Taraxacum Mongolicum Polysaccharides Reverses Mice Obesity via Activation of AKT/mTOR Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Glucose Tolerance Tests (GTT) and Insulin Tolerance Tests (ITT)
2.3. Rectal Temperature and Infrared Image
2.4. Serum Inflammatory Marker Measurement
2.5. Hematoxylin and Eosin Staining (H&E Staining)
2.6. RNA Exaction, cDNA Synthesis, and Quantitative Real-Time PCR (qRT-PCR)
2.7. Western Blotting
2.8. Statistical Analysis
3. Result
3.1. TMPs Alleviate HFD-Induced Obesity in Mice
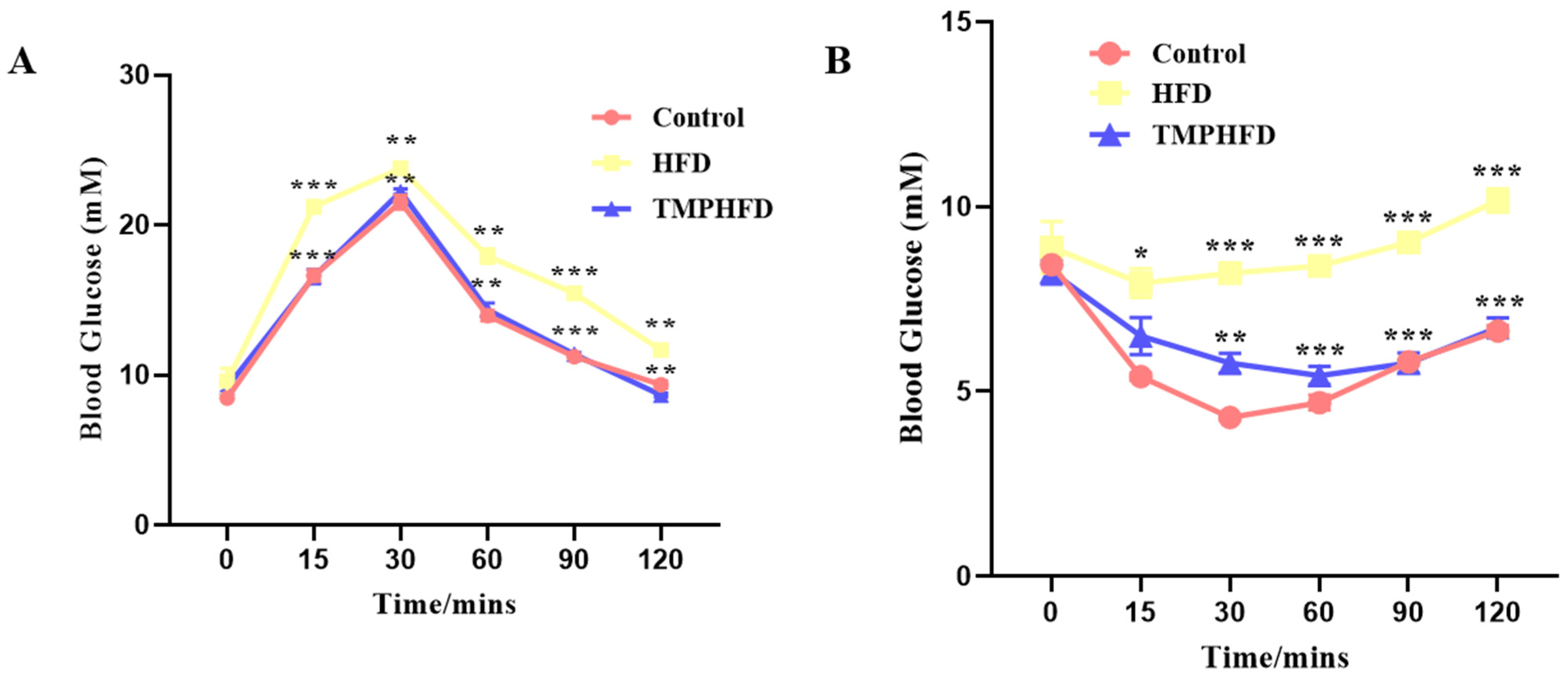
3.2. Administration of TMPs Maintains Glucose Homeostasis
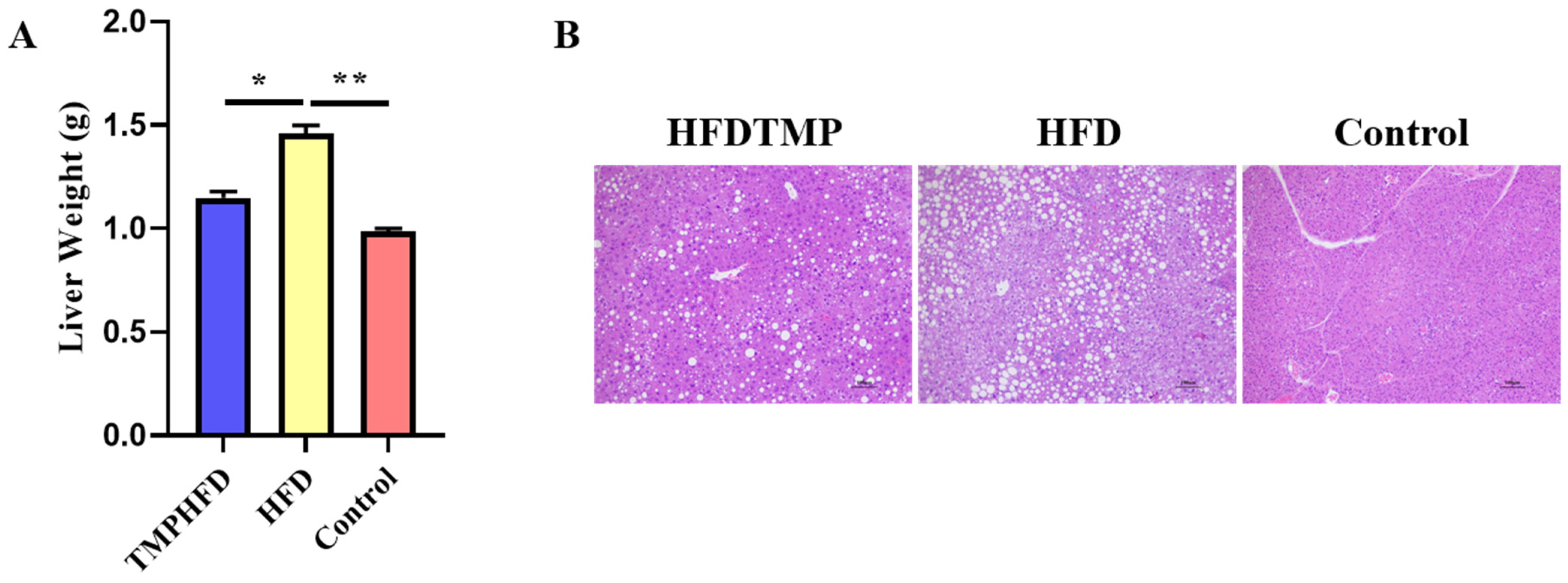
3.3. TMPs Counteract HFD-Induced Hepatic Steatosis
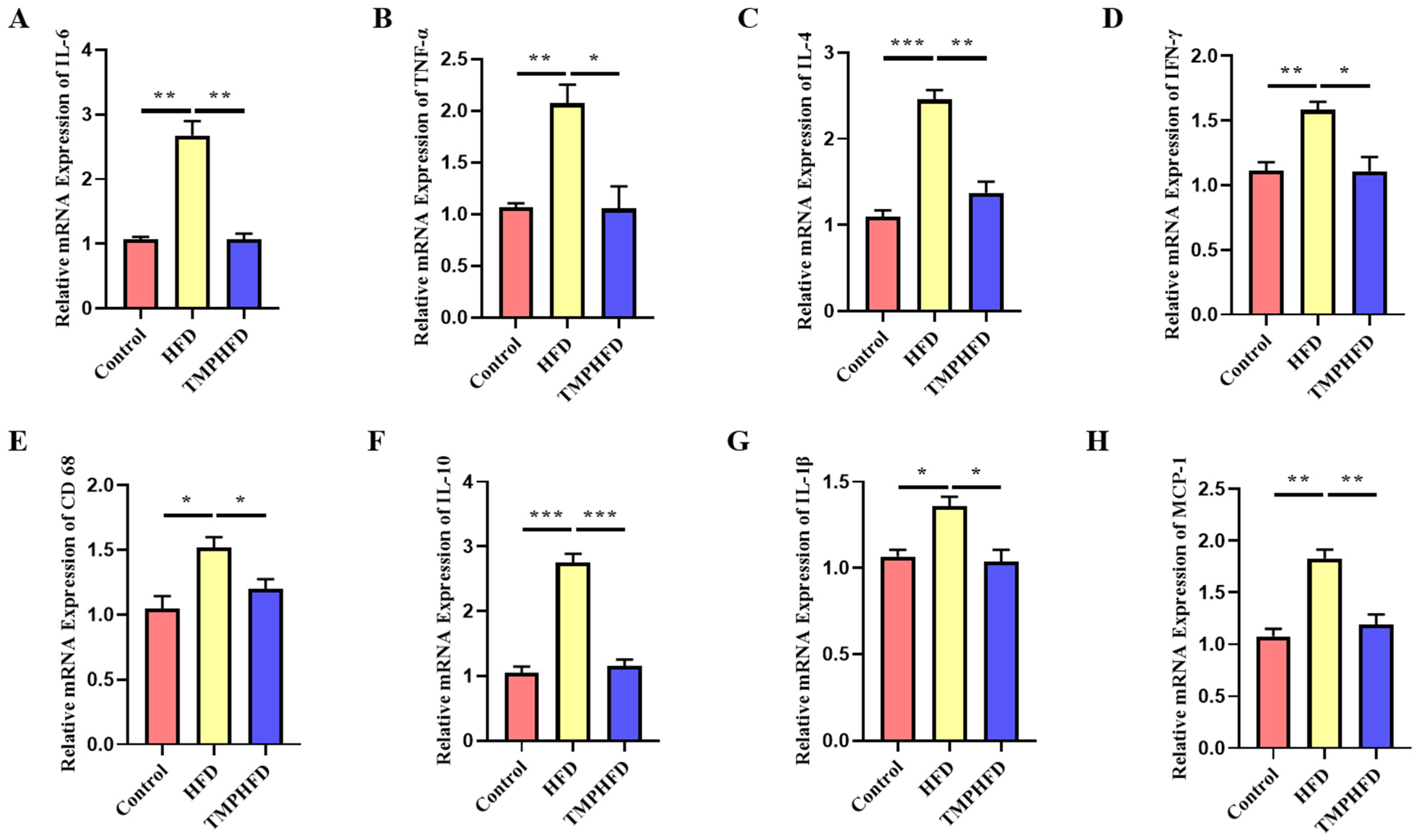
3.4. TMPs Attenuate HFD-Induced Inflammation in Mice
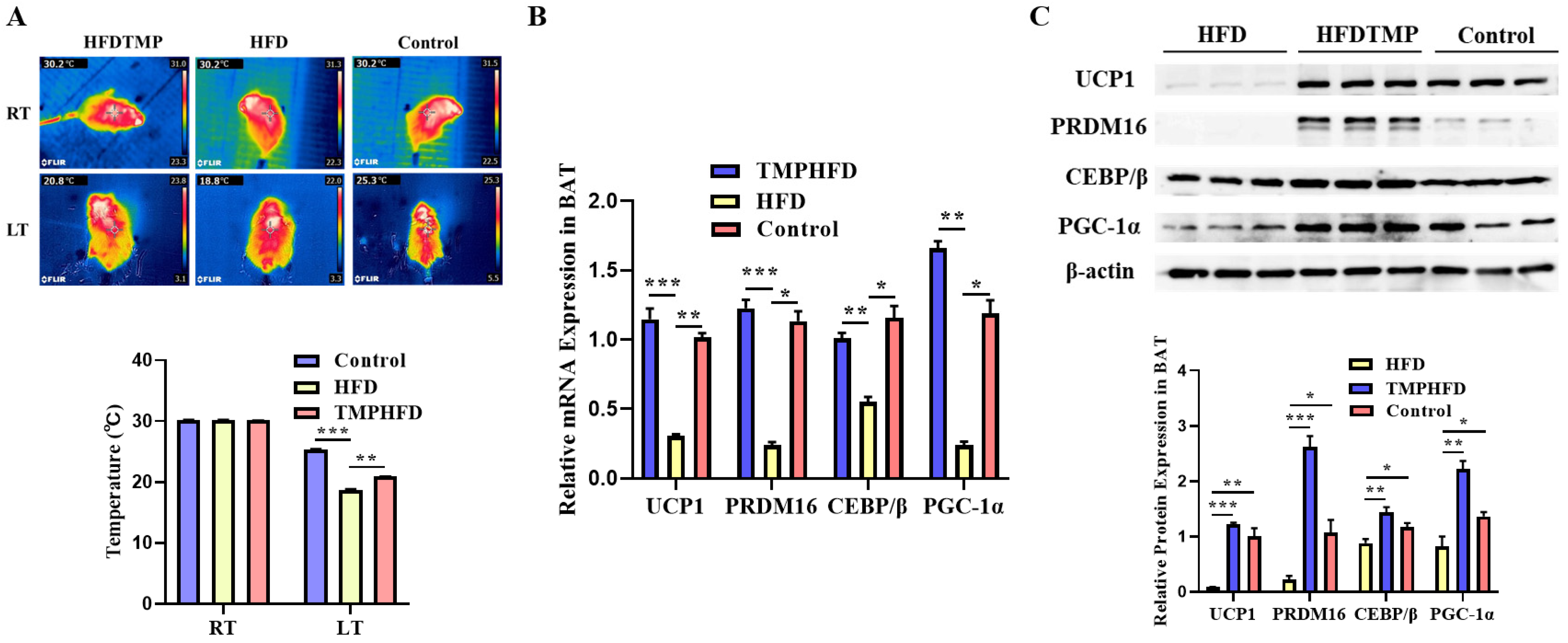
3.5. TMP Intake Restores BAT Function in HFD-Induced Obesity
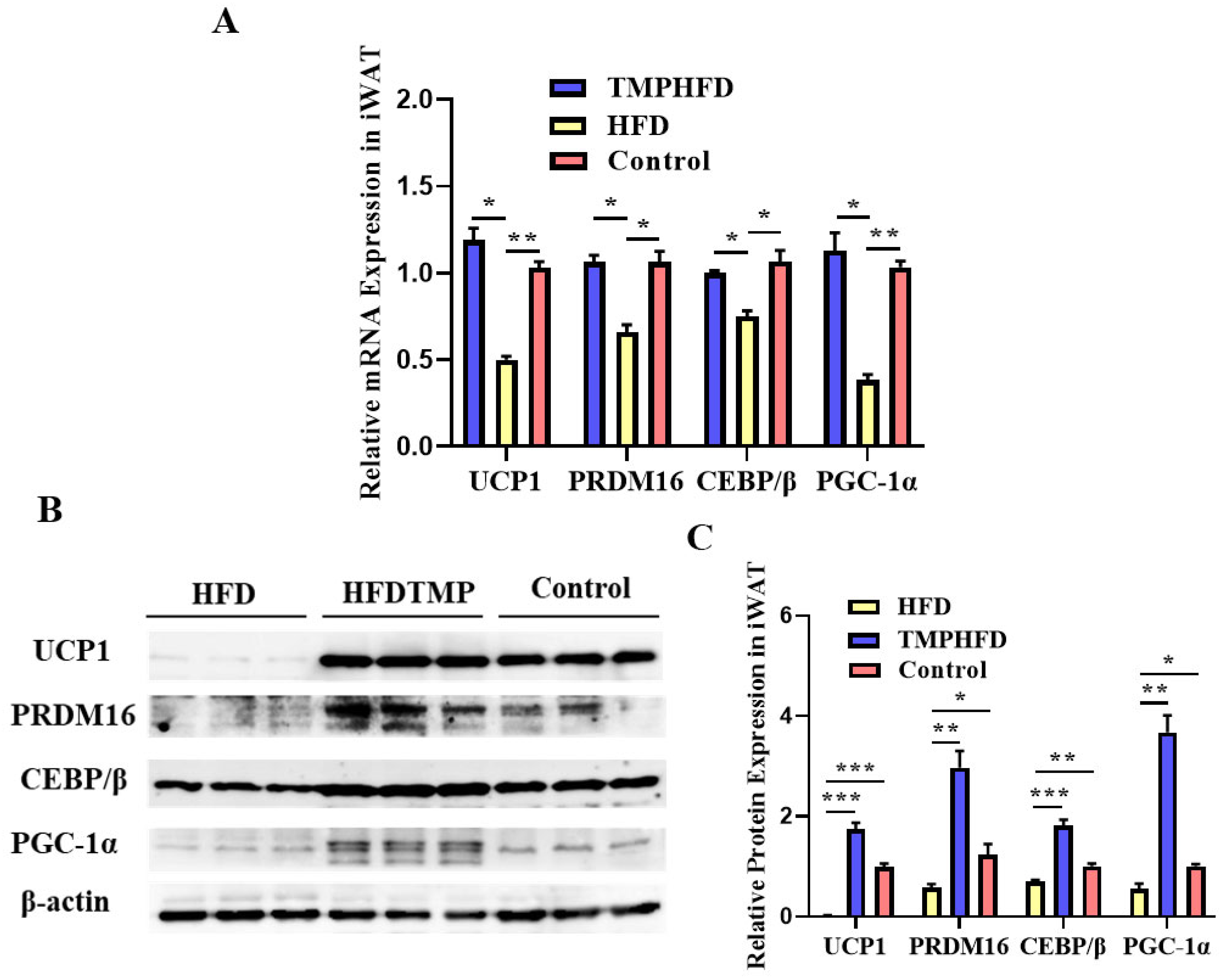
3.6. Dietary TMP Supplementation Potentiated Beige Adipogenesis
3.7. TMPs Induce Browning through AKT/mTOR-Thermogenesis Pathway in Both BAT and iWAT
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Gaal, L.F.; Mertens, I.L.; De Block, C.E. Mechanisms linking obesity with cardiovascular disease. Nature 2006, 444, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Rohr, M.W.; Narasimhulu, C.A.; Rudeski-Rohr, T.A.; Parthasarathy, S. Negative effects of a high-fat diet on intestinal permeability: A review. Adv. Nutr. 2020, 11, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Valentino, M.A.; Lin, J.E.; Waldman, S.A. Central and peripheral molecular targets for antiobesity pharmacotherapy. Clin. Pharmacol. Ther. 2010, 87, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Edwards, I.R.; Aronson, J.K. Adverse drug reactions: Definitions, diagnosis, and management. Lancet 2000, 356, 1255–1259. [Google Scholar] [CrossRef] [PubMed]
- Aabideen, Z.U.; Mumtaz, M.W.; Akhtar, M.T.; Mukhtar, H.; Raza, S.A.; Touqeer, T.; Saari, N. Anti-obesity attributes; UHPLC-QTOF-MS/MS-based metabolite profiling and molecular docking insights of Taraxacum officinale. Molecules 2020, 25, 4935. [Google Scholar] [CrossRef]
- Wen, J.-J.; Gao, H.; Hu, J.-L.; Nie, Q.-X.; Chen, H.-H.; Xiong, T.; Nie, S.-P.; Xie, M.-Y. Polysaccharides from fermented Momordica charantia ameliorate obesity in high-fat induced obese rats. Food Funct. 2019, 10, 448–457. [Google Scholar] [CrossRef]
- Yuan, D.; Huang, Q.; Li, C.; Fu, X. A polysaccharide from Sargassum pallidum reduces obesity in high-fat diet-induced obese mice by modulating glycolipid metabolism. Food Funct. 2022, 13, 7181–7191. [Google Scholar] [CrossRef]
- Zhang, J.; Kang, M.-J.; Kim, M.-J.; Kim, M.-E.; Song, J.-H.; Lee, Y.-M.; Kim, J.-I. Pancreatic lipase inhibitory activity of Taraxacum officinale in vitro and in vivo. Nutr. Res. Pract. 2008, 2, 200–203. [Google Scholar] [CrossRef]
- Raghu Mohan Rao P, R.M.R.P.; Jyothi, Y.; Rabban, S. Anti-obesity activity of Taraxacum officinale in high fat diet induced obese rats. J. Chem. Pharm. Res. 2015, 7, 244–248. [Google Scholar]
- Pena-Leon, V.; Perez-Lois, R.; Seoane, L.M. mTOR pathway is involved in energy homeostasis regulation as a part of the gut–brain axis. Int. J. Mol. Sci. 2020, 21, 5715. [Google Scholar] [CrossRef]
- Fernández-Veledo, S.; Vázquez-Carballo, A.; Vila-Bedmar, R.; Ceperuelo-Mallafré, V.; Vendrell, J. Role of energy-and nutrient-sensing kinases AMP-activated Protein Kinase (AMPK) and Mammalian Target of Rapamycin (mTOR) in Adipocyte Differentiation. IUBMB Life 2013, 65, 572–583. [Google Scholar] [CrossRef] [PubMed]
- Shearin, A.L. Akt Controls Adipocyte Function And Systemic Metabolism. Ph.D. Thesis, University of Pennsylvania, Philadelphia, PA, USA, 2016. [Google Scholar]
- Kwan, J.M. Akt Regulation of Adipogenesis: Implications for Skp2 Involvement. Ph.D. Thesis, University of Illinois at Chicago, Chicago, IL, USA, 2013. [Google Scholar]
- Chondronikola, M.; Volpi, E.; Børsheim, E.; Porter, C.; Annamalai, P.; Enerbäck, S.; Lidell, M.E.; Saraf, M.K.; Labbe, S.M.; Hurren, N.M. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes 2014, 63, 4089–4099. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Plutzky, J. Brown fat and browning for the treatment of obesity and related metabolic disorders. Diabetes Metab. J. 2016, 40, 12. [Google Scholar] [CrossRef] [PubMed]
- Xiang, A.S.; Meikle, P.J.; Carey, A.L.; Kingwell, B.A. Brown adipose tissue and lipid metabolism: New strategies for identification of activators and biomarkers with clinical potential. Pharmacol. Ther. 2018, 192, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Yang, Z.; Peng, T.; Lv, Y. Circular RNAs: Rising stars in lipid metabolism and lipid disorders. J. Cell. Physiol. 2021, 236, 4797–4806. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, Z.; Hao, Y.; An, P.; Luo, J.; Luo, Y. Dandelion polysaccharides ameliorate high-fat-diet-induced atherosclerosis in mice through antioxidant and anti-inflammatory capabilities. Nutrients 2023, 15, 4120. [Google Scholar] [CrossRef]
- Finlin, B.S.; Zhu, B.; Confides, A.L.; Westgate, P.M.; Harfmann, B.D.; Dupont-Versteegden, E.E.; Kern, P.A. Mast cells promote seasonal white adipose beiging in humans. Diabetes 2017, 66, 1237–1246. [Google Scholar] [CrossRef]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef]
- Li, S.; Xu, Y.; Guo, W.; Chen, F.; Zhang, C.; Tan, H.Y.; Wang, N.; Feng, Y. The impacts of herbal medicines and natural products on regulating the hepatic lipid metabolism. Front. Pharmacol. 2020, 11, 351. [Google Scholar] [CrossRef]
- Pamudurti, N.R.; Bartok, O.; Jens, M.; Ashwal-Fluss, R.; Stottmeister, C.; Ruhe, L.; Hanan, M.; Wyler, E.; Perez-Hernandez, D.; Ramberger, E.; et al. Translation of circRNAs. Mol. Cell 2017, 66, 9–21.e27. [Google Scholar] [CrossRef]
- Villiger, A.; Sala, F.; Suter, A.; Butterweck, V. In vitro inhibitory potential of Cynara scolymus, Silybum marianum, Taraxacum officinale, and Peumus boldus on key enzymes relevant to metabolic syndrome. Phytomedicine 2015, 22, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.; Waheed, A.; Qureshi, R.A.; Burdi, D.K.; Verspohl, E.J.; Khan, N.; Hasan, M. The effect of medicinal plants of Islamabad and Murree region of Pakistan on insulin secretion from INS-1 cells. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2004, 18, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ji, J.; Di, L.; Li, J.; Hu, L.; Qiao, H.; Wang, L.; Feng, Y. Resource, chemical structure and activity of natural polysaccharides against alcoholic liver damages. Carbohydr. Polym. 2020, 241, 116355. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fei, S.; Yu, X.; Tan, M. Dandelion (Taraxacum mongolicum) Extract Alleviated H2O2-Induced Oxidative Damage: The Underlying Mechanism Revealed by Metabolomics and Lipidomics. Foods 2023, 12, 3314. [Google Scholar] [CrossRef]
- Harb, E.; Kheder, O.; Poopalasingam, G.; Rashid, R.; Srinivasan, A.; Izzi-Engbeaya, C. Brown adipose tissue and regulation of human body weight. Diabetes/Metab. Res. Rev. 2023, 39, e3594. [Google Scholar] [CrossRef]
- Aquilano, K.; Zhou, B.; Brestoff, J.R.; Lettieri-Barbato, D. Multifaceted mitochondrial quality control in brown adipose tissue. Trends Cell Biol. 2023, 33, 517–529. [Google Scholar] [CrossRef]
- Magro, B.S.; Dias, D.P.M. Brown and beige adipose tissue: New therapeutic targets for metabolic disorders. Health Sci. Rev. 2024, 10, 100148. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Z.; Song, Y.; Xie, H.; Dong, M. An update on brown adipose tissue and obesity intervention: Function, regulation and therapeutic implications. Front. Endocrinol. 2023, 13, 1065263. [Google Scholar] [CrossRef]
- Bertoncini-Silva, C.; Zingg, J.M.; Fassini, P.G.; Suen, V.M.M. Bioactive dietary components—Anti-obesity effects related to energy metabolism and inflammation. BioFactors 2023, 49, 297–321. [Google Scholar] [CrossRef]
- Ye, Y.; Liu, H.; Zhang, F.; Hu, F. mTOR signaling in Brown and Beige adipocytes: Implications for thermogenesis and obesity. Nutr. Metab. 2019, 16, 1–14. [Google Scholar] [CrossRef]


| Gene | Forward | Reverse |
|---|---|---|
| PRDM16 | GCCTGTTTCTCTTCTGTCCCC | GCCAACAGGACGGTGTTATTT |
| UCP1 | TTGCTTCTCTCAGGATCGGC | GTGGGTTGCCCAATGAACAC |
| C/EBP β | GCTGAGCGACGAGTACAAGATGC | CTTGTGCTGCGTCTCCAGGTTG |
| PGC-1α | TGTCGGATGCTTGCTTGAGT | TACGGTTGTAACGCAGGACCT |
| β-actin | AGGCCAACCGCGAGAAGATGACC | GAAGTCCAGGGCGACGTAGCAC |
| Group | TC (mM) | TG (mM) | HDL (mM) | LDL (mM) |
|---|---|---|---|---|
| Control | 8.91 ± 1.29 | 1.14 ± 0.17 | 0.66 ± 0.04 | 2.59 ± 0.11 |
| HFD | 13.82 ± 2.21 * | 2.81 ± 0.12 *** | 0.19 ± 0.03 *** | 7.60 ± 0.28 *** |
| HFDTMP | 8.01 ± 1.77 * | 1.57 ± 0.31 ** | 0.35 ± 0.03 ** | 2.50 ± 0.21 *** |
| Group | TC (mM/g) | TG (mM/g) | HDL (mM/g) | LDL (mM/g) |
|---|---|---|---|---|
| Control | 1.45 ± 0.04 | 0.43 ± 0.02 | 2.99 ± 0.14 | 0.95 ± 0.10 |
| HFD | 3.69 ± 0.12 *** | 1.36 ± 0.04 *** | 0.92 ± 0.08 *** | 1.60 ± 0.07 *** |
| HFDTMP | 1.66 ± 0.08 *** | 0.41 ± 0.02 *** | 2.85 ± 0.13 *** | 1.03 ± 0.03 *** |
| Group | IL-6 (pg/mL) | IL-10 (pg/mL) | TNF-α (pg/mL) | COX-2 (pg/mL) |
|---|---|---|---|---|
| Control | 1.91 ± 0.29 | 0.41 ± 0.07 | 1.26 ± 0.14 | 0.95 ± 0.11 |
| HFD | 3.82 ± 0.21 *** | 2.81 ± 0.11 *** | 0.92 ± 0.08 * | 2.6 ± 0.28 *** |
| HFDTMP | 2.01 ± 0.17 *** | 0.75 ± 0.09 *** | 1.15 ± 0.13 * | 1.5 ± 0.21 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, X.; Yu, S.; Luan, Y.; Wang, J.; Zhao, J.; Zhang, M.; Wang, Q. Taraxacum Mongolicum Polysaccharides Reverses Mice Obesity via Activation of AKT/mTOR Pathway. Nutrients 2024, 16, 3330. https://doi.org/10.3390/nu16193330
Yue X, Yu S, Luan Y, Wang J, Zhao J, Zhang M, Wang Q. Taraxacum Mongolicum Polysaccharides Reverses Mice Obesity via Activation of AKT/mTOR Pathway. Nutrients. 2024; 16(19):3330. https://doi.org/10.3390/nu16193330
Chicago/Turabian StyleYue, Xiaoyu, Shilong Yu, Yue Luan, Jianpeng Wang, Junxing Zhao, Mu Zhang, and Qin Wang. 2024. "Taraxacum Mongolicum Polysaccharides Reverses Mice Obesity via Activation of AKT/mTOR Pathway" Nutrients 16, no. 19: 3330. https://doi.org/10.3390/nu16193330
APA StyleYue, X., Yu, S., Luan, Y., Wang, J., Zhao, J., Zhang, M., & Wang, Q. (2024). Taraxacum Mongolicum Polysaccharides Reverses Mice Obesity via Activation of AKT/mTOR Pathway. Nutrients, 16(19), 3330. https://doi.org/10.3390/nu16193330





