.65 ± 5.96 | 0.52 | 12.71 ± 6.66 | 13.28 ± 7.09 | 0.35 | 0.19 |
| Visceral fat (kg) | 2.76 ± 0.85 | 2.78 ± 0.83 | 0.72 | 2.49 ± 1.12 | 2.41 ± 1.23 | 0.35 | 0.22 |
| ASMM (kg) | 7.43 ± 1.11 | 7.64 ± 1.06 | 0.01 | 7.15 ± 1.49 | 7.29 ± 1.62 | 0.07 | 0.44 |
| Dietary intake | |||||||
| Energy (kcal/day) | 1475.20 ± 422.66 | 1400.32 ± 302.66 | 0.43 | 1475.20 ± 332.14 | 1467.00 ± 406.65 | 0.67 | 0.45 |
| Carbohydrate (%) | 53.50 ± 7.43 | 53.79 ± 10.19 | 0.88 | 52.66 ± 9.14 | 54.20 ± 9.49 | 0.32 | 0.61 |
| Protein (%) | 19.91 ± 4.87 | 18.10 ± 5.59 | 0.08 | 18.95 ± 5.39 | 18.54 ± 4.19 | 0.56 | 0.53 |
| Protein (g) | 75.07 ± 24.23 | 89.93 ± 23.38 | 0.04 | 71.82 ± 27.68 | 79.90 ± 30.67 | 0.04 | 0.42 |
| Protein/kg | 1.22 ± 0.35 | 1.40 ± 0.38 | 0.11 | 1.20 ± 0.49 | 1.27 ± 0.55 | 0.32 | 0.41 |
| Leucine (g/day) | 4.18 ± 1.82 | 12.56 ± 1.90 | 0.01 | 4.22 ± 2.50 | 3.65 ± 2.13 | 0.19 | 0.01 |
| Isoleucine (g/day) | 2.39 ± 1.16 | 2.42 ± 1.00 | 0.94 | 2.51 ± 1.69 | 2.20 ± 1.09 | 0.26 | 0.44 |
| Valine (g/day) | 2.72 ± 1.31 | 2.75 ± 1.22 | 0.92 | 2.92 ± 2.27 | 2.45 ± 1.31 | 0.28 | 0.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soares, J.D.P.; Siqueira, J.M.; Brito, F.d.S.B.; Pimentel, G.D. A Randomized Controlled Trial on the Effects of Leucine-Supplement Combined with Nutritional Counseling on Body Composition in Mix Cancer Older Men. Nutrients 2024, 16, 210. https://doi.org/10.3390/nu16020210
Soares JDP, Siqueira JM, Brito FdSB, Pimentel GD. A Randomized Controlled Trial on the Effects of Leucine-Supplement Combined with Nutritional Counseling on Body Composition in Mix Cancer Older Men. Nutrients. 2024; 16(2):210. https://doi.org/10.3390/nu16020210
Chicago/Turabian StyleSoares, Jéssika D. P., Jéssika M. Siqueira, Flávia dos S. B. Brito, and Gustavo D. Pimentel. 2024. "A Randomized Controlled Trial on the Effects of Leucine-Supplement Combined with Nutritional Counseling on Body Composition in Mix Cancer Older Men" Nutrients 16, no. 2: 210. https://doi.org/10.3390/nu16020210
APA StyleSoares, J. D. P., Siqueira, J. M., Brito, F. d. S. B., & Pimentel, G. D. (2024). A Randomized Controlled Trial on the Effects of Leucine-Supplement Combined with Nutritional Counseling on Body Composition in Mix Cancer Older Men. Nutrients, 16(2), 210. https://doi.org/10.3390/nu16020210





