The Evidence for Diet as a Treatment in Migraine—A Review
Abstract
1. Introduction
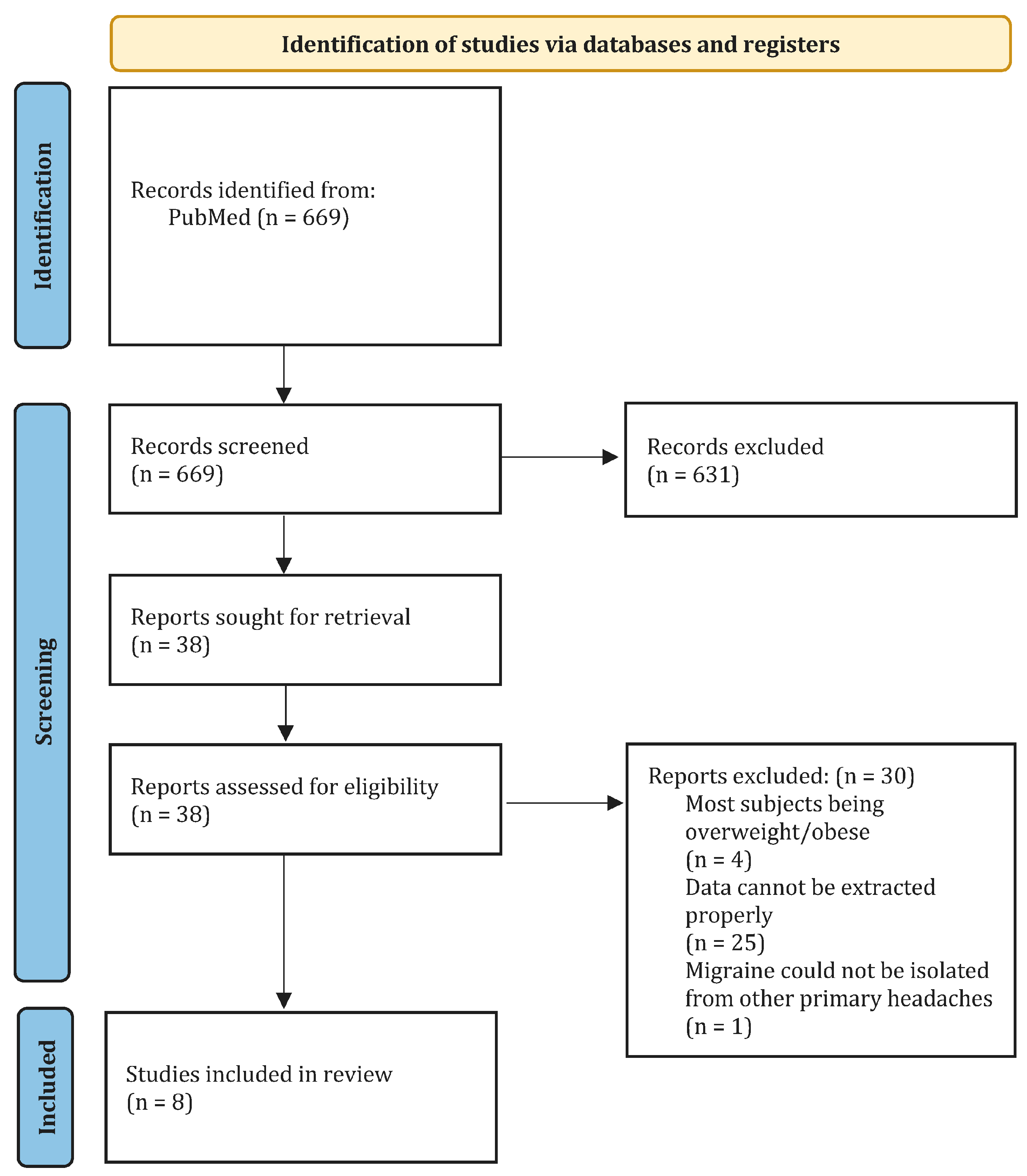
2. Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction
2.4. Quality of Assessment
3. Results
3.1. Diet Interventions
3.1.1. Ketogenic Diet
3.1.2. Low-Carb Diet
3.1.3. DASH Diet
3.1.4. Elimination Diet
3.1.5. Gluten-Free Diet
3.1.6. NOS Scores
4. Discussion
4.1. Proposed Pathophysiological Mechanisms behind Diet Regimens
4.1.1. Ketogenic Diet
4.1.2. DASH Diet
4.1.3. Low-Fat Vegan Diet
4.1.4. Elimination Diet
4.1.5. Gluten-Free Diet
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018, 38, 1–211. [Google Scholar] [CrossRef] [PubMed]
- Safiri, S.; Pourfathi, H.; Eagan, A.; Mansournia, M.A.; Khodayari, M.T.; Sullman, M.J.; Kaufman, J.; Collins, G.; Dai, H.; Bragazzi, N.L.; et al. Global, regional, and national burden of migraine in 204 countries and territories, 1990 to 2019. Pain 2022, 163, e293–e309. [Google Scholar] [CrossRef] [PubMed]
- Steiner, T.J.; Stovner, L.J.; Vos, T. GBD 2015: Migraine is the third cause of disability in under 50s. J. Headache Pain 2016, 17, 104. [Google Scholar] [CrossRef]
- Steiner, T.J.; Birbeck, G.L.; Jensen, R.H.; Katsarava, Z.; Stovner, L.J.; Martelletti, P. Headache disorders are third cause of disability worldwide. J. Headache Pain 2015, 16, 58. [Google Scholar] [CrossRef]
- Eigenbrodt, A.K.; Ashina, H.; Khan, S.; Diener, H.-C.; Mitsikostas, D.D.; Sinclair, A.J.; Pozo-Rosich, P.; Martelletti, P.; Ducros, A.; Lantéri-Minet, M.; et al. Diagnosis and management of migraine in ten steps. Nat. Rev. Neurol. 2021, 17, 501–514. [Google Scholar] [CrossRef]
- Marmura, M.J. Triggers, Protectors, and Predictors in Episodic Migraine. Curr. Pain. Headache Rep. 2018, 22, 81. [Google Scholar] [CrossRef] [PubMed]
- Kelman, L. The triggers or precipitants of the acute migraine attack. Cephalalgia 2007, 27, 394–402. [Google Scholar] [CrossRef]
- Kesserwani, H. Migraine Triggers: An Overview of the Pharmacology, Biochemistry, Atmospherics, and Their Effects on Neural Networks. Cureus 2021, 13, e14243. [Google Scholar] [CrossRef]
- Ramsden, C.E.; Zamora, D.; Faurot, K.R.; MacIntosh, B.; Horowitz, M.; Keyes, G.S.; Yuan, Z.-X.; Miller, V.; Lynch, C.; Honvoh, G.; et al. Dietary alteration of n-3 and n-6 fatty acids for headache reduction in adults with migraine: Randomized controlled trial. BMJ 2021, 374, n1448. [Google Scholar] [CrossRef]
- Bic, Z.; Blix, G.G.; Hopp, H.P.; Leslie, F.M.; Schell, M.J. The influence of a low-fat diet on incidence and severity of migraine headaches. J. Womens Health Gend. Based Med. 1999, 8, 623–630. [Google Scholar] [CrossRef]
- Karimi, E.; Tirani, S.A.; Azimi, E.S.; Askari, G.; As’habi, A.; Arab, A. Is there an association between a plant-based eating pattern and clinical findings of a migraine headache? Front. Nutr. 2023, 10, 1117740. [Google Scholar] [CrossRef]
- Thuraiaiyah, J.; Ashina, H.; Christensen, R.H.; Al-Khazali, H.M.; Wiggers, A.; Amin, F.M.; Steiner, T.J.; Ashina, M. Premonitory symptoms in migraine: A REFORM Study. Cephalalgia 2024, 44, 3331024231223979. [Google Scholar] [CrossRef]
- Furness, J.B.; Callaghan, B.P.; Rivera, L.R.; Cho, H.-J. The enteric nervous system and gastrointestinal innervation: Integrated local and central control. Adv. Exp. Med. Biol. 2014, 817, 39–71. [Google Scholar]
- Arzani, M.; Jahromi, S.R.; Ghorbani, Z.; Vahabizad, F.; Martelletti, P.; Ghaemi, A.; Sacco, S.; Togha, M.; School of Advanced Studies of the European Headache Federation (EHF-SAS). Gut-brain Axis and migraine headache: A comprehensive review. J. Headache Pain. 2020, 21, 15. [Google Scholar] [CrossRef]
- Bongiovanni, D.; Benedetto, C.; Corvisieri, S.; Del Favero, C.; Orlandi, F.; Allais, G.; Sinigaglia, S.; Fadda, M. Effectiveness of ketogenic diet in treatment of patients with refractory chronic migraine. Neurol. Sci. 2021, 42, 3865–3870. [Google Scholar] [CrossRef]
- Lovati, C.; D’alessandro, C.M.; Della Ventura, S.; Muzio, F.; Pantoni, L. Ketogenic diet in refractory migraine: Possible efficacy and role of ketone bodies-a pilot experience. Neurol. Sci. 2022, 43, 6479–6485. [Google Scholar] [CrossRef]
- Arab, A.; Khorvash, F.; Kazemi, M.; Heidari, Z.; Askari, G. Effects of the Dietary Approaches to Stop Hypertension (DASH) diet on clinical, quality of life and mental health outcomes in women with migraine: A randomised controlled trial. Br. J. Nutr. 2022, 128, 1535–1544. [Google Scholar] [CrossRef]
- Alpay, K.; Ertaş, M.; Orhan, E.K.; Üstay, D.K.; Lieners, C.; Baykan, B. Diet restriction in migraine, based on IgG against foods: A clinical double-blind, randomised, cross-over trial. Cephalalgia 2010, 30, 829–837. [Google Scholar] [CrossRef]
- Bunner, A.E.; Agarwal, U.; Gonzales, J.F.; Valente, F.; Barnard, N.D. Nutrition intervention for migraine: A randomized crossover trial. J. Headache Pain 2014, 15, 69. [Google Scholar] [CrossRef]
- Özön, A.; Karadaş, Ö.; Özge, A. Efficacy of Diet Restriction on Migraines. Noro Psikiyatr. Ars. 2018, 55, 233–237. [Google Scholar]
- Özön, A.; Karadaş, Ö. The Effectiveness of Diet Restriction in Elderly with Migraine. Noro Psikiyatr. Ars. 2021, 58, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Ameghino, L.; Farez, M.; Wilken, M.; Goicochea, M. Headache in Patients with Celiac Disease and Its Response to the Gluten-Free Diet. J. Oral. Facial Pain Headache 2019, 33, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Razeghi Jahromi, S.; Ghorbani, Z.; Martelletti, P.; Lampl, C.; Togha, M.; School of Advanced Studies of the European Headache Federation (EHF-SAS). Association of diet and headache. J. Headache Pain 2019, 20, 106. [Google Scholar] [CrossRef] [PubMed]
- Maalouf, M.; Sullivan, P.G.; Davis, L.; Kim, D.Y.; Rho, J.M. Ketones inhibit mitochondrial production of reactive oxygen species production following glutamate excitotoxicity by increasing NADH oxidation. Neuroscience 2007, 145, 256–264. [Google Scholar] [CrossRef]
- Jeong, E.A.; Jeon, B.T.; Shin, H.J.; Kim, N.; Lee, D.H.; Kim, H.J.; Kang, S.S.; Cho, G.J.; Choi, W.S.; Roh, G.S. Ketogenic diet-induced peroxisome proliferator-activated receptor-gamma activation decreases neuroinflammation in the mouse hippocampus after kainic acid-induced seizures. Exp. Neurol. 2011, 232, 195–202. [Google Scholar] [CrossRef]
- Harrington, M.G.; Fonteh, A.N.; Cowan, R.P.; Perrine, K.; Pogoda, J.M.; Biringer, R.G.; Hühmer, A.F. Cerebrospinal fluid sodium increases in migraine. Headache 2006, 46, 1128–1135. [Google Scholar] [CrossRef]
- Nielsen, F.H. Magnesium, inflammation, and obesity in chronic disease. Nutr. Rev. 2010, 68, 333–340. [Google Scholar] [CrossRef]
- Dibaba, D.T.; Xun, P.; He, K. Dietary magnesium intake is inversely associated with serum C-reactive protein levels: Meta-analysis and systematic review. Eur. J. Clin. Nutr. 2014, 68, 510–516. [Google Scholar] [CrossRef]
- Hajjarzadeh, S.; Bakhshimoghaddam, F.; Behrouz, M.; Nikniaz, Z.; Mahdavi, R.; Shalilahmadi, D.; Karandish, M. The relation of adherence to the DASH diet with migraine attack frequency and pain intensity in Iranian women: A cross-sectional study. Nutr. Neurosci. 2023, 27, 353–360. [Google Scholar] [CrossRef]
- Maddock, J.; Ziauddeen, N.; Ambrosini, G.L.; Wong, A.; Hardy, R.; Ray, S. Adherence to a Dietary Approaches to Stop Hypertension (DASH)-type diet over the life course and associated vascular function: A study based on the MRC 1946 British birth cohort. Br. J. Nutr. 2018, 119, 581–589. [Google Scholar] [CrossRef]
- Zemel, M.B.; Sun, X. Dietary calcium and dairy products modulate oxidative and inflammatory stress in mice and humans. J. Nutr. 2008, 138, 1047–1052. [Google Scholar] [CrossRef]
- Sun, X.; Zemel, M.B. Calcitriol and calcium regulate cytokine production and adipocyte-macrophage cross-talk. J. Nutr. Biochem. 2008, 19, 392–399. [Google Scholar] [CrossRef]
- Armstrong, B.K.; Brown, J.B.; Clarke, H.T.; Crooke, D.K.; Hähnel, R.; Masarei, J.R.; Ratajczak, T. Diet and reproductive hormones: A study of vegetarian and nonvegetarian postmenopausal women. J. Natl. Cancer Inst. 1981, 67, 761–767. [Google Scholar]
- Bélanger, A.; Locong, A.; Noel, C.; Cusan, L.; Dupont, A.; Prévost, J.; Caron, S.; Sévigny, J. Influence of diet on plasma steroids and sex hormone-binding globulin levels in adult men. J. Steroid Biochem. 1989, 32, 829–833. [Google Scholar] [CrossRef]
- Mathew, P.G.; Dun, E.C.; Luo, J.J. A cyclic pain: The pathophysiology and treatment of menstrual migraine. Obstet. Gynecol. Surv. 2013, 68, 130–140. [Google Scholar] [CrossRef]
- Scuto, M.; Modafferi, S.; Rampulla, F.; Zimbone, V.; Tomasello, M.; Spano, S.; Ontario, M.; Palmeri, A.; Salinaro, A.T.; Siracusa, R.; et al. Redox modulation of stress resilience by Crocus sativus L. for potential neuroprotective and anti-neuroinflammatory applications in brain disorders: From molecular basis to therapy. Mech. Ageing Dev. 2022, 205, 111686. [Google Scholar] [CrossRef]
- Bakırhan, H.; Pehlivan, M.; Uyar Cankay, T.; Kocak, M. Migraine severity, disability, and duration: Is a good diet quality, high intake of phytochemicals and polyphenols important? Front. Nutr. 2022, 9, 1041907. [Google Scholar] [CrossRef]
- Heidari, H.; Shojaei, M.; Askari, G.; Majeed, M.; Bagherniya, M.; Barreto, G.E.; Sahebkar, A. The impact of curcumin on migraine: A comprehensive review. Biomed. Pharmacother. 2023, 164, 114910. [Google Scholar] [CrossRef]
- Scuto, M.; Rampulla, F.; Reali, G.M.; Spanò, S.M.; Salinaro, A.T.; Calabrese, V. Hormetic Nutrition and Redox Regulation in Gut-Brain Axis Disorders. Antioxidants 2024, 13, 484. [Google Scholar] [CrossRef] [PubMed]
- Lahat, N.; Shapiro, S.; Karban, A.; Gerstein, R.; Kinarty, A.; Lerner, A. Cytokine profile in coeliac disease. Scand. J. Immunol. 1999, 49, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, E.M.; Jahnsen, F.L.; Lundin, K.E.; Johansen, F.; Fausa, O.; Sollid, L.M.; Jahnsen, J.; Scott, H.; Brandtzaeg, P. Gluten induces an intestinal cytokine response strongly dominated by interferon gamma in patients with celiac disease. Gastroenterology 1998, 115, 551–563. [Google Scholar] [CrossRef]
- Bigal, M.E.; Liberman, J.N.; Lipton, R.B. Obesity and migraine: A population study. Neurology 2006, 66, 545–550. [Google Scholar] [CrossRef]
- Bigal, M.E.; Tsang, A.; Loder, E.; Serrano, D.; Reed, M.L.; Lipton, R.B. Body mass index and episodic headaches: A population-based study. Arch. Intern. Med. 2007, 167, 1964–1970. [Google Scholar] [CrossRef]
- Kristoffersen, E.S.; Børte, S.; Hagen, K.; Zwart, J.A.; Winsvold, B.S. Migraine, obesity and body fat distribution—A population-based study. J. Headache Pain 2020, 21, 97. [Google Scholar] [CrossRef]
- Riggins, N.; Ehrlich, A. Episodic Migraine and Older Adults. Curr. Pain. Headache Rep. 2022, 26, 331–335. [Google Scholar] [CrossRef]
| Inclusion Criteria | Exclusion Criteria | |
|---|---|---|
| Population (P) | Adult patients (≥18 years) | Paediatric patients (≤18 years) |
| Intervention (I) | Food, dietary, and nutritional | Supplements, vitamins, medication |
| Comparison (C) | Interventions and placebo/control | Macronutrients, caloric intake, or weight/BMI |
| Outcome (O) | Diets as triggering factors or treatment/prevention of migraine | - |
| Study design (S) | Retrospective or prospective observational studies, RCTs, and non-RCTs | Conference papers, guidelines, opinions, editorials, letters, case reports, book chapters, or comments |
| Language | English | Non-English |
| Other | A diagnosis of migraine must meet the criteria outlines in any iteration of the ICHD | Studies with less than 10 patients If data could not be extracted properly Studies reporting results on overlapping cohorts |
| Author, Year | Diet Intervention | NOS Score |
|---|---|---|
| Arab, A. et al., 2022 [17] | DASH diet | 7 |
| Bunner, A. E. et al., 2014 [19] | Low-fat vegan diet and elimination diet | 4 |
| Alpay, K. et al., 2010 [18] | Elimination diet | 4 |
| Özön, A. Ö. et al., 2018 [20] | Elimination diet | 4 |
| Özön, A. Ö. et al., 2021 [21] | Elimination diet | 4 |
| Ameghino, L. et al., 2019 [22] | Gluten-free diet | 4 |
| Lovati, C. et al., 2022 [16] | Ketogenic diet or low-carb diet | 3 |
| Bongiovanni, D. et al., 2021 [15] | Ketogenic diet | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, K.V.; Schytz, H.W. The Evidence for Diet as a Treatment in Migraine—A Review. Nutrients 2024, 16, 3415. https://doi.org/10.3390/nu16193415
Nguyen KV, Schytz HW. The Evidence for Diet as a Treatment in Migraine—A Review. Nutrients. 2024; 16(19):3415. https://doi.org/10.3390/nu16193415
Chicago/Turabian StyleNguyen, Kattia Valentine, and Henrik Winther Schytz. 2024. "The Evidence for Diet as a Treatment in Migraine—A Review" Nutrients 16, no. 19: 3415. https://doi.org/10.3390/nu16193415
APA StyleNguyen, K. V., & Schytz, H. W. (2024). The Evidence for Diet as a Treatment in Migraine—A Review. Nutrients, 16(19), 3415. https://doi.org/10.3390/nu16193415






