Exploring Nutritional Status and Metabolic Imbalances in Children with FASD: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Ethics
2.3. Laboratory Methods
2.4. Statistical Analysis
3. Results
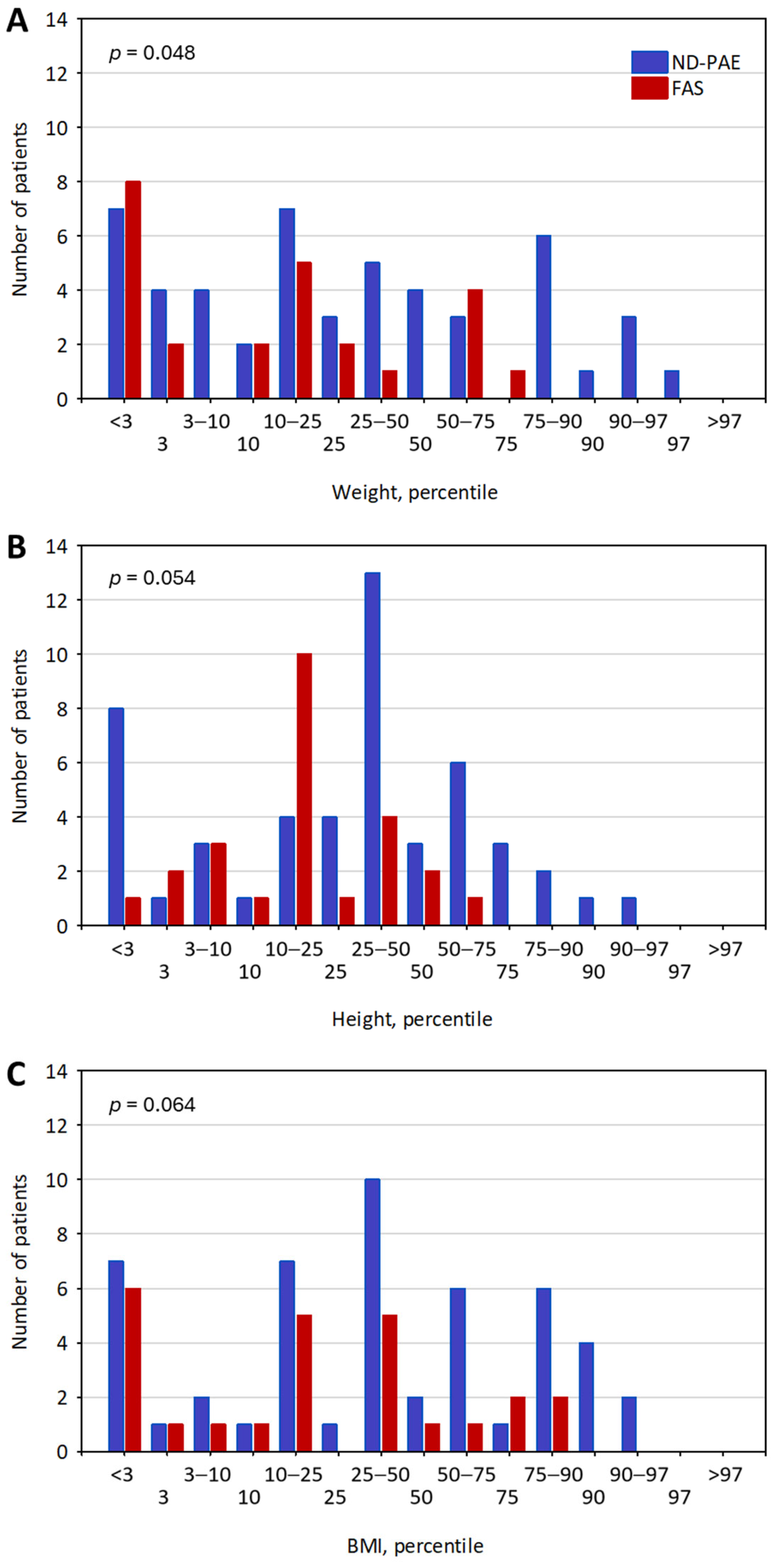
3.1. Sample Characteristics
3.2. Laboratory Test Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lange, S.; Probst, C.; Gmel, G.; Rehm, J.; Burd, L.; Popova, S. Global Prevalence of Fetal Alcohol Spectrum Disorder Among Children and Youth: A Systematic Review and Meta-Analysis. JAMA Pediatr. 2017, 171, 948. [Google Scholar] [CrossRef] [PubMed]
- Roozen, S.; Peters, G.-J.Y.; Kok, G.; TownEQend, D.; Nijhuis, J.; Curfs, L. Worldwide Prevalence of Fetal Alcohol Spectrum Disorders: A Systematic Literature Review Including Meta-Analysis. Alcohol. Clin. Exp. Res. 2016, 40, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Popova, S.; Lange, S.; Probst, C.; Gmel, G.; Rehm, J. Estimation of National, Regional, and Global Prevalence of Alcohol Use during Pregnancy and Fetal Alcohol Syndrome: A Systematic Review and Meta-Analysis. Lancet Glob. Health 2017, 5, e290–e299. [Google Scholar] [CrossRef] [PubMed]
- Donald, K.A.; Eastman, E.; Howells, F.M.; Adnams, C.; Riley, E.P.; Woods, R.P.; Narr, K.L.; Stein, D.J. Neuroimaging Effects of Prenatal Alcohol Exposure on the Developing Human Brain: A Magnetic Resonance Imaging Review. Acta Neuropsychiatr. 2015, 27, 251–269. [Google Scholar] [CrossRef] [PubMed]
- Glass, L.; Ware, A.L.; Mattson, S.N. Neurobehavioral, Neurologic, and Neuroimaging Characteristics of Fetal Alcohol Spectrum Disorders. Handb. Clin. Neurol. 2014, 125, 435–462. [Google Scholar] [CrossRef]
- Cusick, S.E.; Georgieff, M.K. The Role of Nutrition in Brain Development: The Golden Opportunity of the “First 1000 Days. ” J. Pediatr. 2016, 175, 16–21. [Google Scholar] [CrossRef]
- Van Dyke, D.C.; Mackay, L.; Ziaylek, E.N. Management of Severe Feeding Dysfunction in Children with Fetal Alcohol Syndrome. Clin. Pediatr. 1982, 21, 336–339. [Google Scholar] [CrossRef]
- Amos-Kroohs, R.M.; Fink, B.A.; Smith, C.J.; Chin, L.; Van Calcar, S.C.; Wozniak, J.R.; Smith, S.M. Abnormal Eating Behaviors Are Common in Children with Fetal Alcohol Spectrum Disorder. J. Pediatr. 2016, 169, 194–200.e1. [Google Scholar] [CrossRef]
- Werts, R.L.; Van Calcar, S.C.; Wargowski, D.S.; Smith, S.M. Inappropriate Feeding Behaviors and Dietary Intakes in Children with Fetal Alcohol Spectrum Disorder or Probable Prenatal Alcohol Exposure. Alcohol. Clin. Exp. Res. 2014, 38, 871–878. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Risbud, R.D.; Chambers, C.D.; Thomas, J.D. Dietary Nutrient Intake in School-Aged Children With Heavy Prenatal Alcohol Exposure. Alcohol. Clin. Exp. Res. 2016, 40, 1075–1082. [Google Scholar] [CrossRef]
- Fuglestad, A.J.; Fink, B.A.; Eckerle, J.K.; Boys, C.J.; Hoecker, H.L.; Kroupina, M.G.; Zeisel, S.H.; Georgieff, M.K.; Wozniak, J.R. Inadequate Intake of Nutrients Essential for Neurodevelopment in Children with Fetal Alcohol Spectrum Disorders (FASD). Neurotoxicol. Teratol. 2013, 39, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Okulicz-Kozaryn, K.; Maryniak, A.; Borkowska, M.; Śmigiel, R.; Dylag, K.A. Diagnosis of Fetal Alcohol Spectrum Disorders (Fasds): Guidelines of Interdisciplinary Group of Polish Professionals. Int. J. Environ. Res. Public. Health 2021, 18, 7526. [Google Scholar] [CrossRef] [PubMed]
- NHANES Anthropometry Procedures Manual. Available online: https://www.cdc.gov/nchs/data/nhanes/nhanes_07_08/manual_an.pdf (accessed on 10 September 2024).
- Kułaga, Z.; Różdżyńska, A.; Palczewska, I.; Grajda, A.; Gurzkowska, B.; Napieralska, E.; Litwin, M.; Grupa Badaczy OLA. Siatki Centylowe Wysokości, Masy Ciała i Wskaźnika Masy Ciała Dzieci i Młodzieży w Polsce-Wyniki Badania OLAF Percentile Charts of Height, Body Mass and Body Mass Index in Children and Adolescents in Poland-Results of the OLAF Study. Stand. Med. 2010, 7, 690–700. [Google Scholar]
- Colantonio, D.A.; Kyriakopoulou, L.; Chan, M.K.; Daly, C.H.; Brinc, D.; Venner, A.A.; Pasic, M.D.; Armbruster, D.; Adeli, K. Closing the Gaps in Pediatric Laboratory Reference Intervals: A CALIPER Database of 40 Biochemical Markers in a Healthy and Multiethnic Population of Children. Clin. Chem. 2012, 58, 854–868. [Google Scholar] [CrossRef]
- Bailey, D.; Colantonio, D.; Kyriakopoulou, L.; Cohen, A.H.; Chan, M.K.; Armbruster, D.; Adeli, K. Marked Biological Variance in Endocrine and Biochemical Markers in Childhood: Establishment of Pediatric Reference Intervals Using Healthy Community Children from the CALIPER Cohort. Clin. Chem. 2013, 59, 1393–1403. [Google Scholar] [CrossRef]
- Raizman, J.E.; Cohen, A.H.; Teodoro-Morrison, T.; Wan, B.; Khun-Chen, M.; Wilkenson, C.; Bevilaqua, V.; Adeli, K. Pediatric Reference Value Distributions for Vitamins A and E in the CALIPER Cohort and Establishment of Age-Stratified Reference Intervals. Clin. Biochem. 2014, 47, 812–815. [Google Scholar] [CrossRef]
- Rusinska, A.; Płudowski, P.; Walczak, M.; Borszewska-Kornacka, M.K.; Bossowski, A.; Chlebna-Sokół, D.; Czech-Kowalska, J.; Dobrzańska, A.; Franek, E.; Helwich, E.; et al. Vitamin D Supplementation Guidelines for General Population and Groups at Risk of Vitamin D Deficiency in Poland-Recommendations of the Polish Society of Pediatric Endocrinology and Diabetes and the Expert Panel With Participation of National Specialist Consultants and Representatives of Scientific Societies-2018 Update. Front. Endocrinol. 2018, 9, 246. [Google Scholar] [CrossRef]
- Soldin, O.P.; Bierbower, L.H.; Choi, J.J.; Choi, J.J.; Thompson-Hoffman, S.; Soldin, S.J. Serum Iron, Ferritin, Transferrin, Total Iron Binding Capacity, Hs-CRP, LDL Cholesterol and Magnesium in Children; New Reference Intervals Using the Dade Dimension Clinical Chemistry System. Clin. Chim. Acta 2004, 342, 211–217. [Google Scholar] [CrossRef]
- Bohn, M.K.; Hall, A.; Wilson, S.; Henderson, T.; Adeli, K. Pediatric Reference Intervals for Critical Point-of-Care Whole Blood Assays in the CALIPER Cohort of Healthy Children and Adolescents. Am. J. Clin. Pathol. 2021, 156, 1030–1037. [Google Scholar] [CrossRef]
- Van Pelt, J.L.; Klatte, S.; Hwandih, T.; Barcaru, A.; Riphagen, I.J.; Linssen, J.; Bakker, S.J.L. Reference Intervals for Sysmex XN Hematological Parameters as Assessed in the Dutch Lifelines Cohort. Clin. Chem. Lab. Med. 2022, 60, 907–920. [Google Scholar] [CrossRef]
- Katoch, O.R. Determinants of Malnutrition among Children: A Systematic Review. Nutrition 2022, 96, 111565. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, S.M.; Corredor, J.; Fisher-Medina, J.; Cohen, J.; Rabinowitz, S. Diagnosis and Treatment of Feeding Disorders in Children with Developmental Disabilities. Pediatrics 2001, 108, 671–676. [Google Scholar] [CrossRef]
- Beer, S.S.; Juarez, M.D.; Vega, M.W.; Canada, N.L. Pediatric Malnutrition: Putting the New Definition and Standards Into Practice. Nutr. Clin. Pract. 2015, 30, 609–624. [Google Scholar] [CrossRef] [PubMed]
- Becker, P.J.; Gunnell Bellini, S.; Wong Vega, M.; Corkins, M.R.; Spear, B.A.; Spoede, E.; Hoy, M.K.; Piemonte, T.A.; Rozga, M. Validity and Reliability of Pediatric Nutrition Screening Tools for Hospital, Outpatient, and Community Settings: A 2018 Evidence Analysis Center Systematic Review. J. Acad. Nutr. Diet 2020, 120, 288–318.e2. [Google Scholar] [CrossRef]
- Domin, A.; Mazur, A. Nutritional Status of a Group of Polish Children with FASD: A Retrospective Study. Front. Nutr. 2023, 10, 1111545. [Google Scholar] [CrossRef] [PubMed]
- Castells, S.; Mark, E.; Abaci, F.; Schwartz, E. Growth Retardation in Fetal Alcohol Syndrome. Unresponsiveness to Growth-Promoting Hormones. Dev. Pharmacol. Ther. 1981, 3, 232–241. [Google Scholar] [CrossRef]
- Carter, R.C.; Jacobson, J.L.; Sokol, R.J.; Avison, M.J.; Jacobson, S.W. Fetal Alcohol-Related Growth Restriction from Birth through Young Adulthood and Moderating Effects of Maternal Prepregnancy Weight. Alcohol. Clin. Exp. Res. 2013, 37, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Pielage, M.; El Marroun, H.; Odendaal, H.J.; Willemsen, S.P.; Hillegers, M.H.J.; Steegers, E.A.P.; Rousian, M. Alcohol Exposure before and during Pregnancy Is Associated with Reduced Fetal Growth: The Safe Passage Study. BMC Med. 2023, 21, 318. [Google Scholar] [CrossRef]
- Kuehn, D.; Aros, S.; Cassorla, F.; Avaria, M.; Unanue, N.; Henriquez, C.; Kleinsteuber, K.; Conca, B.; Avila, A.; Carter, T.C.; et al. A Prospective Cohort Study of the Prevalence of Growth, Facial, and Central Nervous System Abnormalities in Children with Heavy Prenatal Alcohol Exposure. Alcohol. Clin. Exp. Res. 2012, 36, 1811–1819. [Google Scholar] [CrossRef]
- Astley, S.J.; Bledsoe, J.M.; Davies, J.K. The Essential Role of Growth Deficiency in the Diagnosis of Fetal Alcohol Spectrum Disorder. Adv. Pediatr. Res. 2016, 3, 9. [Google Scholar] [CrossRef]
- Keller, U. Nutritional Laboratory Markers in Malnutrition. J. Clin. Med. 2019, 8, 775. [Google Scholar] [CrossRef] [PubMed]
- Keller, U.; Szinnai, G.; Bilz, S.; Berneis, K. Effects of Changes in Hydration on Protein, Glucose and Lipid Metabolism in Man: Impact on Health. Eur. J. Clin. Nutr. 2003, 57 (Suppl. S2), S69–S74. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.B.; Hammo, B.; Barry, J.; Radhakrishnan, K. Overview of Albumin Physiology and Its Role in Pediatric Diseases. Curr. Gastroenterol. Rep. 2021, 23, 11. [Google Scholar] [CrossRef]
- Narayanan, V.; Gaudiani, J.L.; Mehler, P.S. Serum Albumin Levels May Not Correlate with Weight Status in Severe Anorexia Nervosa. Eat. Disord. 2009, 17, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Börjeson, M.; Wretlind, A. The Protein Metabolism in Anorexia Nervosa. AMA Arch. Gen. Psychiatry 1959, 1, 283–287. [Google Scholar] [CrossRef]
- Smith, G.; Robinson, P.H.; Fleck, A. Serum Albumin Distribution in Early Treated Anorexia Nervosa. Nutrition 1996, 12, 4–684. [Google Scholar] [CrossRef]
- Ellmerer, M.; Schaupp, L.; Brunner, G.A.; Sendlhofer, G.; Wutte, A.; Wach, P.; Pieber, T.R. Measurement of Interstitial Albumin in Human Skeletal Muscle and Adipose Tissue by Open-Flow Microperfusion. Am. J. Physiol. Endocrinol. Metab. 2000, 278, E352–E356. [Google Scholar] [CrossRef]
- Deo, M.G.; Bhan, A.K.; Ramalingaswami, V. Metabolism of Albumin and Body Fluid Compartments in Protein Deficiency: An Experimental Study in the Rhesus Monkey. J. Nutr. 1974, 104, 858–864. [Google Scholar] [CrossRef]
- Colin Carter, R.; Jacobson, J.L.; Molteno, C.D.; Jiang, H.; Meintjes, E.M.; Jacobson, S.W.; Duggan, C. Effects of Heavy Prenatal Alcohol Exposure and Iron Deficiency Anemia on Child Growth and Body Composition through Age 9 Years. Alcohol. Clin. Exp. Res. 2012, 36, 1973–1982. [Google Scholar] [CrossRef]
- Pausova, Z.; Paus, T.; Abrahamowicz, M.; Almerigi, J.; Arbour, N.; Bernard, M.; Gaudet, D.; Hanzalek, P.; Hamet, P.; Evans, A.C.; et al. Genes, Maternal Smoking, and the Offspring Brain and Body during Adolescence: Design of the Saguenay Youth Study. Hum. Brain Mapp. 2007, 28, 502–518. [Google Scholar] [CrossRef]
- Young, S.L.; Gallo, L.A.; Brookes, D.S.K.; Hayes, N.; Maloney, M.; Liddle, K.; James, A.; Moritz, K.M.; Reid, N. Altered Bone and Body Composition in Children and Adolescents with Confirmed Prenatal Alcohol Exposure. Bone 2022, 164, 116510. [Google Scholar] [CrossRef]
- Akison, L.K.; Reid, N.; Wyllie, M.; Moritz, K.M. Adverse Health Outcomes in Offspring Associated With Fetal Alcohol Exposure: A Systematic Review of Clinical and Preclinical Studies With a Focus on Metabolic and Body Composition Outcomes. Alcohol. Clin. Exp. Res. 2019, 43, 1324–1343. [Google Scholar] [CrossRef] [PubMed]
- Drozdz, D.; Sancewicz-Pach, K.; Wierzchowska-Słowiaczek, E. [Value of bioelectrical impedance analysis in the assessment of body water in children with nephrotic syndrome: Initial results]. Pol. Merkur. Lekarski 2000, 8, 224–225. [Google Scholar] [PubMed]
- Nishikawa, H.; Yoh, K.; Enomoto, H.; Ishii, N.; Iwata, Y.; Nakano, C.; Takata, R.; Nishimura, T.; Aizawa, N.; Sakai, Y.; et al. Extracellular Water to Total Body Water Ratio in Viral Liver Diseases: A Study Using Bioimpedance Analysis. Nutrients 2018, 10, 1072. [Google Scholar] [CrossRef]
- Nishino, T.; Takahashi, K.; Ochiai, C.; Tomori, S.; Ono, S.; Mimaki, M. Association between Serum Albumin and Body Water Using a Bioelectrical Impedance Analyzer: A Case Report of Longitudinal Variation in a Child with Initial Idiopathic Nephrotic Syndrome. AME Case Rep. 2024, 8, 62. [Google Scholar] [CrossRef]
- Gaudiani, J.L.; Sabel, A.L.; Mehler, P.S. Low Prealbumin Is a Significant Predictor of Medical Complications in Severe Anorexia Nervosa. Int. J. Eat. Disord. 2014, 47, 148–156. [Google Scholar] [CrossRef]
- Gonzales, S.; Schultz, L.; Dykes, J.C.; Chen, S.; Wujcik, K.; Kaufman, B.; Chen, C.; Maeda, K.; McElhinney, D.B.; Almond, C. Association between Pre-Albumin and Malnutrition in Children with Advanced Heart Failure. J. Heart Lung Transplant. 2021, 40, S203. [Google Scholar] [CrossRef]
- Xia, L.P.; Shen, L.; Kou, H.; Zhang, B.J.; Zhang, L.; Wu, Y.; Li, X.J.; Xiong, J.; Yu, Y.; Wang, H. Prenatal Ethanol Exposure Enhances the Susceptibility to Metabolic Syndrome in Offspring Rats by HPA Axis-Associated Neuroendocrine Metabolic Programming. Toxicol. Lett. 2014, 226, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Himmelreich, M.; Lutke, C.J.; Hargrove, E.T. The Lay of the Land: Fetal Alcohol Spectrum Disorder (FASD) as a Whole-Body Diagnosis. In The Routledge Handbook of Social Work and Addictive Behaviors; Routledge: London, UK, 2020; pp. 191–215. ISBN 9780429203121. [Google Scholar]
- Derme, M.; Briante, M.; Ceccanti, M.; Giannini, G.; Vitali, M.; Messina, M.P.; Piccioni, M.G.; Mattia, A.; Nicotera, S.; Crognale, A. Prenatal Alcohol Exposure and Metabolic Disorders in Pediatrics: The Role of the Oxidative Stress—A Review of the Literature. Children 2024, 11, 269. [Google Scholar] [CrossRef]
- Moore, E.M.; Riley, E.P. What Happens When Children with Fetal Alcohol Spectrum Disorders Become Adults? Curr. Dev. Disord. Rep. 2015, 2, 219–227. [Google Scholar] [CrossRef]
- Liu, Q.; Gao, F.; Liu, X.; Li, J.; Wang, Y.; Han, J.; Wang, X. Prenatal Alcohol Exposure and Offspring Liver Dysfunction: A Systematic Review and Meta-Analysis. Arch. Gynecol. Obstet. 2016, 294, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Asiedu, B.; Nyakudya, T.T.; Lembede, B.W.; Chivandi, E. Early-Life Exposure to Alcohol and the Risk of Alcohol-Induced Liver Disease in Adulthood. Birth Defects Res. 2021, 113, 451–468. [Google Scholar] [CrossRef] [PubMed]
- Kable, J.A.; Mehta, P.K.; Rashid, F.; Coles, C.D. Path Analysis of the Impact of Prenatal Alcohol on Adult Vascular Function. Alcohol 2023, 47, 116. [Google Scholar] [CrossRef]
- Zhu, Z.; Cao, F.; Li, X. Epigenetic Programming and Fetal Metabolic Programming. Front. Endocrinol. 2019, 10, 764. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hsu, C.N. Metabolic Syndrome Programming and Reprogramming: Mechanistic Aspects of Oxidative Stress. Antioxidants 2022, 11, 2108. [Google Scholar] [CrossRef]
- Cho, K.; Kobayashi, S.; Araki, A.; Miyashita, C.; Itoh, S.; Saijo, Y.; Ito, Y.; Sengoku, K.; Baba, T.; Minakami, H.; et al. Prenatal Alcohol Exposure and Adverse Fetal Growth Restriction: Findings from the Japan Environment and Children’s Study. Pediatr. Res. 2022, 92, 291–298. [Google Scholar] [CrossRef]
- Hope, S.; Nærland, T.; Høiland, A.L.; Torske, T.; Malt, E.; Abrahamsen, T.; Nerhus, M.; Wedervang–Resell, K.; Lonning, V.; Johannessen, J.; et al. Higher Vitamin B12 Levels in Neurodevelopmental Disorders than in Healthy Controls and Schizophrenia. FASEB J. 2020, 34, 8114–8124. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.B.; Audhya, T.; McDonough-Means, S.; Rubin, R.A.; Quig, D.; Geis, E.; Gehn, E.; Loresto, M.; Mitchell, J.; Atwood, S.; et al. Nutritional and Metabolic Status of Children with Autism vs. Neurotypical Children, and the Association with Autism Severity. Nutr. Metab. 2011, 8, 34. [Google Scholar] [CrossRef]
- Nielsen, M.J.; Rasmussen, M.R.; Andersen, C.B.F.; Nexø, E.; Moestrup, S.K. Vitamin B12 Transport from Food to the Body’s Cells—A Sophisticated, Multistep Pathway. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 345–354. [Google Scholar] [CrossRef]
- Dyląg, K.; Sikora-Sporek, A.; Bańdo, B.; Boroń-Zyss, J.; Drożdż, D.; Dumnicka, P.; Przybyszewska, K.; Sporek, M.; Walocha, J.W.; Wojciechowski, W.; et al. Magnetic Resonance Imaging (MRI) Findings among Children with Fetal Alcohol Syndrome (FAS), Partial Fetal Alcohol Syndrome (PFAS) and Alcohol Related Neurodevelopmental Disorders (ARND). Przegl Lek. 2016, 73, 605–609. [Google Scholar]
- Kilpatrick, L.A.; Alger, J.R.; O’Neill, J.; Joshi, S.H.; Narr, K.L.; Levitt, J.G.; O’Connor, M.J. Impact of Prenatal Alcohol Exposure on Intracortical Myelination and Deep White Matter in Children with Attention Deficit Hyperactivity Disorder. Neuroimage. Rep. 2022, 2, 100082. [Google Scholar] [CrossRef] [PubMed]
- Niedzwiedz-Massey, V.M.; Douglas, J.C.; Rafferty, T.; Wight, P.A.; Kane, C.J.M.; Drew, P.D. Ethanol Modulation of Hippocampal Neuroinflammation, Myelination, and Neurodevelopment in a Postnatal Mouse Model of Fetal Alcohol Spectrum Disorders. Neurotoxicol Teratol. 2021, 87, 107015. [Google Scholar] [CrossRef]
- Niedzwiedz-Massey, V.M.; Douglas, J.C.; Rafferty, T.; Kane, C.J.M.; Drew, P.D. Ethanol Effects on Cerebellar Myelination in a Postnatal Mouse Model of Fetal Alcohol Spectrum Disorders. Alcohol 2021, 96, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Milbocker, K.A.; LeBlanc, G.L.; Brengel, E.K.; Hekmatyar, K.S.; Kulkarni, P.; Ferris, C.F.; Klintsova, A.Y. Reduced and Delayed Myelination and Volume of Corpus Callosum in an Animal Model of Fetal Alcohol Spectrum Disorders Partially Benefit from Voluntary Exercise. Sci. Rep. 2022, 12, 10653. [Google Scholar] [CrossRef]
- Guest, J.; Bilgin, A.; Hokin, B.; Mori, T.A.; Croft, K.D.; Grant, R. Novel Relationships between B12, Folate and Markers of Inflammation, Oxidative Stress and NAD(H) Levels, Systemically and in the CNS of a Healthy Human Cohort. Nutr. Neurosci. 2015, 18, 355–364. [Google Scholar] [CrossRef]
- Koop, D.R. Alcohol Metabolism’s Damaging Effects on the Cell: A Focus on Reactive Oxygen Generation by the Enzyme Cytochrome P450 2E1. Alcohol Res. Health 2006, 29, 274. [Google Scholar] [PubMed]
- Gupta, K.K.; Gupta, V.K.; Shirasaka, T. An Update on Fetal Alcohol Syndrome-Pathogenesis, Risks, and Treatment. Alcohol. Clin. Exp. Res. 2016, 40, 1594–1602. [Google Scholar] [CrossRef] [PubMed]
- Micangeli, G.; Menghi, M.; Profeta, G.; Tarani, F.; Mariani, A.; Petrella, C.; Barbato, C.; Ferraguti, G.; Ceccanti, M.; Tarani, L.; et al. The Impact of Oxidative Stress on Pediatrics Syndromes. Antioxidants 2022, 11, 1983. [Google Scholar] [CrossRef]
- Brocardo, P.S.; Gil-Mohapel, J.; Christie, B.R. The Role of Oxidative Stress in Fetal Alcohol Spectrum Disorders. Brain Res. Rev. 2011, 67, 209–225. [Google Scholar] [CrossRef]
- Wells, P.G.; Bhatia, S.; Drake, D.M.; Miller-Pinsler, L. Fetal Oxidative Stress Mechanisms of Neurodevelopmental Deficits and Exacerbation by Ethanol and Methamphetamine. Birth Defects Res. C Embryo Today 2016, 108, 108–130. [Google Scholar] [CrossRef]
- Ehrhart, F.; Roozen, S.; Verbeek, J.; Koek, G.; Kok, G.; van Kranen, H.; Evelo, C.T.; Curfs, L.M.G. Review and Gap Analysis: Molecular Pathways Leading to Fetal Alcohol Spectrum Disorders. Mol. Psychiatry 2019, 24, 10. [Google Scholar] [CrossRef] [PubMed]
- Logan-Garbisch, T.; Bortolazzo, A.; Luu, P.; Ford, A.; Do, D.; Khodabakhshi, P.; French, R.L. Developmental Ethanol Exposure Leads to Dysregulation of Lipid Metabolism and Oxidative Stress in Drosophila. G3: Genes Genomes Genet. 2015, 5, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Raghul Kannan, S.; Latha Laxmi, I.P.; Ahmad, S.F.; Tamizhselvi, R. Embryonic Ethanol Exposure Induces Oxidative Stress and Inflammation in Zebrafish Model: A Dose-Dependent Study. Toxicology 2024, 506, 153876. [Google Scholar] [CrossRef] [PubMed]
- Coll, T.A.; Chaufan, G.; Pérez-Tito, L.G.; Ventureira, M.R.; Ríos de Molina, M.d.C.; Cebral, E. Cellular and Molecular Oxidative Stress-Related Effects in Uterine Myometrial and Trophoblast-Decidual Tissues after Perigestational Alcohol Intake up to Early Mouse Organogenesis. Mol. Cell Biochem. 2018, 440, 89–104. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.J.; Paiva, M.; Heaton, M.B. The Antioxidants Vitamin E and Beta-Carotene Protect against Ethanol-Induced Neurotoxicity in Embryonic Rat Hippocampal Cultures. Alcohol 1999, 17, 163–168. [Google Scholar] [CrossRef]
- Mitchell, J.J.; Paiva, M.; Heaton, M.B. Vitamin E and Beta-Carotene Protect against Ethanol Combined with Ischemia in an Embryonic Rat Hippocampal Culture Model of Fetal Alcohol Syndrome. Neurosci. Lett. 1999, 263, 189–192. [Google Scholar] [CrossRef]
- Zhang, X.; Sliwowska, J.H.; Weinberg, J. Prenatal Alcohol Exposure and Fetal Programming: Effects on Neuroendocrine and Immune Function. Exp. Biol. Med. 2005, 230, 376–388. [Google Scholar] [CrossRef]
- Shabtai, Y.; Fainsod, A. Competition between Ethanol Clearance and Retinoic Acid Biosynthesis in the Induction of Fetal Alcohol Syndrome. Biochem. Cell Biol. 2018, 96, 148–160. [Google Scholar] [CrossRef]
- Ballard, M.S.; Sun, M.; Ko, J. Vitamin A, Folate, and Choline as a Possible Preventive Intervention to Fetal Alcohol Syndrome. Med. Hypotheses 2012, 78, 489–493. [Google Scholar] [CrossRef]
- Ford, L.; Farr, J.; Morris, P.; Berg, J. The Value of Measuring Serum Cholesterol-Adjusted Vitamin E in Routine Practice. Ann. Clin. Biochem. 2006, 43, 130–134. [Google Scholar] [CrossRef]
- Murillo-Fuentes, M.L.; Artillo, R.; Ojeda, M.L.; Delgado, M.J.; Murillo, M.L.; Carreras, O. Effects of Prenatal or Postnatal Ethanol Consumption on Zinc Intestinal Absorption and Excretion in Rats. Alcohol Alcohol. 2007, 42, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Keen, C.L.; Uriu-Adams, J.Y.; Skalny, A.; Grabeklis, A.; Grabeklis, S.; Green, K.; Yevtushok, L.; Wertelecki, W.W.; Chambers, C.D. The Plausibility of Maternal Nutritional Status Being a Contributing Factor to the Risk for Fetal Alcohol Spectrum Disorders: The Potential Influence of Zinc Status as an Example. Biofactors 2010, 36, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Flynn, A.; Martier, S.S.; Sokol, R.J.; Miller, S.I.; Golden, N.L.; Del Villano, B.C. Zinc Status of Pregnant Alcoholic Women: A Determinant of Fetal Outcome. Lancet 1981, 1, 572–575. [Google Scholar] [CrossRef] [PubMed]

| Laboratory Test | Method and Analyzer | Age and Sex Subgroups | Age- and Sex-Specific Reference Intervals | Source of Reference Intervals |
|---|---|---|---|---|
| Total protein | Biuret, Abbott Architect | 1–<6 years | 61–75 g/L | Colantonio D.A. et al. [15] |
| 6–<9 years | 64–77 g/L | |||
| ≥9 years, females | 65–81 g/L | |||
| Albumin | BCG, Abbott Architect | 1–<8 years | 38–47 g/L | Colantonio D.A. et al. [15] |
| 8–<15 years | 41–48 g/L | |||
| ≥15 years, females | 40–49 g/L | |||
| ≥15 years, males | 41–51 g/L | |||
| Prealbumin | Immunoturbidimetric, Abbott Alinity | 1–<12 years, females | 0.12–0.30 g/L | Laboratory information |
| 1–<12 years, females | 0.11–0.34 g/L | |||
| ≥12 years, females | 0.16–0.38 g/L | |||
| ≥12 years, males | 0.18–0.45 g/L | |||
| Cholesterol | Enzymatic, Abbott Architect | ≥1 year | 2.90–5.40 mmol/L | Colantonio D.A. et al. [15] |
| Ferritin | CMIA, Abbott Architect | 1–<5 years | 5.3–99.9 ng/dL | Bailey D. et al. [16] |
| 5–<14 years | 13.7–78.8 ng/dL | |||
| ≥14 years, females | 5.5–67.4 ng/dL | |||
| 14–<16 years, males | 12.7–82.2 ng/dL | |||
| ≥16 years, males | 11.1–171.9 ng/dL | |||
| Vitamin B12 | CMIA, Abbott Alinity | 1–<9 years | 209–1190 pmol/L | Bailey D. et al. [16] |
| 9–<14 years | 186–830 pmol/L | |||
| 14–<17 years | 180–655 pmol/L | |||
| Vitamin E | HPLC | ≥1 year | 6–14 mg/L | Raizman E.J. et al. [17] |
| Vitamin A | HPLC | <11 years | 0.275–0.444 mg/L | Raizman E.J. et al. [17] |
| 11–<16 years | 0.249–0.550 mg/L | |||
| ≥13 years | 0.287–0.751 mg/L | |||
| Vitamin D | CLIA, Diasorin Liaison XL | All | 30–100 ng/mL | Rusińska A. et al. [18] |
| Parathormone | CMIA, Abbott Architect | 1–<9 years | 16.2–63.0 ng/mL | Bailey D. et al. [16] |
| 9–<17 years | 21.9–87.6 pg/mL | |||
| Phosphate | Phosphomolybdate, Abbott Architect | 1–<5 years | 1.38–2.19 mmol/L | Colantonio D.A. et al. [15] |
| 5–<13 years | 1.33–1.92 mmol/L | |||
| 13–<16 years, females | 1.02–1.79 mmol/L | |||
| 13–<16 years, males | 1.14–1.99 mmol/L | |||
| ≥16 years | 0.95–1.62 mmol/L | |||
| Magnesium | Enzymatic, Abbott Architect | 1–<4 years, females | 0.62–0.90 mmol/L | Soldin P. et al. [19] |
| 1–<4 years, males | 0.65–0.90 mmol/L | |||
| 4–<11 years, females | 0.66–1.03 mmol/L | |||
| 4–<11 years, males | 0.61–0.90 mmol/L | |||
| 11–<16 years, females | 0.66–0.86 mmol/L | |||
| 11–<16 years, males | 0.55–0.84 mmol/L | |||
| ≥16 years, females | 0.61–0.78 mmol/L | |||
| ≥16 years, males | 0.64–0.86 mmol/L | |||
| Total calcium | Arsenazo III, Abbott Architect | ≥1 year | 2.29–2.63 mmol/L | Colantonio D.A. et al. [15] |
| Ionised calcium | Potentiometric, Radiometer ABL90 Flex | All | 1.19–1.33 mmol/L | Bohn M.K. [20] |
| Zinc | ICP-MS | <14 years | 7.7–15.0 μmol/L | Laboratory information |
| ≥14 years | 9.0–18.0 μmol/L | |||
| Red blood cells | Impedance, Sysmex XN 550 | 2 months–<15 years | 3.8–5.2 × 106/μL | Pelt J.L. et al. [21] |
| ≥15 years, females | 4.0–5.2 × 106/μL | |||
| ≥15 years, males | 4.4–5.7 × 106/μL | |||
| Haemoglobin | Sodium lauryl sulphate, Sysmex XN 550 | 1–<2 years | 10.0–13.5 g/dL | |
| 2–<5 years | 10.5–13.5 g/dL | |||
| 5–<10 years | 10.9–14.9 g/dL | |||
| 10–<15 years | 11.4–15.4 g/dL | |||
| ≥15 years, females | 11.8–15.2 g/dL | |||
| ≥15 years, males | 13.4–17.0 g/dL | |||
| Haematocrit | Impedance, Sysmex XN 550 | 6 months–<2 years | 33–39% | |
| 2–<7 years | 34–40% | |||
| 7–<13 years | 35–45% | |||
| ≥13 years, females | 37–46% | |||
| ≥13 years, males | 41–50% | |||
| MCV | Calculated, Sysmex XN 550 | 6 months–<2 years | 70.0–90.0 fL | |
| 2–<5 years | 74.0–94.0 fL | |||
| 5–<8 years | 76.0–96.0 fL | |||
| 8–<15 years | 78.0–98.0 fL | |||
| ≥15 years | 82.5–97.4 fL | |||
| White blood cells | Flow cytometry, Sysmex XN 550 | 6 months–<2 years | 6.0–17.5 × 103/μL | |
| 2–<6 years | 5.0–15.5 × 103/μL | |||
| 6–<10 years | 4.5–14.5 × 103/μL | |||
| 10–<15 years | 4.5–13.5 × 103/μL | |||
| ≥15 years | 3.7–9.2 × 103/μL | |||
| Neutrophils | Flow cytometry, Sysmex XN 550 | 2 weeks–<2 years | 1.0–8.5 × 103/μL | |
| 2–<7 years | 1.5–8.0 × 103/μL | |||
| 7–<11 years | 1.5–8.5 × 103/μL | |||
| 11–16 years | 1.8–8.0 × 103/μL | |||
| Lymphocytes | Flow cytometry, Sysmex XN 550 | 1–<2 years | 1.1–8.6 × 103/μL | |
| 2–<6 years | 1.5–7.0 × 103/μL | |||
| 6–<12 years | 0.9–3.4 × 103/μL | |||
| 12–16 years | 1.2–5.2 × 103/μL | |||
| Monocytes | Flow cytometry, Sysmex XN 550 | 6 months–<2 years | 0.25–1.15 × 103/μL | |
| 2–<6 years | 0.19–0.94 × 103/μL | |||
| 6–<12 years | 0.19–0.85 × 103/μL | |||
| ≥12 years | 0.18–0.78 × 103/μL |
| Variable | Whole Cohort (n = 75) | FAS (n = 25) | ND-PAE (n = 50) | p-Value |
|---|---|---|---|---|
| Age, years | 7 (3; 9) | 8 (3; 9) | 6 (4; 9) | 0.8 |
| Male sex, n (%) | 39 (52) | 14 (56) | 25 (50) | 0.6 |
| Institutional care, n (%) | 3 (4) | 0 | 3 (6) | 0.093 |
| Foster family, n (%) | 34 (45) | 10 (40) | 24 (48) | |
| Adoptive family, n (%) | 34 (44) | 15 (60) | 18 (36) | |
| Biological family, n (%) | 5 (7) | 0 | 5 (10) | |
| Total protein, g/L | 71.8 ± 4.5 | 72.0 ± 3.9 | 71.7 ± 4.8 | 0.7 |
| Albumin, g/L | 46.9 ± 2.7 | 46.7 ± 2.3 | 47.1 ± 2.9 | 0.5 |
| Prealbumin, g/L | 0.19 (0.17; 0.22) | 0.20 (0.18; 0.23) | 0.19 (0.17; 0.21) | 0.3 |
| Cholesterol, mmol/L | 4.36 ± 0.65 | 4.48 ± 0.63 | 4.31 ± 0.66 | 0.3 |
| Ferritin, ng/mL | 25.7 (19.1; 37.4) | 25.0 (18.6; 39.5) | 25.7 (19.3; 34.5) | 0.7 |
| Vitamin B12, pg/mL | 531 (435; 696) | 526 (464; 728) | 540 (427; 682) | 0.7 |
| Vitamin E, mg/L | 12.1 ± 2.7 | 12.3 ± 2.5 | 12.0 ± 2.8 | 0.6 |
| Vitamin A, mg/L | 0.33 (0.29; 0.37) | 0.34 (0.29; 0.41) | 0.32 (0.29; 0.37) | 0.2 |
| Vitamin D, ng/mL | 34.5 ± 10.7 | 35.0 ± 7.9 | 34.3 ± 12.0 | 0.8 |
| Parathormone, pg/mL | 35.2 (29.5; 42.8) | 35.4 (30.6; 42.6) | 34.9 (29.5; 43.6) | 0.8 |
| Phosphate, mmol/L | 1.58 ± 0.19 | 1.61 ± 0.20 | 1.57 ± 0.19 | 0.4 |
| Magnesium, mmol/L | 0.85 (0.80; 0.88) | 0.85 (0.80; 0.88) | 0.85 (0.81; 0.88) | 0.9 |
| Total calcium, mmol/L | 2.46 ± 0.10 | 2.44 ± 0.09 | 2.47 ± 0.11 | 0.2 |
| Ionised calcium, mmol/L | 1.26 (1.23; 1.29) | 1.25 (1.23; 1.28) | 1.26 (1.23; 1.29) | 0.3 |
| Zinc, μmol/L | 14.0 ± 2.5 | 14.3 ± 2.6 | 13.9 ± 2.5 | 0.5 |
| Red blood cells, ×106/μL | 4.71 ± 0.33 | 4.66 ± 0.32 | 4.74 ± 0.34 | 0.4 |
| Haemoglobin, mg/dL | 12.8 ± 0.9 | 12.6 ± 0.9 | 12.8 ± 0.9 | 0.4 |
| Haematocrit, % | 37.2 ± 2.4 | 36.6 ± 2.5 | 37.5 ± 2.3 | 0.1 |
| MCV, fL | 78.6 (76.9; 80.5) | 78.4 (76.7; 80.3) | 78.6 (77.1; 80.8) | 0.4 |
| White blood cells, ×103/μL | 7.35 (5.48; 8.53) | 6.08 (5.23; 7.94) | 7.56 (5.76; 9.06) | 0.1 |
| Neutrophils, ×103/μL | 3.11 (2.38; 4.00) | 3.14 (2.27; 3.60) | 3.04 (2.38; 4.14) | 0.6 |
| Lymphocytes, ×103/μL | 2.80 (2.23; 4.01) | 2.67 (2.23; 3.77) | 2.85 (2.24; 4.08) | 0.7 |
| Monocytes, ×103/μL | 0.54 (0.45; 0.67) | 0.51 (0.44; 0.62) | 0.60 (0.46; 0.71) | 0.9 |
| Variable | Whole Cohort (n = 75) | FAS (n = 25) | ND-PAE (n = 50) | p-Value | |||
|---|---|---|---|---|---|---|---|
| Below | Above | Below | Above | Below | Above | ||
| Total protein, n (%) | 0 | 13 (17) | 0 | 3 (12) | 0 | 10 (22) | 0.3 |
| Albumin, n (%) | 0 | 23 (31) | 0 | 7 (28) | 0 | 16 (32) | 0.6 |
| Prealbumin, n (%) | 0 | 0 | 0 | 0 | 0 | 0 | - |
| Cholesterol, n (%) | 0 | 13 (17) | 0 | 5 (20) | 0 | 8 (16) | 0.7 |
| Ferritin, n (%) | 4 (5) | 1 (1) | 1 (4) | 1 (4) | 3 (6) | 0 | 0.3 |
| Vitamin B12, n (%) | 0 | 6 (8) | 0 | 4 (16) | 0 | 2 (4) | 0.071 |
| Vitamin E, n (%) | 0 | 14 (19) | 0 | 5 (20) | 0 | 9 (18) | 0.7 |
| Vitamin A, n (%) | 2 (3) | 5 (7) | 1 (4) | 2 (9) | 1 (2) | 3 (6) | 0.8 |
| Vitamin D, n (%) | 26 (35) | 0 | 6 (24) | 0 | 20 (40) | 0 | 0.2 |
| Parathormone, n (%) | 0 | 2 (3) | 0 | 0 | 0 | 2 (4) | 0.3 |
| Phosphate, n (%) | 4 (5) | 0 | 2 (8) | 0 | 2 (4) | 0 | 0.5 |
| Magnesium, n (%) | 0 | 3 (4) | 0 | 1 (4) | 0 | 2 (4) | 1.0 |
| Total calcium, n (%) | 1 (1) | 3 (4) | 1 (4) | 1 (4) | 0 | 2 (4) | 0.4 |
| Ionised calcium, n (%) | 2 (3) | 6 (8) | 2 (8) | 2 (8) | 0 | 4 (8) | 0.1 |
| Zinc, n (%) | 1 (1) | 14 (19) | 1 (4) | 5 (20) | 0 | 9 (19) | 0.4 |
| Red blood cells, n (%) | 0 | 4 (5) | 0 | 1 (4) | 0 | 14 (30) | 0.7 |
| Haemoglobin, n (%) | 0 | 1 (1) | 0 | 0 | 0 | 1 (2) | 0.5 |
| Haematocrit, n (%) | 5 (7) | 1 (1) | 4 (16) | 0 | 1 (2) | 1 (2) | 0.067 |
| MCV, n (%) | 10 (13) | 3 (4) | 4 (16) | 0 | 6 (12) | 3 (6) | 0.4 |
| White blood cells, n (%) | 3 (4) | 2 (3) | 2 (8) | 1 (4) | 1 (2) | 1 (2) | 0.4 |
| Neutrophils, n (%) | 3 (4) | 3 (4) | 1 (4) | 0 | 2 (4) | 3 (6) | 0.4 |
| Lymphocytes, n (%) | 1 (1) | 10 (13) | 0 | 2 (8) | 1 (2) | 8 (16) | 0.4 |
| Monocytes, n (%) | 0 | 6 (8) | 0 | 2 (8) | 0 | 4 (8) | 1.0 |
| Weight Percentile | Height Percentile | BMI Percentile | ||||
|---|---|---|---|---|---|---|
| R | p-Value | R | p-Value | R | p-Value | |
| Prealbumin | 0.24 | 0.039 | NS | NS | ||
| Vitamin B12 | 0.30 | 0.009 | NS | 0.30 | 0.010 | |
| Vitamin D | −0.23 | 0.050 | NS | NS | ||
| Zinc | 0.24 | 0.042 | 0.27 | 0.021 | 0.29 | 0.014 |
| Haemoglobin | 0.27 | 0.022 | NS | NS | ||
| Haematocrit | 0.23 | 0.046 | NS | NS | ||
| White blood cells | −0.36 | 0.002 | NS | −0.26 | 0.025 | |
| Neutrophils | −0.28 | 0.015 | NS | NS | ||
| Lymphocytes | −0.40 | <0.001 | NS | −0.34 | 0.003 | |
| Laboratory Test Result | Age | Type of Custody | ||||
|---|---|---|---|---|---|---|
| ≤2 Years (n = 11) | >2 Years (n = 64) | p-Value | Foster or Institutional (n = 37) | Adoptive (n = 34) | p-Value | |
| Increased albumin, n (%) | 8 (73) | 15 (24) | 0.001 | 17 (46) | 3 (9) | <0.001 |
| Increased zinc, n (%) | NS | 11 (30) | 0 | 0.002 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dylag, K.A.; Wieczorek-Stawinska, W.; Burkot, K.; Drzewiecki, L.; Przybyszewska, K.; Tokarz, A.; Dumnicka, P. Exploring Nutritional Status and Metabolic Imbalances in Children with FASD: A Cross-Sectional Study. Nutrients 2024, 16, 3401. https://doi.org/10.3390/nu16193401
Dylag KA, Wieczorek-Stawinska W, Burkot K, Drzewiecki L, Przybyszewska K, Tokarz A, Dumnicka P. Exploring Nutritional Status and Metabolic Imbalances in Children with FASD: A Cross-Sectional Study. Nutrients. 2024; 16(19):3401. https://doi.org/10.3390/nu16193401
Chicago/Turabian StyleDylag, Katarzyna Anna, Wiktoria Wieczorek-Stawinska, Katarzyna Burkot, Lukasz Drzewiecki, Katarzyna Przybyszewska, Aleksandra Tokarz, and Paulina Dumnicka. 2024. "Exploring Nutritional Status and Metabolic Imbalances in Children with FASD: A Cross-Sectional Study" Nutrients 16, no. 19: 3401. https://doi.org/10.3390/nu16193401
APA StyleDylag, K. A., Wieczorek-Stawinska, W., Burkot, K., Drzewiecki, L., Przybyszewska, K., Tokarz, A., & Dumnicka, P. (2024). Exploring Nutritional Status and Metabolic Imbalances in Children with FASD: A Cross-Sectional Study. Nutrients, 16(19), 3401. https://doi.org/10.3390/nu16193401








