Association between Poor Outcomes and Risk of Refeeding Syndrome among Patients Urgently Admitted to the High Dependency Unit: A Single-Center Cohort Study in Japan
Abstract
1. Background
2. Materials and Methods
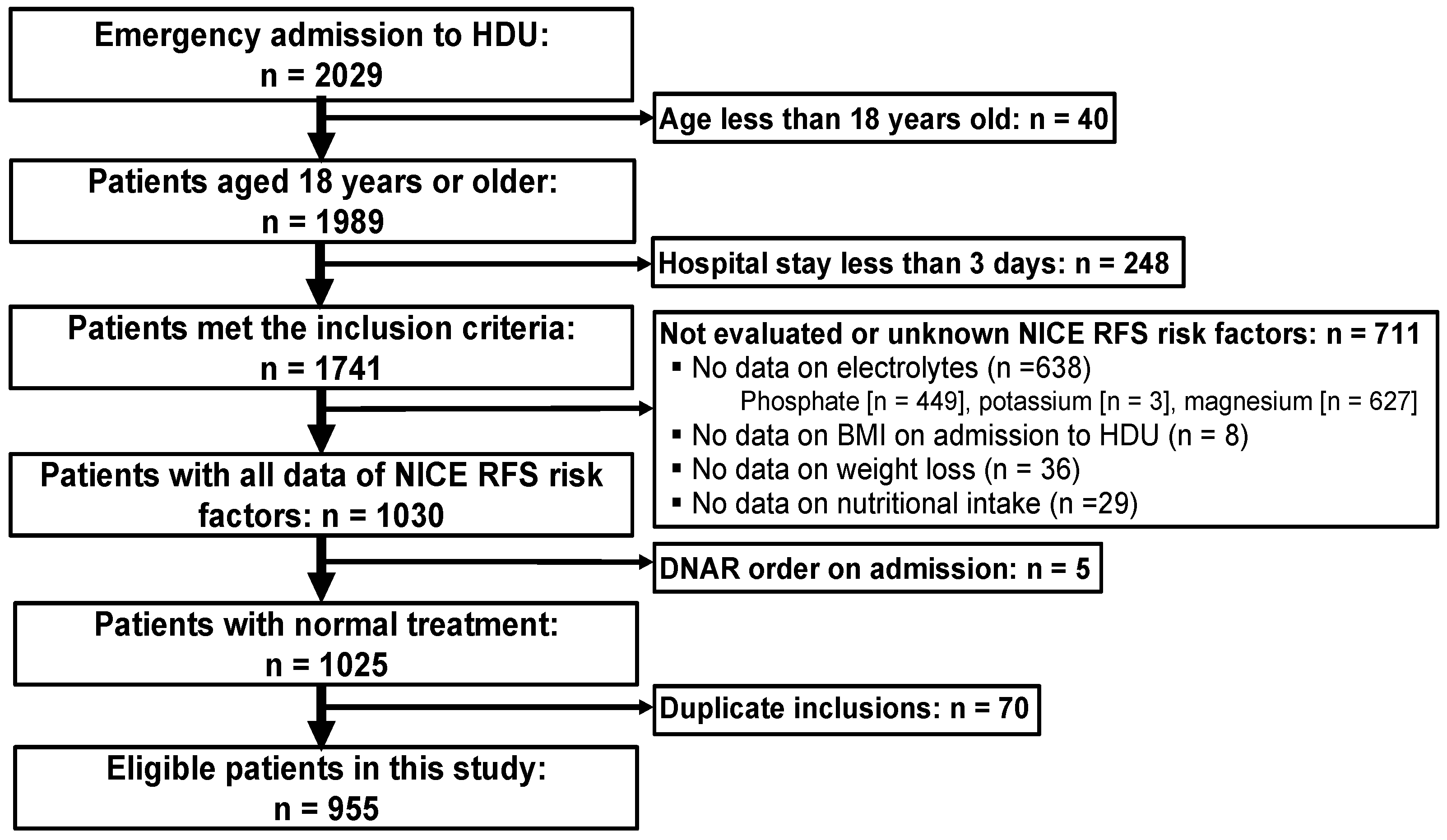
2.1. Study Design and Participants
2.2. Data Collection
2.3. Outcomes
2.4. Sample Size
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
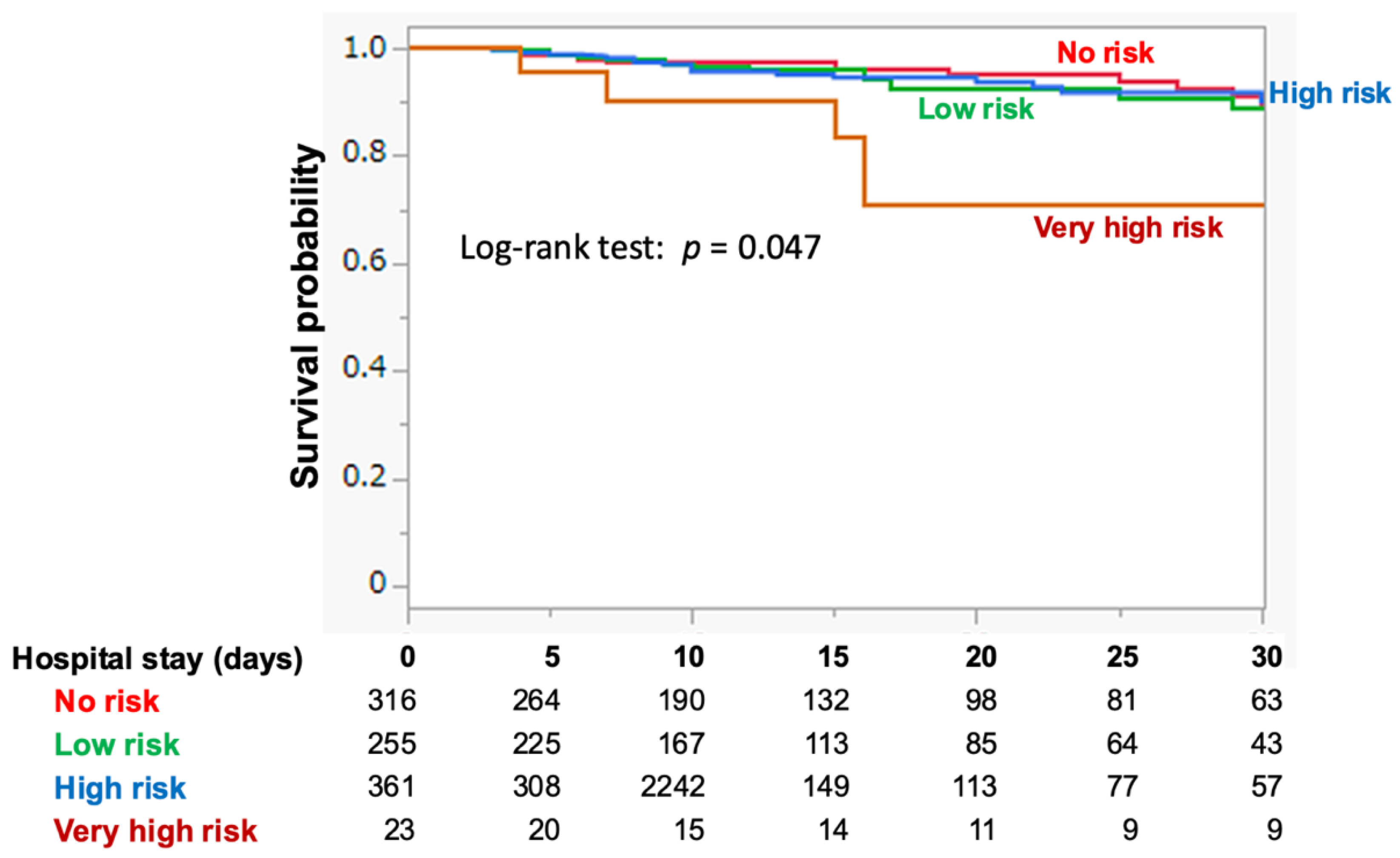
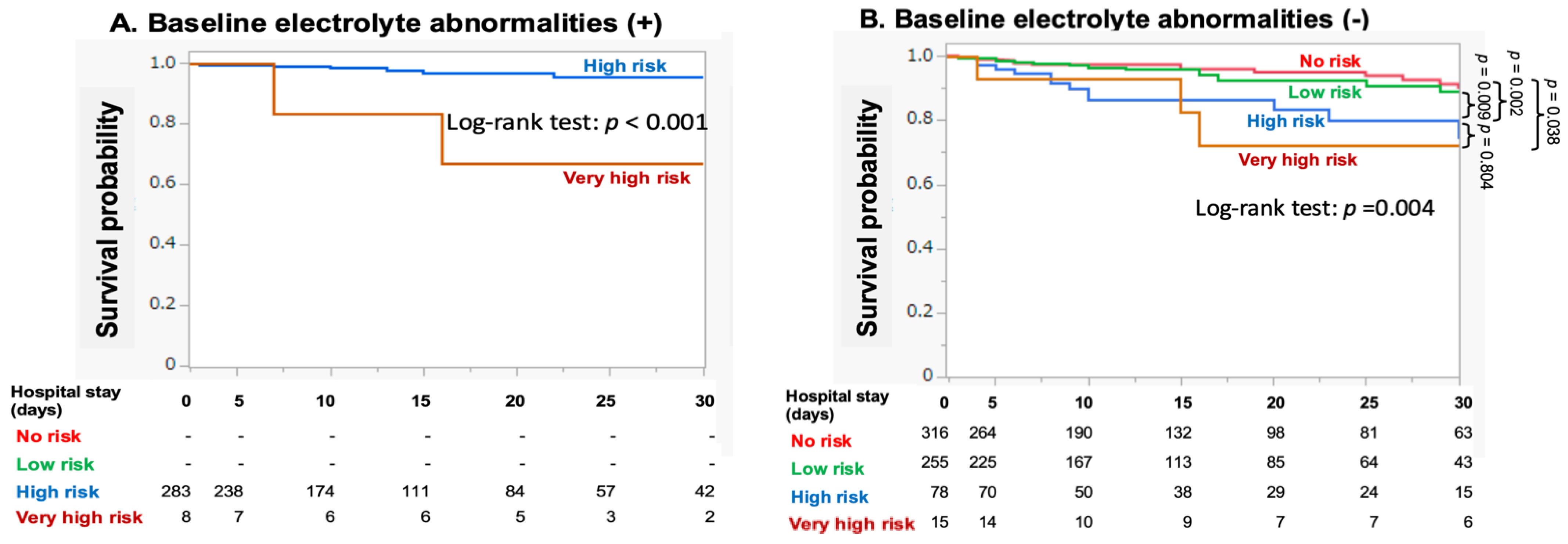
3.2. RFS Risk Categorization and Outcomes
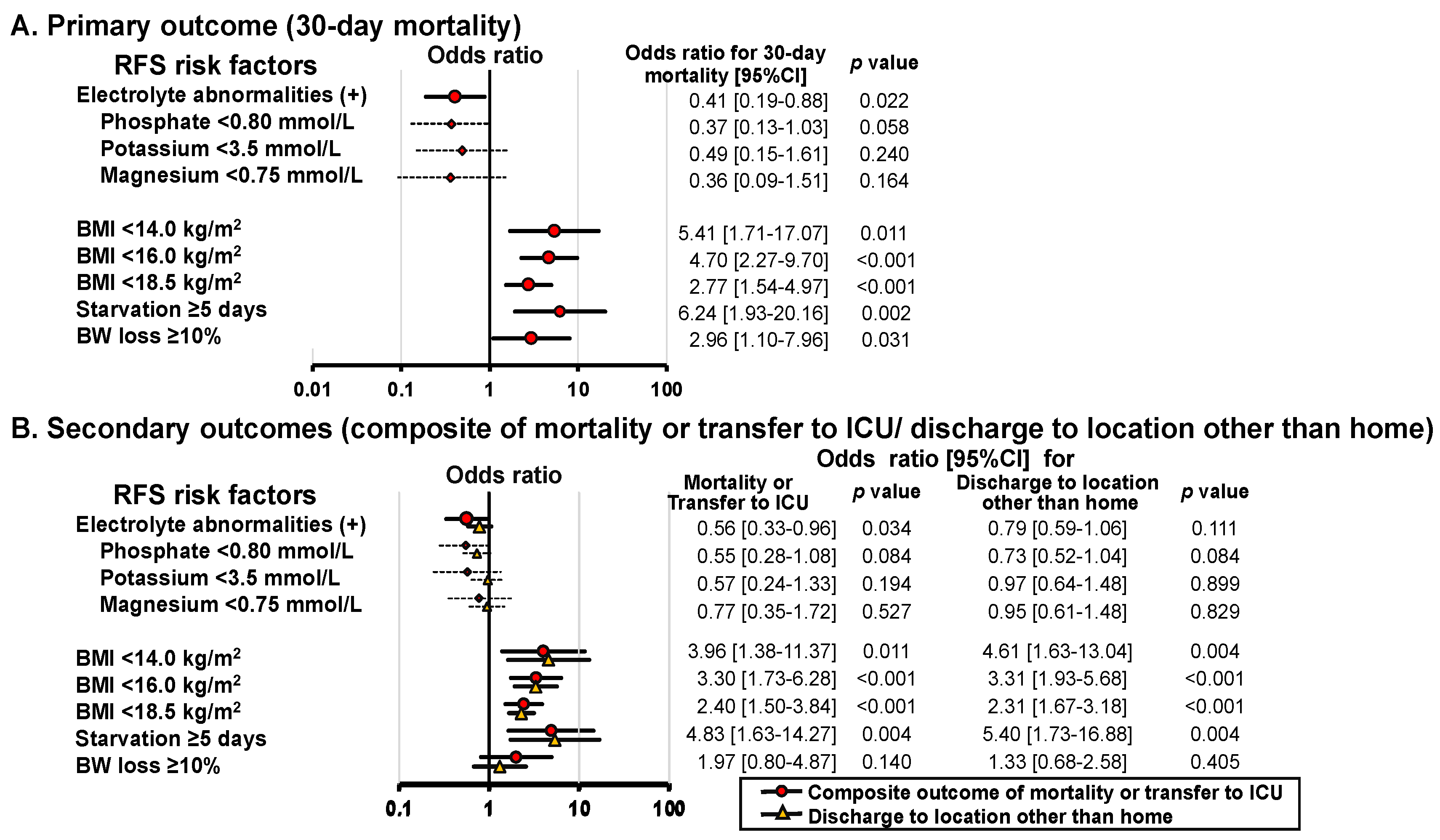
3.3. Association between RFS Risk Factors and Outcomes
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mehanna, H.M.; Moledina, J.; Travis, J. Refeeding syndrome: What it is, and how to prevent and treat it. BMJ 2008, 336, 1495–1498. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, I.; Ponzo, V.; Pellegrini, M.; Evangelista, A.; Bioletto, F.; Ciccone, G.; Pasanisi, F.; Ghigo, E.; Bo, S. The incidence of the refeeding syndrome. A systematic review and meta-analyses of literature. Clin. Nutr. 2021, 40, 3688–3701. [Google Scholar] [CrossRef] [PubMed]
- Boot, R.; Koekkoek, K.; van Zanten, A.R.H. Refeeding syndrome: Relevance for the critically ill patient. Curr. Opin. Crit. Care 2018, 24, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Doig, G.S.; Simpson, F.; Heighes, P.T.; Bellomo, R.; Chesher, D.; Caterson, I.D.; Reade, M.C.; Harrigan, P.W. Refeeding Syndrome Trial Investigators, G. Restricted versus continued standard caloric intake during the management of refeeding syndrome in critically ill adults: A randomised, parallel-group, multicentre, single-blind controlled trial. Lancet Respir. Med. 2015, 3, 943–952. [Google Scholar] [CrossRef]
- da Silva, J.S.V.; Seres, D.S.; Sabino, K.; Adams, S.C.; Berdahl, G.J.; Citty, S.W.; Cober, M.P.; Evans, D.C.; Greaves, J.R.; Gura, K.M.; et al. ASPEN Consensus Recommendations for Refeeding Syndrome. Nutr. Clin. Pract. 2020, 35, 178–195. [Google Scholar] [CrossRef] [PubMed]
- Friedli, N.; Stanga, Z.; Culkin, A.; Crook, M.; Laviano, A.; Sobotka, L.; Kressig, R.W.; Kondrup, J.; Mueller, B.; Schuetz, P. Management and prevention of refeeding syndrome in medical inpatients: An evidence-based and consensus-supported algorithm. Nutrition 2018, 47, 13–20. [Google Scholar] [CrossRef]
- Friedli, N.; Baumann, J.; Hummel, R.; Kloter, M.; Odermatt, J.; Fehr, R.; Felder, S.; Baechli, V.; Geiser, M.; Deiss, M.; et al. Refeeding syndrome is associated with increased mortality in malnourished medical inpatients: Secondary analysis of a randomized trial. Medicine 2020, 99, e18506. [Google Scholar] [CrossRef]
- Xiong, R.; Huang, H.; Wu, Y.; Wang, S.; Wang, D.; Ji, Z.; Lin, Z.; Zang, N.; Pan, S.; Huang, K. Incidence and outcome of refeeding syndrome in neurocritically ill patients. Clin. Nutr. 2021, 40, 1071–1076. [Google Scholar] [CrossRef]
- Coskun, R.; Gundogan, K.; Baldane, S.; Guven, M.; Sungur, M. Refeeding hypophosphatemia: A potentially fatal danger in the intensive care unit. Turk. J. Med. Sci. 2014, 44, 369–374. [Google Scholar] [CrossRef]
- National Collaborating Centre for Acute Care. Nutrition Support for Adults: Oral Nutrition Support, Enteral Tube Feeding and Parenteral Nutrition. Available online: https://www.nice.org.uk/guidance/cg32/evidence/full-guideline-194889853 (accessed on 11 July 2024).
- Friedli, N.; Stanga, Z.; Sobotka, L.; Culkin, A.; Kondrup, J.; Laviano, A.; Mueller, B.; Schuetz, P. Revisiting the refeeding syndrome: Results of a systematic review. Nutrition 2017, 35, 151–160. [Google Scholar] [CrossRef]
- Yoshida, M.; Izawa, J.; Wakatake, H.; Saito, H.; Kawabata, C.; Matsushima, S.; Suzuki, A.; Nagatomi, A.; Yoshida, T.; Masui, Y.; et al. Mortality associated with new risk classification of developing refeeding syndrome in critically ill patients: A cohort study. Clin. Nutr. 2021, 40, 1207–1213. [Google Scholar] [CrossRef] [PubMed]
- Singer, P.; Blaser, A.R.; Berger, M.M.; Calder, P.C.; Casaer, M.; Hiesmayr, M.; Mayer, K.; Montejo-Gonzalez, J.C.; Pichard, C.; Preiser, J.C.; et al. ESPEN practical and partially revised guideline: Clinical nutrition in the intensive care unit. Clin. Nutr. 2023, 42, 1671–1689. [Google Scholar] [CrossRef]
- Persico, R.S.; Franzosi, O.S. Patients with enteral nutrition at risk of refeeding syndrome show electrolyte abnormalities at admission in the Emergency Department. Nutr. Hosp. 2021, 38, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Kraaijenbrink, B.V.; Lambers, W.M.; Mathus-Vliegen, E.M.; Siegert, C.E. Incidence of refeeding syndrome in internal medicine patients. Neth. J. Med. 2016, 74, 116–121. [Google Scholar] [PubMed]
- Sanchez-Craig, M.; Wilkinson, D.A.; Davila, R. Empirically based guidelines for moderate drinking: 1-year results from three studies with problem drinkers. Am. J. Public Health 1995, 85, 823–828. [Google Scholar] [CrossRef][Green Version]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic. Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Charlson, M.E.; Carrozzino, D.; Guidi, J.; Patierno, C. Charlson Comorbidity Index: A Critical Review of Clinimetric Properties. Psychother. Psychosom. 2022, 91, 8–35. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5); American Psychiatric Publication: Washington, DC, USA, 2013. [Google Scholar]
- Shankar-Hari, M.; Phillips, G.S.; Levy, M.L.; Seymour, C.W.; Liu, V.X.; Deutschman, C.S.; Angus, D.C.; Rubenfeld, G.D.; Singer, M.; Sepsis Definitions Task, F. Developing a New Definition and Assessing New Clinical Criteria for Septic Shock: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 775–787. [Google Scholar] [CrossRef]
- Geerse, D.A.; Bindels, A.J.; Kuiper, M.A.; Roos, A.N.; Spronk, P.E.; Schultz, M.J. Treatment of hypophosphatemia in the intensive care unit: A review. Crit. Care 2010, 14, R147. [Google Scholar] [CrossRef]
- Lee, J.W. Fluid and electrolyte disturbances in critically ill patients. Electrolyte Blood Press 2010, 8, 72–81. [Google Scholar] [CrossRef]
- Lakatos, E. Sample sizes based on the log-rank statistic in complex clinical trials. Biometrics 1988, 44, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann-Struzek, C.; Mellhammar, L.; Rose, N.; Cassini, A.; Rudd, K.E.; Schlattmann, P.; Allegranzi, B.; Reinhart, K. Incidence and mortality of hospital- and ICU-treated sepsis: Results from an updated and expanded systematic review and meta-analysis. Intensive Care Med. 2020, 46, 1552–1562. [Google Scholar] [CrossRef]
- Imaeda, T.; Nakada, T.A.; Takahashi, N.; Yamao, Y.; Nakagawa, S.; Ogura, H.; Shime, N.; Umemura, Y.; Matsushima, A.; Fushimi, K. Trends in the incidence and outcome of sepsis using data from a Japanese nationwide medical claims database-the Japan Sepsis Alliance (JaSA) study group. Crit. Care 2021, 25, 338. [Google Scholar] [CrossRef]
- Krutkyte, G.; Wenk, L.; Odermatt, J.; Schuetz, P.; Stanga, Z.; Friedli, N. Refeeding Syndrome: A Critical Reality in Patients with Chronic Disease. Nutrients 2022, 14, 2859. [Google Scholar] [CrossRef]
- Aubry, E.; Friedli, N.; Schuetz, P.; Stanga, Z. Refeeding syndrome in the frail elderly population: Prevention, diagnosis and management. Clin. Exp. Gastroenterol. 2018, 11, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Kagansky, N.; Levy, S.; Koren-Morag, N.; Berger, D.; Knobler, H. Hypophosphataemia in old patients is associated with the refeeding syndrome and reduced survival. J. Intern. Med. 2005, 257, 461–468. [Google Scholar] [CrossRef]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Portet, S. A primer on model selection using the Akaike Information Criterion. Infect. Dis. Model. 2020, 5, 111–128. [Google Scholar] [CrossRef] [PubMed]
- Rio, A.; Whelan, K.; Goff, L.; Reidlinger, D.P.; Smeeton, N. Occurrence of refeeding syndrome in adults started on artificial nutrition support: Prospective cohort study. BMJ Open 2013, 3, e002173. [Google Scholar] [CrossRef]
- Meira, A.P.C.; Santos, C.O.D.; Lucho, C.L.C.; Kasmirscki, C.; Silva, F.M. Refeeding Syndrome in Patients Receiving Parenteral Nutrition Is Not Associated to Mortality or Length of Hospital Stay: A Retrospective Observational Study. Nutr. Clin. Pract. 2021, 36, 673–678. [Google Scholar] [CrossRef]
- Doig, G.S.; Simpson, F.; Sweetman, E.A.; Finfer, S.R.; Cooper, D.J.; Heighes, P.T.; Davies, A.R.; O’Leary, M.; Solano, T.; Peake, S.; et al. Early parenteral nutrition in critically ill patients with short-term relative contraindications to early enteral nutrition: A randomized controlled trial. JAMA 2013, 309, 2130–2138. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xiao, C.; Chen, L.; Zhang, X.; Kou, Q. Impact of hypophosphatemia on outcome of patients in intensive care unit: A retrospective cohort study. BMC Anesthesiol. 2019, 19, 86. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Li, Y.; Zhang, C.; Guo, X.; Liang, X.; Huang, Y.; Zhang, F.; Li, J.; Liu, Q. Prognostic value of serum phosphate levels in sepsis: A systematic review and meta-analysis. PeerJ 2023, 11, e16241. [Google Scholar] [CrossRef]
- Shor, R.; Halabe, A.; Rishver, S.; Tilis, Y.; Matas, Z.; Fux, A.; Boaz, M.; Weinstein, J. Severe hypophosphatemia in sepsis as a mortality predictor. Ann. Clin. Lab. Sci. 2006, 36, 67–72. [Google Scholar] [PubMed]
- Liu, Z.; Li, T.; Du, Y.; Li, C.; Chong, W. Both hypophosphatemia and hyperphosphatemia are associated with increased mortality in septic patients. Front. Nephrol. 2022, 2, 935288. [Google Scholar] [CrossRef]
- Berger, M.M.; Reintam-Blaser, A.; Calder, P.C.; Casaer, M.; Hiesmayr, M.J.; Mayer, K.; Montejo, J.C.; Pichard, C.; Preiser, J.C.; van Zanten, A.R.H.; et al. Monitoring nutrition in the ICU. Clin. Nutr. 2019, 38, 584–593. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Correia, M.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 38, 1–9. [Google Scholar] [CrossRef]
- Kondrup, J.; Allison, S.P.; Elia, M.; Vellas, B.; Plauth, M. ESPEN guidelines for nutrition screening 2002. Clin. Nutr. 2003, 22, 415–421. [Google Scholar] [CrossRef]
- Stratton, R.J.; Hackston, A.; Longmore, D.; Dixon, R.; Price, S.; Stroud, M.; King, C.; Elia, M. Malnutrition in hospital outpatients and inpatients: Prevalence, concurrent validity and ease of use of the ‘malnutrition universal screening tool’ (‘MUST’) for adults. Br. J. Nutr. 2004, 92, 799–808. [Google Scholar] [CrossRef]
- Liu, P.; Chen, L.; Zhong, T.; Zhang, M.; Ma, T.; Tian, H. Impact of calorie intake and refeeding syndrome on the length of hospital stay of patients with malnutrition: A systematic review and meta-analysis. Clin. Nutr. 2022, 41, 2003–2012. [Google Scholar] [CrossRef]
- Schuetz, P.; Fehr, R.; Baechli, V.; Geiser, M.; Deiss, M.; Gomes, F.; Kutz, A.; Tribolet, P.; Bregenzer, T.; Braun, N.; et al. Individualised nutritional support in medical inpatients at nutritional risk: A randomised clinical trial. Lancet 2019, 393, 2312–2321. [Google Scholar] [CrossRef] [PubMed]
| Total | No Risk | Low Risk | High Risk | Very High Risk | p Value | |
|---|---|---|---|---|---|---|
| Number of patients in each risks, n (%) | n = 955 | n = 316 (33.1) | n = 255 (26.7) | n = 361 (37.8) | n = 23 (2.4) | |
| Age, median [IQR], year | 75 [62–83] | 70 [52–80] | 76 [68–84] | 77 [65–84] | 75 [65–80] | <0.001 |
| Male sex, n (%) | 528 (55.3) | 177 (56.0) | 136 (53.3) | 202 (56.0) | 13 (56.5) | 0.909 |
| BMI, median [IQR], kg/m2 | 22.0 [19–24] | 23.0 [21–25] | 22.0 [19–24] | 20.9 [18–24] | 13.6 [13–14] | <0.001 |
| CCI, median [IQR] | 1 [0–1] | 1 [0–2] | 1 [1–2] | 1 [1–2] | 1 [1–2] | <0.001 |
| Pre-existing illness | ||||||
| Chronic kidney disease on dialysis, n (%) | 44 (4.6) | 8 (2.5) | 20 (7.8) | 16 (4.4) | 0 (0.0) | 0.016 |
| Malignancy, n (%) | 37 (3.9) | 14 (4.4) | 7 (2.8) | 16 (4.4) | 0 (0.0) | 0.500 |
| Psychiatric, n (%) | 7 (8.1) | 30 (9.5) | 22 (8.6) | 22 (6.1) | 3 (13.0) | 0.303 |
| Anorexia nervosa, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Living place before HDU admission | 0.892 | |||||
| Home, n (%) | 927 (97.1) | 309 (97.8) | 245 (96.1) | 350 (97.0) | 23 (100.0) | |
| Other hospital, n (%) | 10 (1.0) | 3 (1.0) | 3 (1.2) | 4 (1.1) | 0 (0) | |
| Nursing facility, n (%) | 17 (1.8) | 4 (1.3) | 7 (2.8) | 6 (1.7) | 0 (0) | |
| Ambulance use, n (%) | 495 (52.8) | 153 (48.4) | 137 (53.7) | 195 (54.0) | 10 (43.5) | 0.365 |
| Emergency surgery, n (%) | 71 (7.4) | 35 (11.1) | 16 (6.3) | 19 (5.3) | 1 (4.4) | 0.024 |
| Primary diagnosis | <0.001 | |||||
| Cardiovascular, n (%) | 144 (15.1) | 34 (10.8) | 59 (23.1) | 47 (13.0) | 4 (17.4) | |
| Respiratory, n (%) | 144 (15.1) | 41 (13.0) | 33 (12.9) | 63 (17.5) | 7 (30.4) | |
| Digestive, n (%) | 67 (7.0) | 21 (6.6) | 15 (5.9) | 29 (8.0) | 2 (8.7) | |
| Neurology, n (%) | 257 (26.9) | 108 (34.2) | 63 (24.7) | 83 (23.0) | 3 (13.0) | |
| Infection, n (%) | 33 (3.5) | 6 (1.9) | 8 (3.1) | 18 (5.0) | 1 (4.3) | |
| Trauma, n (%) | 90 (9.4) | 46 (14.6) | 17 (6.7) | 26 (7.2) | 1 (4.3) | |
| Metabolic, n (%) | 100 (10.5) | 28 (8.9) | 26 (102) | 43 (11.9) | 3 (13.0) | |
| Hematologic, n (%) | 10 (1.1) | 0 (0.0) | 6 (2.4) | 3 (0.8) | 1 (4.3) | |
| Genitourinary, n (%) | 28 (2.9) | 8 (28.6) | 4 (1.6) | 16 (4.4) | 0 (0.0) | |
| Skin musculoskeletal, n (%) | 82 (8.6) | 24 (29.3) | 24 (9.4) | 33 (9.1) | 1 (4.3) | |
| Diagnosis of sepsis, n (%) | 99 (10.4) | 22 (7.0) | 26 (10.2) | 48 (13.3) | 3 (13.0) | 0.059 |
| Treatment on admission | ||||||
| Mechanical ventilation with intubation and circulatory support | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Modified NICE RFS Risk Group | ||||||
|---|---|---|---|---|---|---|
| Total | No Risk | Low Risk | High Risk | Very High Risk | p-Value | |
| n (%) | 955 | 316 (33.1) | 255 (26.7) | 361 (37.8) | 23 (2.4) | |
| BMI (kg/m2) | <0.001 | |||||
| 16 kg/m2 ≤ BMI < 18.5 kg/m2 | 128 (13.4) | 0 | 49 (5.1) | 79 (8.3) | 0 | |
| 14 kg/m2 ≤ BMI < 16 kg/m2 | 43 (4.5) | 0 | 0 | 40 (4.2) | 3 (0.3) | |
| BMI < 14 kg/m2 | 18 (1.9) | 0 | 0 | 0 | 18 (1.9) | |
| Weight loss during the 6 months | <0.001 | |||||
| >10% and ≤15% | 12 (1.3) | 0 | 3 (0.3) | 6 (0.6) | 3 (13.0) | |
| >15% and ≤20% | 5 (0.5) | 0 | 0 | 4 (0.4) | 1 (0.1) | |
| >20% | 5 (0.5) | 0 | 0 | 0 | 5 (0.5) | |
| Little or no nutritional intake (duration) | <0.001 | |||||
| >5 and ≤10 days | 9 (0.9) | 0 | 2 (0.2) | 6 (0.6) | 1 (0.1) | |
| >10 and ≤15 days | 3 (0.3) | 0 | 0 | 3 (0.3) | 0 | |
| >15 days | 3 (0.3) | 0 | 0 | 0 | 3 (0.3) | |
| Low baseline levels of electrolytes on admission | 291 (30.5) | 0 | 0 | 283 (29.6) | 8 (0.8) | <0.001 |
| Phosphate < 0.80 mmol/L | 174 (18.2) | 0 | 0 | 173 (18.1) | 1 (0.1) | <0.001 |
| Potassium < 3.5 mmol/L | 105 (11.0) | 0 | 0 | 99 (10.4) | 6 (0.6) | <0.001 |
| Magnesium < 0.75 mmol/L | 95 (9.9) | 0 | 0 | 91 (9.5) | 4 (0.4) | <0.001 |
| Alcohol abuse † | 15 (1.6) | 0 | 8 (0.8) | 5 (0.5) | 2 (0.2) | <0.001 |
| Insulin treatment | 66 (6.9) | 0 | 40 (4.2) | 25 (2.6) | 1 (0.1) | <0.001 |
| Chemotherapy | 12 (1.3) | 0 | 6 (0.6) | 6 (0.6) | 0 | 0.064 |
| Antacids | 201 (21.0) | 0 | 99 (10.4) | 89 (9.3) | 13 (1.4) | <0.001 |
| Diuretics | 176 (18.4) | 0 | 110 (11.5) | 65 (6.8) | 1 (0.1) | <0.001 |
| 30-day mortality, n [% of each risk group] | 51 [5.3%] | 14 [4.4%] | 14 [5.5%] | 18 [5.0%] | 5 [21.7%] | 0.005 |
| Odds ratio [95%CI] | ||||||
| Univariate | 1.0 (Ref.) | 1.25 [0.58–2.67] | 1.13 [0.55–2.31] | 5.99 [1.94–18.48] | ||
| Multivariate, adjusted with sepsis | 1.0 (Ref.) | 1.17 [0.54–2.51] | 0.98 [0.47–2.03] | 5.63 [1.77–17.84] | ||
| Multivariate, adjusted with sepsis and CCI | 1.0 (Ref.) | 0.91 [0.41–2.02] | 0.91 [0.43–1.89] | 5.53 [1.73–17.66] | ||
| Multivariate, adjusted with sepsis, CCI, and age (Primary analysis) | 1.0 (Ref.) | 0.86 [0.38–1.88] | 0.81 [0.38–1.68] | 5.54 [1.73–17.79] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshida, M.; Suzuki, M.; Wakatake, H.; Kurisu, M.; Saito, H.; Ohshima, Y.; Kaneko, M.; Fujiwara, K.; Masui, Y.; Hayashi, K.; et al. Association between Poor Outcomes and Risk of Refeeding Syndrome among Patients Urgently Admitted to the High Dependency Unit: A Single-Center Cohort Study in Japan. Nutrients 2024, 16, 3287. https://doi.org/10.3390/nu16193287
Yoshida M, Suzuki M, Wakatake H, Kurisu M, Saito H, Ohshima Y, Kaneko M, Fujiwara K, Masui Y, Hayashi K, et al. Association between Poor Outcomes and Risk of Refeeding Syndrome among Patients Urgently Admitted to the High Dependency Unit: A Single-Center Cohort Study in Japan. Nutrients. 2024; 16(19):3287. https://doi.org/10.3390/nu16193287
Chicago/Turabian StyleYoshida, Minoru, Masako Suzuki, Haruaki Wakatake, Miyuki Kurisu, Hiroki Saito, Yuki Ohshima, Mayumi Kaneko, Kuniyasu Fujiwara, Yoshihiro Masui, Koichi Hayashi, and et al. 2024. "Association between Poor Outcomes and Risk of Refeeding Syndrome among Patients Urgently Admitted to the High Dependency Unit: A Single-Center Cohort Study in Japan" Nutrients 16, no. 19: 3287. https://doi.org/10.3390/nu16193287
APA StyleYoshida, M., Suzuki, M., Wakatake, H., Kurisu, M., Saito, H., Ohshima, Y., Kaneko, M., Fujiwara, K., Masui, Y., Hayashi, K., & Fujitani, S. (2024). Association between Poor Outcomes and Risk of Refeeding Syndrome among Patients Urgently Admitted to the High Dependency Unit: A Single-Center Cohort Study in Japan. Nutrients, 16(19), 3287. https://doi.org/10.3390/nu16193287






