Nutrigenetic Investigations in Preeclampsia
Abstract
1. Introduction
2. Obesity and Preeclampsia
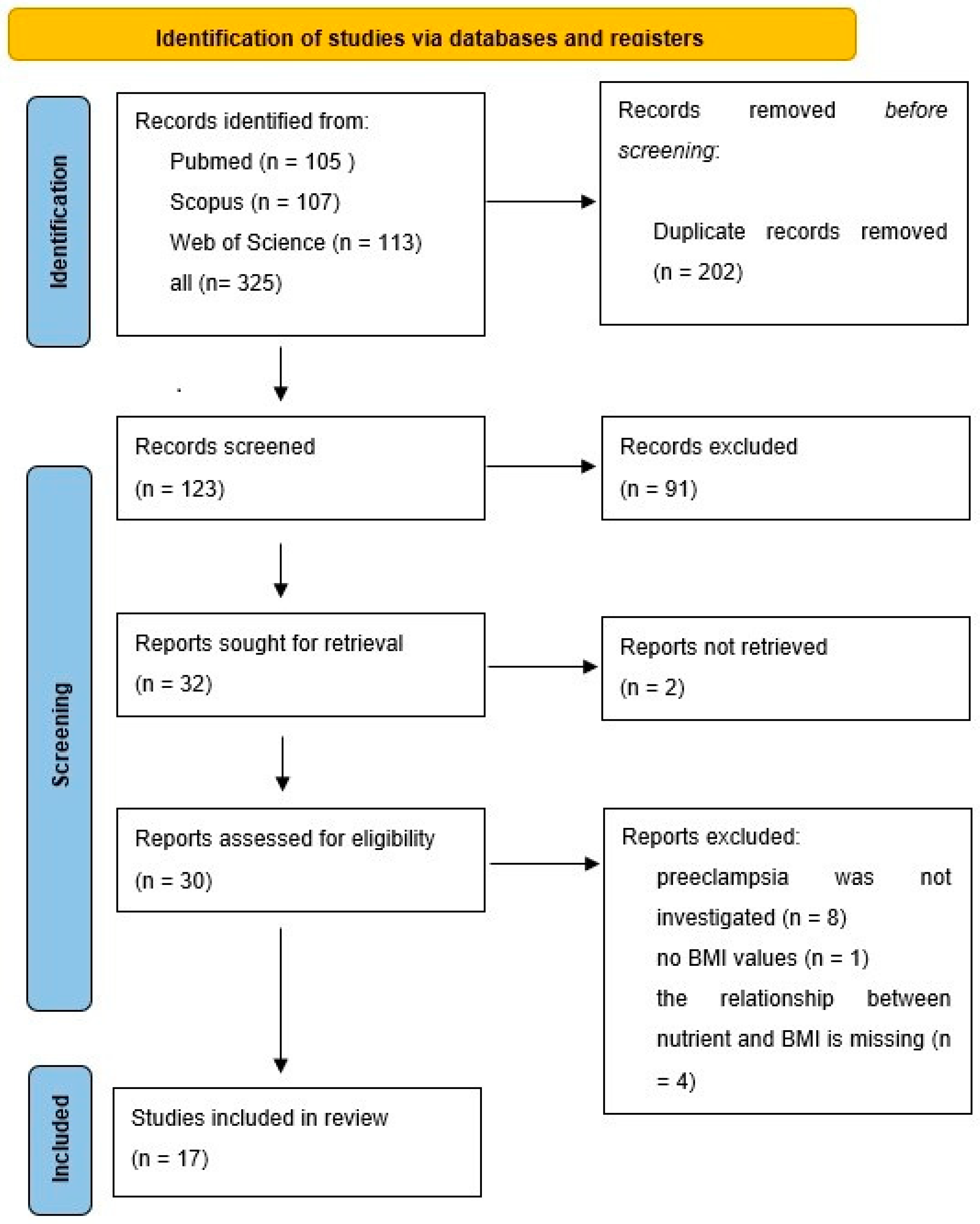
2.1. Method of Searching
2.2. REN (Renin Gene)
2.3. AGT (Angiotensinogen Gene) and AGTR1/2 (Angiotensin II Receptor Type 1 and 2 Genes)
2.4. ACE2 (Angiotensin-Converting Enzyme 2 Gene)
2.5. RGS2 (Regulator of G-Protein Signaling 2 Gene)
2.6. VDR (Vitamin D Receptor Gene)
2.7. AVP (Arginine Vasopressin Gene)
2.8. ACVR2 (Activin A Receptor Type 2A Gene)
2.9. LGALS13 (Placental Protein 13 Gene)
2.10. miR-27a
2.11. LIPC (Hepatic Lipase Gene)
2.12. MIF (Macrophage Migration Inhibitory Factors Gene)
2.13. GWAS Studies
3. Micronutrients and Preeclampsia
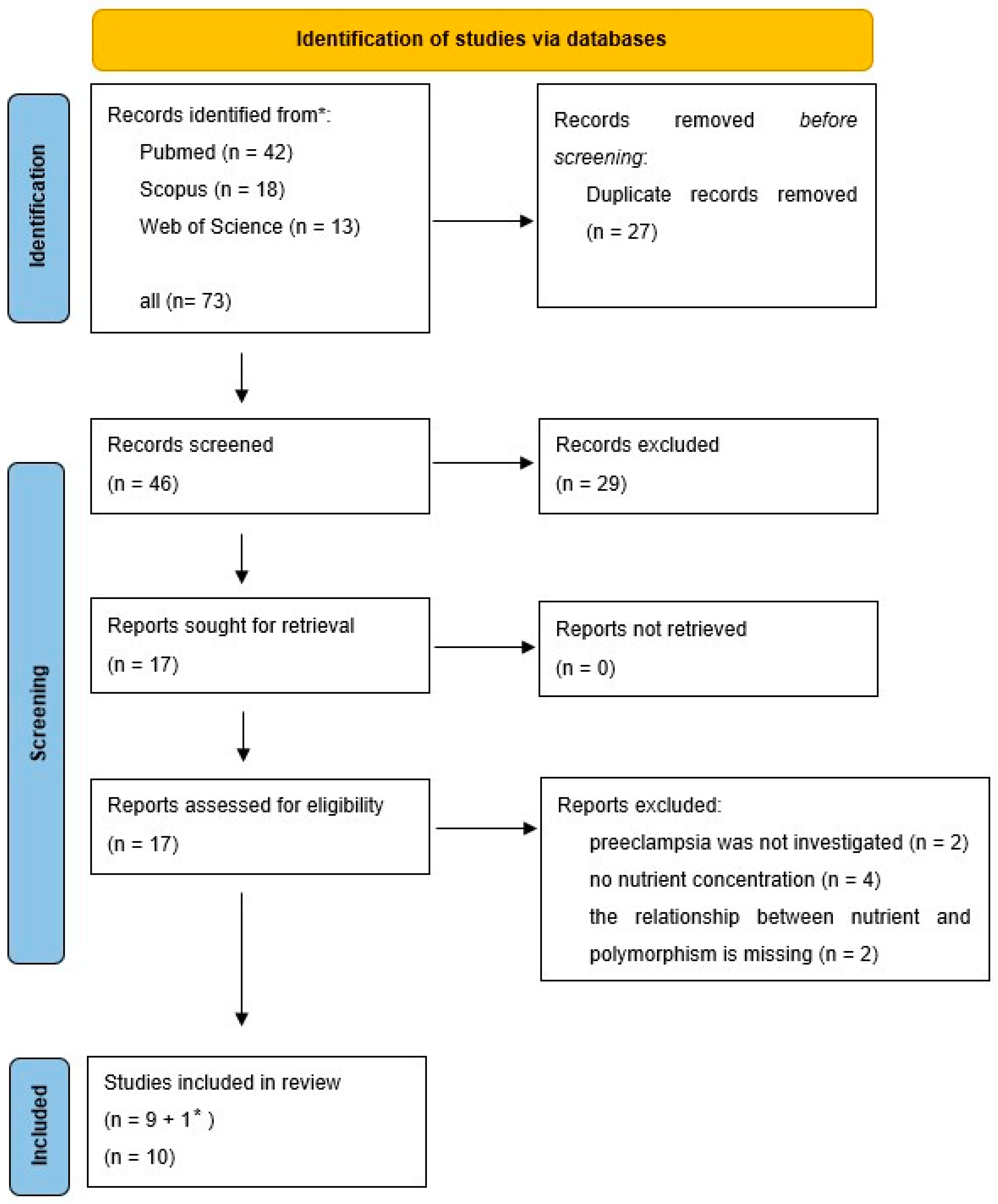
3.1. Method of Searching
3.2. Methylenetetrahydrofolate Reductase (MTHFR) and Folic Acid
- -
- For the CC genotype (n = 97), the incidence of low folate levels was similar in both preeclamptic and control cases (10% in each group). However, in the normal folate range, the percentage of preeclamptic cases (28%) was significantly lower compared to the control group (52%).
- -
- For the TC or TT genotypes (n = 200), similar results were found. The incidence of preeclampsia with low folate levels was 17% in both preeclamptic and control groups, whereas with normal folate levels, preeclamptic cases were 26%, compared to 40% in the control group.
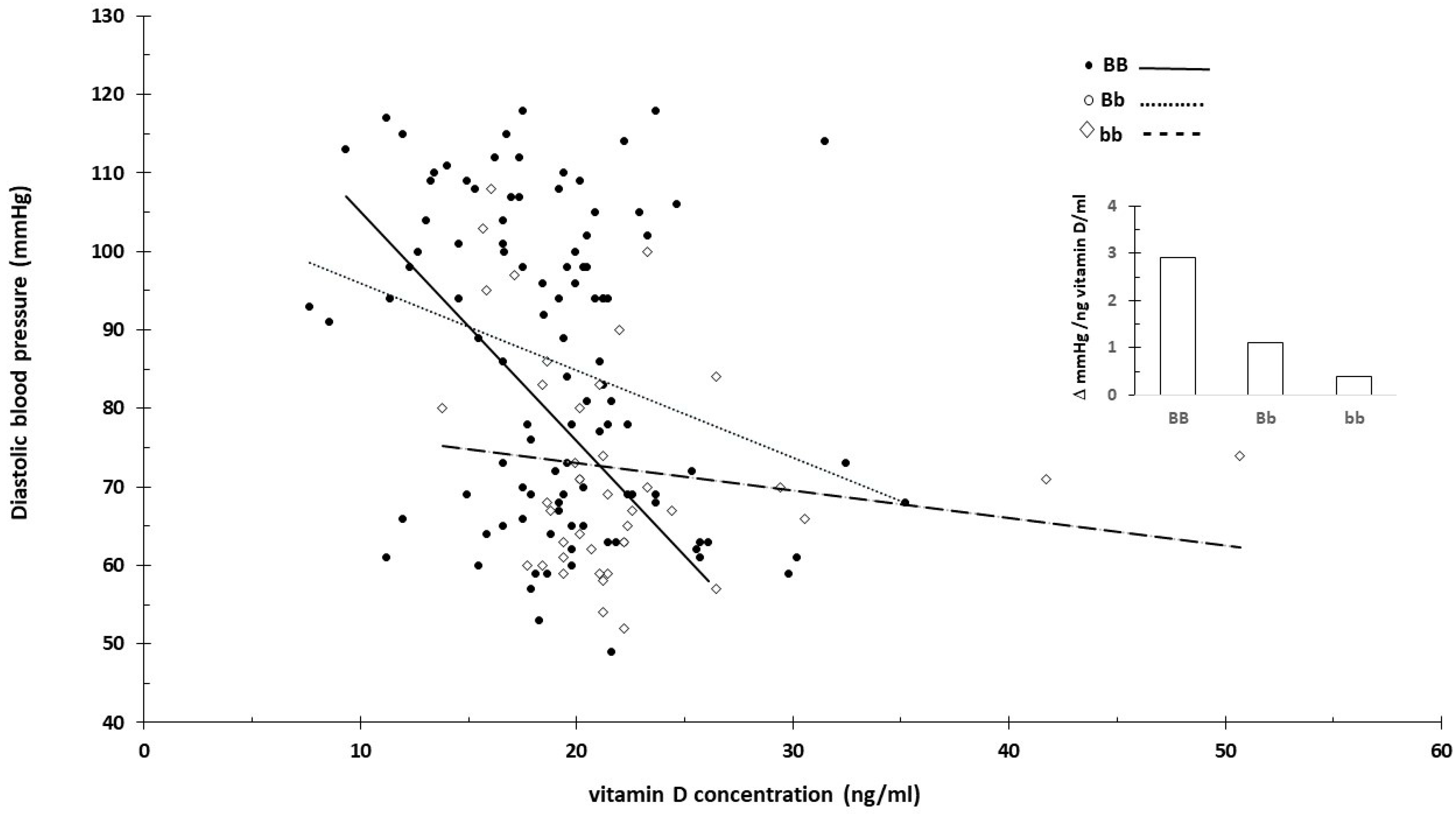
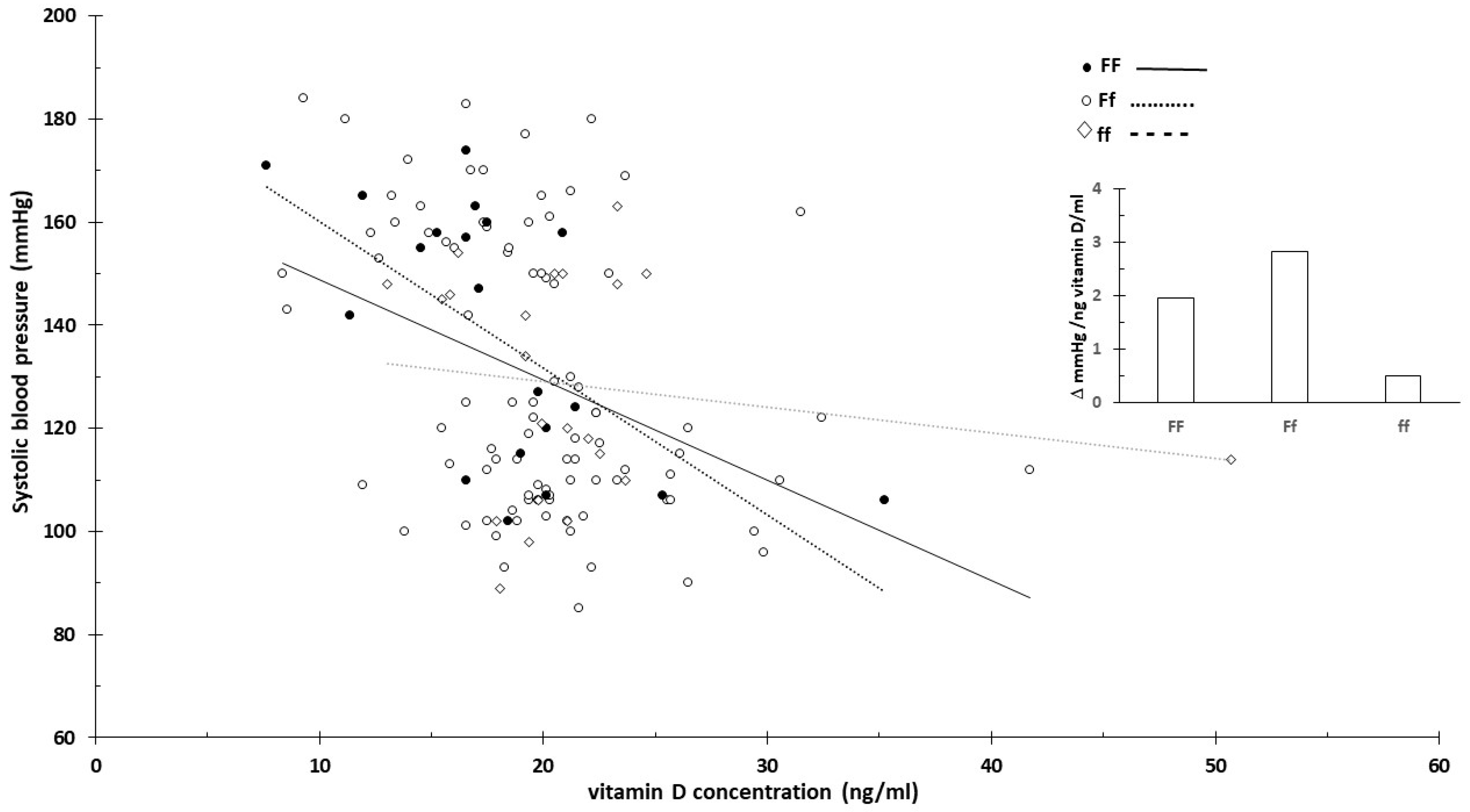
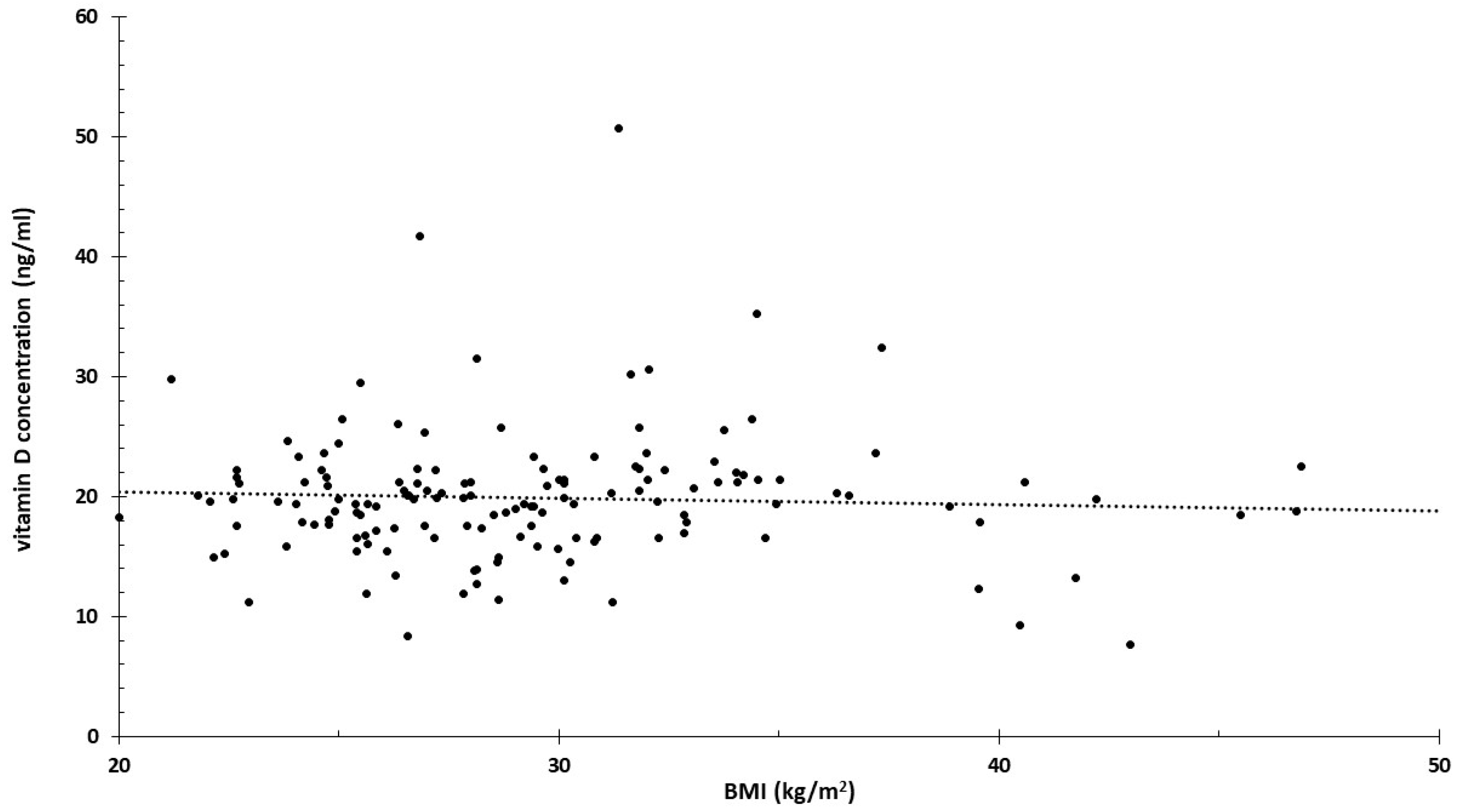
3.3. Vitamin D Receptor and Vitamin D
3.4. Proposed New Analysis
3.5. SIRT 1 Polymorphisms and Levels of Micronutrients
4. Discussion
4.1. Obesity
4.2. Micronutrients
4.3. Future Nutrigenetic Studies
4.4. Target Gene and Metabolite Selection
4.5. Data Evaluation
5. Strengths and Weaknesses
6. Conclusions
Funding
Conflicts of Interest
References
- Xu, M.; Wang, H.X.; Zu, P.; Jiang, N.; Bian, J.F.; Xu, J.R.; Luo, W.; Zhu, P. Association Between Preeclampsia and Blood Pressure in Offspring: A Systematic Review and Meta-Analysis. Curr. Hypertens. Rep. 2024, 26, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Schiavone, M.J.; Pérez, M.P.; Aquieri, A.; Nosetto, D.; Pronotti, M.V.; Mazzei, M.; Kudrle, C.; Avaca, H. The Role of Obesity in the Development of Preeclampsia. Curr. Hypertens. Rep. 2024, 26, 247–258. [Google Scholar] [CrossRef]
- Brown, M.A.; Magee, L.A.; Kenny, L.C.; Karumanchi, S.A.; McCarthy, F.P.; Saito, S.; Hall, D.R.; Warren, C.E.; Adoyi, G.; Ishaku, S.; et al. The Hypertensive Disorders of Pregnancy: ISSHP Classification, Diagnosis & Management Recommendations for International Practice. Pregnancy Hypertens. 2018, 13, 291–310. [Google Scholar] [CrossRef]
- ACOG Practice Bulletin No. 202 Summary: Gestational Hypertension and Preeclampsia. Obstet. Gynecol. 2019, 133, 211–214.
- Lai, J.; Syngelaki, A.; Nicolaides, K.H.; von Dadelszen, P.; Magee, L.A. Impact of New Definitions of Preeclampsia at Term on Identification of Adverse Maternal and Perinatal Outcomes. Am. J. Obstet. Gynecol. 2021, 224, 518.e1–518.e11. [Google Scholar] [CrossRef]
- Tiruneh, S.A.; Vu, T.T.T.; Moran, L.J.; Callander, E.J.; Allotey, J.; Thangaratinam, S.; Rolnik, D.L.; Teede, H.J.; Wang, R.; Enticott, J. Externally Validated Prediction Models for Pre-Eclampsia: Systematic Review and Meta-Analysis. Ultrasound Obstet. Gynecol. 2024, 63, 592–604. [Google Scholar] [CrossRef] [PubMed]
- Shafiul Hossen, M.; Abdul Aziz, M.; Abdul Barek, M.; Safiqul Islam, M. Investigation of the Linkage Between TNF-Alpha rs1800629 Polymorphism and Preeclampsia Risk: A Meta-Analysis. Cytokine 2024, 175, 156499. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.; Tang, X.; Liu, X.; Xu, H. Association Between Vitamin D Receptor (VDR) Gene Polymorphisms and Hypertensive Disorders of Pregnancy: A Systematic Review and Meta-Analysis. PeerJ 2023, 11, e15181. [Google Scholar] [CrossRef]
- Lu, M.; Nie, J.; Shen, H.; Jiao, W.; Men, Z.; Meng, Y.; Xu, H.; Zhu, L.; Yang, X.; Gao, S. The Forkhead Box Protein P3 Gene rs3761548 Promoter Polymorphism Confers a Genetic Contribution to the Risk of Preeclampsia: A Systematic Review and Meta-Analysis. Cytokine 2023, 164, 156164. [Google Scholar] [CrossRef]
- Abbasi, H.; Dastgheib, S.A.; Hadadan, A.; Karimi-Zarchi, M.; Javaheri, A.; Meibodi, B.; Zanbagh, L.; Tabatabaei, R.S.; Neamatzadeh, H. Association of Endothelial Nitric Oxide Synthase 894G > T Polymorphism with Preeclampsia Risk: A Systematic Review and Meta-Analysis Based on 35 Studies. Fetal Pediatr. Pathol. 2021, 40, 455–470. [Google Scholar] [CrossRef]
- Veisian, M.; Javaheri, A.; Amjadi, N.; Tabatabaei, R.S.; Zanbagh, L.; Hadadan, A.; Abbasi, H.; Salimi, E.; Dastgheib, S.A.; Neamatzadeh, H. Association of IL-6 -176G > C Polymorphism with Susceptibility to Preeclampsia: A Systematic Review and Meta-Analysis. Fetal Pediatr. Pathol. 2020, 39, 491–502. [Google Scholar] [CrossRef]
- Veisian, M.; Tabatabaei, R.S.; Javaheri, A.; Abbasi, H.; Salimi, E.; Hadadan, A.; Zanbagh, L.; Dastgheib, S.A.; Neamatzadeh, H. Association of Interleukin-10 -1082G > A Polymorphism with Susceptibility to Preeclampsia: A Systematic Review and Meta-Analysis Based on 21 Studies. Fetal Pediatr. Pathol. 2020, 39, 518–532. [Google Scholar] [CrossRef]
- Esquivel, M.K. Nutritional Status and Nutrients Related to Pre-Eclampsia Risk. Am. J. Lifestyle Med. 2022, 17, 41–45. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, M.; Yi, Y.; Xia, L.; Zhang, R.; Li, C.; Liu, P. Influence of maternal body mass index on pregnancy com-plications and outcomes: A systematic review and meta-analysis. Front. Endocrinol. 2024, 15, 1280692. [Google Scholar] [CrossRef]
- Yan, J.Y.; Jiang, L.L. Expression of advanced glycation end products in placenta and concentration in maternal and umbilical serum in pre-eclampsia. J. Obstet. Gynaecol. Res. 2015, 41, 843–849. [Google Scholar] [CrossRef]
- Endresen, M.J.; Morris, J.M.; Nobrega, A.C.; Buckley, D.; Linton, E.A.; Redman, C.W. Serum from preeclamptic women induces vascular cell adhesion molecule-1 expression on human endothelial cells in vitro: A possible role of increased circulating levels of free fatty acids. Am. J. Obstet. Gynecol. 1998, 179, 665–670. [Google Scholar] [CrossRef]
- Veiga, E.C.A.; Korkes, H.A.; Salomão, K.B.; Cavalli, R.C. Association of LEPTIN and other inflammatory markers with preeclampsia: A systematic review. Front. Pharmacol. 2022, 13, 966400. [Google Scholar] [CrossRef]
- Puttaiah, A.; Kirthan, J.P.A.; Sadanandan, D.M.; Somannavar, M.S. Inflammatory markers and their association with preeclampsia among pregnant women: A systematic review and meta-analysis. Clin. Biochem. 2024, 129, 110778. [Google Scholar] [CrossRef]
- Nugrahani, A.D.; Aziz, M.A.; Santoso, D.P.J.; Siddiq, A.; Pramatirta, A.Y.; Irianti, S.; Pribadi, A.; Anwar, A.D.; Syamsunarno, M.R.A.A. Adiponectin and leptin profiles among obese pregnant women with preeclampsia vs. non-preeclampsia: A systematic review and meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 2024, 28, 3922–3933. [Google Scholar] [CrossRef]
- Danielli, M.; Thomas, R.C.; Quinn, L.M.; Tan, B.K. Vascular adhesion protein-1 (VAP-1) in vascular inflammatory diseases. Vasa 2022, 51, 341–350. [Google Scholar] [CrossRef]
- Trivett, C.; Lees, Z.J.; Freeman, D.J. Adipose tissue function in healthy pregnancy, gestational diabetes mellitus and pre-eclampsia. Eur. J. Clin. Nutr. 2021, 75, 1745–1756. [Google Scholar] [CrossRef]
- Vats, H.; Saxena, R.; Sachdeva, M.P.; Walia, G.K.; Gupta, V. Impact of Maternal Pre-Pregnancy Body Mass Index on Maternal, Fetal and Neonatal Adverse Outcomes in the Worldwide Populations: A Systematic Review and Meta-Analysis. Obes. Res. Clin. Pract. 2021, 15, 536–545. [Google Scholar] [CrossRef]
- Elawad, T.; Scott, G.; Bone, J.N.; Elwell, H.; Lopez, C.E.; Filippi, V.; Green, M.; Khalil, A.; Kinshella, M.W.; Mistry, H.D.; et al. Risk Factors for Pre-Eclampsia in Clinical Practice Guidelines: Comparison with the Evidence. BJOG 2024, 131, 46–62. [Google Scholar] [CrossRef]
- WHO. 6 Facts on Obesity. Available online: https://www.who.int/news-room/facts-in-pictures/detail/6-facts-on-obesity (accessed on 1 July 2024).
- NCD Risk Factor Collaboration (NCD-RisC). Trends in Adult Body-Mass Index in 200 Countries from 1975 to 2014: A Pooled Analysis of 1698 Population-Based Measurement Studies with 19.2 Million Participants. Lancet 2016, 387, 1377–1396. [Google Scholar] [CrossRef]
- Socol, F.G.; Bernad, E.S.; Craina, M.; Abu-Awwad, S.A.; Bernad, B.C.; Socol, I.D.; Farcas, S.S.; Abu-Awwad, A.; Andreescu, N.I. Genetic Insights and Neonatal Outcomes in Preeclampsia and Eclampsia: A Detailed Analysis of the RS5707 Genotype. Diagnostics 2024, 14, 1366. [Google Scholar] [CrossRef]
- Mansego, M.L.; Redon, J.; Marin, R.; González-Albert, V.; Martin-Escudero, J.C.; Fabia, M.J.; Martinez, F.; Chaves, F.J. Renin polymorphisms and haplotypes are associated with blood pressure levels and hypertension risk in postmenopausal women. J. Hypertens. 2008, 26, 230–237. [Google Scholar] [CrossRef]
- Fragoso, J.M.; Alvarez-León, E.; Delgadillo-Rodríguez, H.; Arellano-González, M.; López-Pacheco, F.C.; Cruz-Robles, D.; Peña-Duque, M.A.; Pérez-Méndez, O.; Martínez-Ríos, M.A.; Vargas-Alarcón, G. The C4280A (rs5705) gene polymorphism of the renin (REN) gene is associated with risk of developing coronary artery disease, but not with restenosis after coronary stenting. Exp. Mol. Pathol. 2015, 99, 128–132. [Google Scholar] [CrossRef]
- ACOG Committee on Obstetric Practice. ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. American College of Obstetricians and Gynecologists. Int. J. Gynaecol. Obstet. 2002, 77, 67–75. [Google Scholar]
- Yu, S.; Peng, W.; Zhang, H.; Li, C.; Chen, X.; Wei, M.; Yan, W. The association between maternal and foetal REN gene polymorphisms and preeclampsia/eclampsia: A hybrid design study. Pregnancy Hypertens. 2019, 18, 150–155. [Google Scholar] [CrossRef]
- He, D.; Peng, X.; Xie, H.; Peng, R.; Li, Q.; Guo, Y.; Wang, W.; He, H.; Chen, Y. Genetic variations in angiotensinogen gene and risk of preeclampsia: A pilot study. J. Clin. Med. 2023, 12, 1509. [Google Scholar] [CrossRef] [PubMed]
- Novák, J.; Maceková, S.; Héžová, R.; Máchal, J.; Zlámal, F.; Hlinomaz, O.; Rezek, M.; Souček, M.; Vašků, A.; Slabý, O.; et al. Polymorphism rs7079 in miR-31/-584 binding site in angiotensinogen gene associates with earlier onset of coronary artery disease in Central European population. Genes 2022, 13, 1981. [Google Scholar] [CrossRef]
- Zhou, A.; Dekker, G.A.; Lumbers, E.R.; Lee, S.Y.; Thompson, S.D.; McCowan, L.M.; Roberts, C.T.; SCOPE Consortium. The association of AGTR2 polymorphisms with preeclampsia and uterine artery bilateral notching is modulated by maternal BMI. Placenta 2013, 34, 75–81. [Google Scholar] [CrossRef]
- Lozano-Gonzalez, K.; Padilla-Rodríguez, E.; Texis, T.; Gutiérrez, M.N.; Rodríguez-Dorantes, M.; Cuevas-Córdoba, B.; Ramírez-García, E.; Mino-León, D.; Sánchez-García, S.; Gonzalez-Covarrubias, V. Allele frequency of ACE2 intron variants and its association with blood pressure. DNA Cell Biol. 2020, 39, 2095–2101. [Google Scholar] [CrossRef]
- Huang, G.; Wang, Y.; Qin, L.; Huang, B.; Yu, X. Association and functional analysis of angiotensin-converting enzyme 2 genetic variants with the pathogenesis of pre-eclampsia. Front. Endocrinol. 2022, 13, 926512. [Google Scholar] [CrossRef]
- Karppanen, T.; Kaartokallio, T.; Klemetti, M.M.; Heinonen, S.; Kajantie, E.; Kere, J.; Kivinen, K.; Pouta, A.; Staff, A.C.; Laivuori, H. An RGS2 3’UTR polymorphism is associated with preeclampsia in overweight women. BMC Genet. 2016, 17, 121. [Google Scholar] [CrossRef]
- Ghorbani, Z.; Shakiba, M.; Rezavand, N.; Rahimi, Z.; Vaisi-Raygani, A.; Rahimi, Z.; Shakiba, E. Gene variants and haplotypes of vitamin D biosynthesis, transport, and function in preeclampsia. Hypertens. Pregnancy 2021, 40, 1–8. [Google Scholar] [CrossRef]
- Erfanian, S.; Yazdanpour, L.; Javeshghani, D.; Roustazadeh, A. Association of arginine vasopressin (AVP) promoter polymorphisms with preeclampsia. Pregnancy Hypertens. 2019, 18, 122–125. [Google Scholar] [CrossRef]
- Amosco, M.D.; Tavera, G.R.; Villar, V.A.M.; Naniong, J.M.A.; David-Bustamante, L.M.G.; Williams, S.M.; Jose, P.A.; Palmes-Saloma, C.P. Non-additive effects of ACVR2A in preeclampsia in a Philippine population. BMC Pregnancy Childbirth 2019, 19, 11. [Google Scholar] [CrossRef]
- Madar-Shapiro, L.; Karady, I.; Trahtenherts, A.; Syngelaki, A.; Akolekar, R.; Poon, L.; Cohen, R.; Sharabi-Nov, A.; Huppertz, B.; Sammar, M.; et al. Predicting the Risk to Develop Preeclampsia in the First Trimester Combining Promoter Variant -98A/C of LGALS13 (Placental Protein 13), Black Ethnicity, Previous Preeclampsia, Obesity, and Maternal Age. Fetal Diagn. Ther. 2018, 43, 250–265. [Google Scholar] [CrossRef]
- Zhang, C.; Williams, M.A.; Edwards, K.L.; Austin, M.A. Trp64Arg polymorphism of the beta3-adrenergic receptor gene, pre-pregnancy obesity and risk of pre-eclampsia. J. Matern. Fetal Neonatal Med. 2005, 17, 19–28. [Google Scholar] [CrossRef]
- Li, Y.Y.; Lu, X.Z.; Wang, H.; Zhou, Y.H.; Yang, X.X.; Geng, H.Y.; Gong, G.; Kim, H.J. ADRB3 Gene Trp64Arg Polymorphism and Essential Hypertension: A Meta-Analysis Including 9,555 Subjects. Front. Genet. 2018, 9, 106. [Google Scholar] [CrossRef]
- Maharaj, N.R.; Ramkaran, P.; Pillay, S.; Chuturgoon, A.A. microRNA-27a rs895819 is associated with obesity in HIV infected preeclamptic Black South African women on HAART. BMC Med. Genet. 2016, 17, 92. [Google Scholar] [CrossRef]
- Lin, H.; Yin, Z.; Yu, X.Y.; Lin, N.; Lin, Y.; Chen, J.; Chen, Y.Z.; Lu, K.P.; Liu, H.K. Variants -250G/A and -514C/T in the LIPC gene are associated with hypertensive disorders of pregnancy in Chinese women. Genet. Mol. Res. 2014, 13, 6126–6134. [Google Scholar] [CrossRef]
- Enquobahrie, D.A.; Sanchez, S.E.; Muy-Rivera, M.; Qiu, C.; Zhang, C.; Austin, M.A.; Williams, M.A. Hepatic lipase gene polymorphism, pre-pregnancy overweight status and risk of preeclampsia among Peruvian women. Gynecol. Endocrinol. 2005, 21, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhan, Y.; Li, G.Z.; Liu, S.G. Association between severe preeclampsia and single nucleotide polymorphism of macrophage migration inhibitory factors -173G/C. Zhonghua Fu Chan Ke Za Zhi 2012, 47, 342–346. (In Chinese) [Google Scholar]
- Ardissino, M.; Geddes-Barton, M.; Banerjee, A. Genetically predicted body mass index and maternal outcomes of pregnancy: A two-sample Mendelian randomisation study. BJOG 2024, 131, 493–499. [Google Scholar] [CrossRef]
- Pattingre, S.; Turtoi, A. BAG Family Members as Mitophagy Regulators in Mammals. Cells 2022, 11, 681. [Google Scholar] [CrossRef]
- Ferro, E.; Trabalzini, L. RalGDS family members couple Ras to Ral signalling and that’s not all. Cell Signal. 2010, 22, 1804–1810. [Google Scholar] [CrossRef]
- Abramova, M.; Churnosova, M.; Efremova, O.; Aristova, I.; Reshetnikov, E.; Polonikov, A.; Churnosov, M.; Ponomarenko, I. Effects of Pre-Pregnancy Overweight/Obesity on the Pattern of Association of Hypertension Susceptibility Genes with Preeclampsia. Life 2022, 12, 2018. [Google Scholar] [CrossRef]
- Oh, C.; Keats, E.C.; Bhutta, Z.A. Vitamin and Mineral Supplementation During Pregnancy on Maternal, Birth, Child Health and Development Outcomes in Low- and Middle-Income Countries: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 491. [Google Scholar] [CrossRef]
- Rocha, T.; Allotey, J.; Palacios, A.; Vogel, J.P.; Smits, L.; Carroli, G.; Mistry, H.; Young, T.; Qureshi, Z.P.; Cormick, G.; et al. Calcium supplementation to prevent pre-eclampsia: Protocol for an individual participant data meta-analysis, network meta-analysis and health economic evaluation. BMJ Open 2023, 13, e065538. [Google Scholar] [CrossRef]
- Woo Kinshella, M.L.; Sarr, C.; Sandhu, A.; Bone, J.N.; Vidler, M.; Moore, S.E.; Elango, R.; Cormick, G.; Belizan, J.M.; Hofmeyr, G.J.; et al. Calcium for pre-eclampsia prevention: A systematic review and network meta-analysis to guide personalised antenatal care. BJOG 2022, 129, 1833–1843. [Google Scholar] [CrossRef]
- Liu, C.; Liu, C.; Wang, Q.; Zhang, Z. Supplementation of folic acid in pregnancy and the risk of preeclampsia and gestational hypertension: A meta-analysis. Arch. Gynecol. Obstet. 2018, 298, 697–704. [Google Scholar] [CrossRef]
- Bulloch, R.E.; Lovell, A.L.; Jordan, V.M.B.; McCowan, L.M.E.; Thompson, J.M.D.; Wall, C.R. Maternal folic acid supplementation for the prevention of preeclampsia: A systematic review and meta-analysis. Paediatr. Perinat. Epidemiol. 2018, 32, 346–357. [Google Scholar] [CrossRef]
- Fogacci, S.; Fogacci, F.; Banach, M.; Michos, E.D.; Hernandez, A.V.; Lip, G.Y.H.; Blaha, M.J.; Toth, P.P.; Borghi, C.; Cicero, A.F.G.; et al. Vitamin D supplementation and incident preeclampsia: A systematic review and meta-analysis of randomized clinical trials. Clin. Nutr. 2020, 39, 1742–1752. [Google Scholar] [CrossRef]
- Khaing, W.; Vallibhakara, S.A.; Tantrakul, V.; Vallibhakara, O.; Rattanasiri, S.; McEvoy, M.; Attia, J.; Thakkinstian, A. Calcium and Vitamin D Supplementation for Prevention of Preeclampsia: A Systematic Review and Network Meta-Analysis. Nutrients 2017, 9, 1141. [Google Scholar] [CrossRef]
- Lo, A.C.Q.; Lo, C.C.W. Vitamin D supplementation and incident preeclampsia: An updated meta-analysis of randomized clinical trials. Clin. Nutr. 2022, 41, 1852–1853. [Google Scholar] [CrossRef]
- Molloy, A.M. Genetic aspects of folate metabolism. Subcell. Biochem. 2012, 56, 105–130. [Google Scholar] [CrossRef]
- Yamada, K.; Chen, Z.; Rozen, R.; Matthews, R.G. Effects of common polymorphisms on the properties of recombinant human methylenetetrahydrofolate reductase. Proc. Natl. Acad. Sci. USA 2001, 98, 14853–14858. [Google Scholar] [CrossRef]
- Guenther, B.D.; Sheppard, C.A.; Tran, P.; Rozen, R.; Matthews, R.G.; Ludwig, M.L. The structure and properties of methylenetetrahydrofolate reductase from Escherichia coli suggest how folate ameliorates human hyperhomocysteinemia. Nat. Struct. Biol. 1999, 6, 359–365. [Google Scholar] [CrossRef]
- Mislanova, C.; Martsenyuk, O.; Huppertz, B.; Obolenskaya, M. Placental markers of folate-related metabolism in preeclampsia. Reproduction 2011, 142, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.A.; Sanchez, S.E.; Zhang, C.; Bazul, V. Methylenetetrahydrofolate reductase 677 C–>T polymorphism and plasma folate in relation to pre-eclampsia risk among Peruvian women. J. Matern. Fetal Neonatal Med. 2004, 15, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Powers, R.W.; Dunbar, M.S.; Gallaher, M.J.; Roberts, J.M. The 677 C-T methylenetetrahydrofolate reductase mutation does not predict increased maternal homocysteine during pregnancy. Obstet. Gynecol. 2003, 101, 762–766. [Google Scholar] [CrossRef]
- Also-Rallo, E.; Lopez-Quesada, E.; Urreizti, R.; Vilaseca, M.A.; Lailla, J.M.; Balcells, S.; Grinberg, D. Polymorphisms of genes involved in homocysteine metabolism in preeclampsia and in uncomplicated pregnancies. Eur. J. Obstet. Gynecol. Reprod. Biol. 2005, 120, 45–52. [Google Scholar] [CrossRef]
- Rajkovic, A.; Mahomed, K.; Rozen, R.; Malinow, M.R.; King, I.B.; Williams, M.A. Methylenetetrahydrofolate reductase 677 C® T polymorphism, plasma folate, vitamin B(12) concentrations, and risk of preeclampsia among black African women from Zimbabwe. Mol. Genet. Metab. 2000, 69, 33–39. [Google Scholar] [CrossRef]
- Knabl, J.; Vattai, A.; Ye, Y.; Jueckstock, J.; Hutter, S.; Kainer, F.; Mahner, S.; Jeschke, U. Role of Placental VDR Expression and Function in Common Late Pregnancy Disorders. Int. J. Mol. Sci. 2017, 18, 2340. [Google Scholar] [CrossRef] [PubMed]
- Aziz, A.; Shah, M.; Siraj, S.; Iqbal, W.; Jan, A.; Khan, I.; Ahmed, S.; Vitale, S.G.; Angioni, S. Association of vitamin D deficiency and vitamin D receptor (VDR) gene single-nucleotide polymorphism (rs7975232) with risk of preeclampsia. Gynecol. Endocrinol. 2023, 39, 2146089. [Google Scholar] [CrossRef]
- Rezavand, N.; Tabarok, S.; Rahimi, Z.; Vaisi-Raygani, A.; Mohammadi, E.; Rahimi, Z. The effect of VDR gene polymorphisms and vitamin D level on blood pressure, risk of preeclampsia, gestational age, and body mass index. J. Cell Biochem. 2019, 120, 6441–6448. [Google Scholar] [CrossRef]
- Fondjo, L.A.; Mensah, J.B.; Awuah, E.O.; Sakyi, S.A. Interplay between vitamin D status, vitamin D receptor gene variants and preeclampsia risk in Ghanaian women: A case-control study. PLoS ONE 2024, 19, e0303778. [Google Scholar] [CrossRef]
- Vimaleswaran, K.S.; Berry, D.J.; Lu, C.; Tikkanen, E.; Pilz, S.; Hiraki, L.T.; Cooper, J.D.; Dastani, Z.; Li, R.; Houston, D.K.; et al. Causal relationship between obesity and vitamin D status: Bi-directional Mendelian randomization analysis of multiple cohorts. PLoS Med. 2013, 10, e1001383. [Google Scholar] [CrossRef]
- Shimoyama, Y.; Suzuki, K.; Hamajima, N.; Niwa, T. Sirtuin 1 gene polymorphisms are associated with body fat and blood pressure in Japanese. Transl. Res. 2011, 157, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Khadir, F.; Rahimi, Z.; Vaisi-Raygani, A.; Shakiba, E.; Pouramir, M.; Bahrehmand, F. Variants and Haplotypes of SIRT1 (rs7895833, rs7069102, and rs2273773) are Associated with the Risk of Preeclampsia and Affect the Trace Elements and Antioxidant Enzymes Levels. Biochem. Genet. 2024, 62, 2667–2685. [Google Scholar] [CrossRef] [PubMed]
- Junien, C.; Dupret, J.M.; Gallou, C.; Longuemaux, S.; Richard, S.; Saquet, C.; Krishnamoorty, R.; Delomenie, C.; Droz, D.; Bouvier, R.; et al. Prévention du carcinoma rénal: L’approche nutrigénétique [Prevention of renal carcinoma: The nutri-genetic approach]. J. Soc. Biol. 2000, 194, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.J.; Nakura, J.; Wu, Z.; Yamamoto, M.; Abe, M.; Chen, Y.; Tabara, Y.; Yamamoto, Y.; Igase, M.; Bo, X.; et al. Association of angiotensin II type 2 receptor gene variant with hypertension. Hypertens. Res. 2003, 26, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Reilly, R.; McNulty, H.; Pentieva, K.; Strain, J.J.; Ward, M. MTHFR 677TT genotype and disease risk: Is there a modulating role for B-vitamins? Proc. Nutr. Soc. 2014, 73, 47–56. [Google Scholar] [CrossRef]
- Czeizel, A.E. Periconceptional folic acid and multivitamin supplementation for the prevention of neural tube defects and other congenital abnormalities. Birth Defects Res. A Clin. Mol. Teratol. 2009, 85, 260–268. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, N.; An, J.; Zeng, X.; Zhao, Y.; Sun, X.; Bu, H.; Wang, H. Maternal folic acid supplementation to prevent preeclampsia: A systematic review and meta-analysis. Complement. Ther. Med. 2024, 82, 103052. [Google Scholar] [CrossRef]
- Orabona, R.; Zanardini, C.; Zatti, S.; Sartori, E.; Prefumo, F. Folic Acid Supplementation in Pregnancy: A Matter of Doses? Hypertension 2020, 76, 30–31. [Google Scholar] [CrossRef]
- Yu, X.; Diao, L.; Du, B.; Wang, Y.; Xu, X.; Yu, A.; Zhao, J. Individualized folic acid supplementation based on MTHFR and MTRR gene polymorphisms reduces the risk of gestational diabetes mellitus in a Chinese population. Int. J. Clin. Exp. Pathol. 2023, 16, 150–157. [Google Scholar]
- Wien, T.N.; Pike, E.; Wisløff, T.; Staff, A.; Smeland, S.; Klemp, M. Cancer risk with folic acid supplements: A system-atic review and meta-analysis. BMJ Open 2012, 2, e000653. [Google Scholar] [CrossRef]
- van Etten, R.W.; de Koning, E.J.; Verhaar, M.C.; Gaillard, C.A.; Rabelink, T.J. Impaired NO-dependent vasodilation in patients with Type II (non-insulin-dependent) diabetes mellitus is restored by acute administration of folate. Diabetologia 2002, 45, 1004–1010. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.ncbi.nlm.nih.gov/clinvar?term=607093[MIM] (accessed on 3 July 2024).
- Shaheen, G.; Jahan, S.; Bibi, N.; Ullah, A.; Faryal, R.; Almajwal, A.; Afsar, T.; Al-Disi, D.; Abulmeaty, M.; Al Khuraif, A.A.; et al. Association of endothelial nitric oxide synthase gene variants with preeclampsia. Reprod. Health 2021, 18, 163. [Google Scholar] [CrossRef] [PubMed]
- Tesfa, E.; Munshea, A.; Nibret, E.; Tebeje Gizaw, S. Association of endothelial nitric oxide synthase gene variants in pre-eclampsia: An updated systematic review and meta-analysis. J. Matern. Fetal Neonatal Med. 2023, 36, 2290918. [Google Scholar] [CrossRef] [PubMed]
- Kukor, Z.; Valent, S. A nitrogén-monoxid-szintézis zavarai praeeclampsiában [Nitric oxide and preeclampsia]. Orv. Hetil. 2010, 151, 2125–2135. [Google Scholar] [CrossRef] [PubMed]
- Kukor, Z.; Tóth, M. Ca(2+)-dependent and Ca(2+)-independent NO-synthesizing activities of human primordial placenta. Acta Physiol. Hung. 1994, 82, 313–319. [Google Scholar]
- Sharma, D.; Hussain, S.A.; Akhter, N.; Singh, A.; Trivedi, S.S.; Bhattacharjee, J. Endothelial nitric oxide synthase (eNOS) gene Glu298Asp polymorphism and expression in North Indian preeclamptic women. Pregnancy Hypertens. 2014, 4, 65–69. [Google Scholar] [CrossRef]
- Kim, Y.J.; Park, H.S.; Lee, H.Y.; Ha, E.H.; Suh, S.H.; Oh, S.K.; Yoo, H.S. Reduced L-arginine level and decreased placental eNOS activity in preeclampsia. Placenta 2006, 27, 438–444. [Google Scholar] [CrossRef]
- Facchinetti, F.; Longo, M.; Piccinini, F.; Neri, I.; Volpe, A. L-arginine infusion reduces blood pressure in preeclamptic women through nitric oxide release. J. Soc. Gynecol. Investig. 1999, 6, 202–207. [Google Scholar] [CrossRef]
- Ortiz-Cerda, T.; Mosso, C.; Alcudia, A.; Vázquez-Román, V.; González-Ortiz, M. Pathophysiology of Preeclampsia and L-Arginine/L-Citrulline Supplementation as a Potential Strategy to Improve Birth Outcomes. Adv. Exp. Med. Biol. 2023, 1428, 127–148. [Google Scholar] [CrossRef]
- Gui, S.; Jia, J.; Niu, X.; Bai, Y.; Zou, H.; Deng, J.; Zhou, R. Arginine supplementation for improving maternal and neonatal outcomes in hypertensive disorder of pregnancy: A systematic review. J. Renin Angiotensin Aldosterone Syst. 2014, 15, 88–96. [Google Scholar] [CrossRef]
- Kukor, Z.; Valent, S.; Tóth, M. Regulation of nitric oxide synthase activity by tetrahydrobiopterin in human placentae from normal and pre-eclamptic pregnancies. Placenta 2000, 21, 763–772. [Google Scholar] [CrossRef]
- Kukor, Z.; Mészáros, G.; Hertelendy, F.; Tóth, M. Calcium-dependent nitric oxide synthesis is potently stimulated by tetrahydrobiopterin in human primordial placenta. Placenta 1996, 17, 69–73. [Google Scholar] [CrossRef]
- Sahin-Tóth, M.; Kukor, Z.; Tóth, M. Tetrahydrobiopterin preferentially stimulates activity and promotes subunit aggregation of membrane-bound calcium-dependent nitric oxide synthase in human placenta. Mol. Hum. Reprod. 1997, 3, 293–298. [Google Scholar] [CrossRef][Green Version]
- Pánczél, Z.; Supák, D.; Kovács, B.; Kukor, Z.; Valent, S. A pravasztatin hatása tetrahidrobiopterin-érzékeny és -rezisztens praeeclampsiás placenták NO-szintáz-aktivitására [Effect of pravastatin on tetrahydrobiopterin-sensitive and -resistant NO synthase activity of preeclamptic placentas]. Orv. Hetil. 2020, 161, 389–395. (In Hungarian) [Google Scholar] [CrossRef]
- Pánczél, Z.; Kukor, Z.; Supák, D.; Kovács, B.; Kecskeméti, A.; Czizel, R.; Djurecz, M.; Alasztics, B.; Csomó, K.B.; Hrabák, A.; et al. Pravastatin induces NO synthesis by enhancing microsomal arginine uptake in healthy and preeclamptic placentas. BMC Pregnancy Childbirth 2019, 19, 426. [Google Scholar] [CrossRef]
- Fürst, P.; Stehle, P. What are the essential elements needed for the determination of amino acid requirements in humans? J. Nutr. 2004, 134 (Suppl. S6), 1558S–1565S. [Google Scholar] [CrossRef]
- Calder, P.C.; Yaqoob, P. Glutamine and the immune system. Amino Acids 1999, 17, 227–241. [Google Scholar] [CrossRef]
- Wu, G. Intestinal mucosal amino acid catabolism. J. Nutr. 1998, 128, 1249–1252. [Google Scholar] [CrossRef]
- Prameswari, N.; Irwinda, R.; Wibowo, N.; Saroyo, Y.B. Maternal Amino Acid Status in Severe Preeclampsia: A Cross-Sectional Study. Nutrients 2022, 14, 1019. [Google Scholar] [CrossRef]



| Reference | Polymorphism | Gene | Region | Effect of Allele | BMI |
|---|---|---|---|---|---|
| Socol (2024) [26] | rs5707 | renin (REN) | intron | protective: AA | BMI < 24 * |
| Yu (2019) [30] | rs5707 | renin (REN) | intron | risk: AC | BMI ≥ 24 * |
| He (2023) [31] | rs7079 | angiotensinogen (AGT) | 3′UTR side | risk: TT | BMI < 25 * |
| Amosco (2019) [39] | rs1014064 | ACVR2 | intron | risk: G | BMI ≥ 25.1 * |
| Madar-Shapiro (2018) [40] | rs3764843 | LGALS13 | promoter | risk: AA | BMI ≥ 35 * |
| Karppanen (2016) [36] | rs4606 | RGS2 | 3′UTR side | risk: GG/CG | 25 ≤ BMI < 30 * |
| Zhou (2013) [33] | C4599A | AGTR2 | 3′UTR side | risk: A | BMI ≥ 25 * BMI < 25 N.E. |
| Zhang (2005) [41] | rs4994 | ADRB3 | Trp64Arg | protective: AA | BMI < 30 * |
| Enquobahrie (2005) [45] | rs1800588 | LIPC | promoter | risk: TT | BMI ≥ 25 * BMI < 25 N.E |
| Erfanian (2019) [38] | rs3729965 | AVP (arginine vasopressin) | promoter | risk: T | BMI ≥ 30 * |
| Huang (2022) [35] | rs2106809 | ACE2 | intron | risk: AA | BMI ≥ 23 * |
| Abramova (2022) [50] | rs805303 | BAG6 | intron | protective: A | BMI ≥ 25 * BMI < 25 N.E. |
| Abramova (2022) [50] | rs167479 | RGL3 | Pro162His | risk: G | BMI ≥ 25 * BMI < 25 N.E. |
| Reference | Polymorphism | Gene | Region | Effect of Allele |
|---|---|---|---|---|
| Mislanova (2011) [62] | rs1801133 | MTHFR | Ala222Val | C/T: lower folate concentration |
| Williams (2004) [63] | rs1801133 | MTHFR | Ala222Val | polymorphism is not an important risk marker |
| Powers (2003) [64] | rs1801133 | MTHFR | Ala222Val | no difference in folate concentration between the alleles |
| Also-Rallo (2005) [65] | rs1801133 | MTHFR | Ala222Val | alleles have no effect |
| Also-Rallo (2005) [65] | rs1801133 | MTHFR | Glu429Ala | alleles have no effect |
| Rajkovic (2000) [66] | rs1801133 | MTHFR | Ala222Val | no difference in folate concentration between the alleles |
| Aziz (2023) [68] | rs7975232 | VDR | intron | alleles have no effect |
| Rezavand (2019) [69] | rs10735810 rs1544410 rs731236 | VDR | exon intron exon | no correlation was investigated |
| Ghorbani (2021) [37] | rs7975232 | VDR | intron | vitamin D concentrations lower in GT/GG than the TT genotype |
| Fondjo (2024) [70] | rs228570 rs1544410 | VDR | intron intron | no vitamin D concentration test according to genotype |
| Khadir (2024) [73] | rs7895833 rs7069102 rs2273773 | SIRT1 | promoter intron exon | zinc decreasing AA GG TT |
| Khadir (2024) [73] | rs7069102 | SIRT1 | intron | Cu level increased: TT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kukor, Z. Nutrigenetic Investigations in Preeclampsia. Nutrients 2024, 16, 3248. https://doi.org/10.3390/nu16193248
Kukor Z. Nutrigenetic Investigations in Preeclampsia. Nutrients. 2024; 16(19):3248. https://doi.org/10.3390/nu16193248
Chicago/Turabian StyleKukor, Zoltán. 2024. "Nutrigenetic Investigations in Preeclampsia" Nutrients 16, no. 19: 3248. https://doi.org/10.3390/nu16193248
APA StyleKukor, Z. (2024). Nutrigenetic Investigations in Preeclampsia. Nutrients, 16(19), 3248. https://doi.org/10.3390/nu16193248




