Abstract
Background: The vitamin D metabolite ratio (VMR) has recently been identified as a potentially better indicator of vitamin D deficiency than 25-hydroxyvitamin D (25(OH)D) alone. This study aims to validate these findings by demonstrating that VMR is more strongly correlated with parathyroid hormone (PTH) levels than 25(OH)D and 24,25-dihydroxyvitamin D (24,25(OH)2D). In addition, the study investigates VMR as a more effective predictor of mortality than 25(OH)D and 24,25(OH)2D. Methods: The SarcoPhAge cohort is a Belgian cohort of community-dwelling older adults. Levels of 25(OH)D and 24,25(OH)2D were measured in 204 serum samples collected at the second year of follow-up using liquid chromatography–tandem mass spectrometry (LC–MS/MS), and VMR was calculated using the formula: VMR = (24,25(OH)D/25(OH)D) × 100. Vitamin D deficiency cut-offs were defined at 25(OH)D < 20 ng/mL, 24,25(OH)2D < 1.2 ng/mL, or VMR < 4% according to previously proposed cut-offs. Participants were followed for up to 9 years. Results: A total of 35 individuals (17.2%) had 25(OH)D < 20 ng/mL, 40 individuals (19.6%) had 24,25(OH)2D < 1.2 ng/mL, and 14 individuals (7.0%) had VMR < 4%. All three markers, 25(OH)D, 24,25(OH)2D, and VMR, were independently associated with PTH levels, with VMR showing the strongest correlation (rho: −0.292; p < 0.0001). When categorized into quartiles, only 24,25(OH)2D and VMR showed significant increases in PTH levels across quartiles (p = 0.002 and p < 0.0001, respectively). When cut-offs for low vitamin D status were applied, patients with low VMR had the highest rate of all-cause mortality. However, in a Cox proportional hazard regression model, both low VMR profile and low 25(OH)D profile were risk factors for all-cause mortality. Conclusions: This study confirms that VMR is an efficient biomarker for assessing functional vitamin D deficiency.
1. Introduction
Vitamin D is a critical nutrient that plays an important role in maintaining bone, and its deficiency can lead to many adverse outcomes such as rickets or osteomalacia. Its importance extends beyond bone health to influence several physiological processes, including immune function, cardiovascular health, several cancers, diabetes, cognitive function, and muscle [,,]. Despite large research and public health initiatives, worldwide prevalence of vitamin D deficiency remains high in many regions [,,,,]. In addition, screening of the general population for vitamin D deficiency has shown some limitations in preventing outcomes []. Active screening for vitamin D deficiency at the population level, in the absence of a clinical presentation, does not appear to be justified, but screening and/or routine supplementation may be appropriate in high-risk populations, for example, older individuals in residential care and those with pigmented skin living in northerly latitudes [,]. Therefore, more effective diagnostic strategies for vitamin D deficiency are required.
The current standard for assessing vitamin D status involves measuring serum levels of 25-hydroxyvitamin D [25(OH)D]. Indeed, total 25(OH)D, the sum of 25(OH)D2 and 25(OH)D3, is usually considered the best indicator of vitamin D in the body, as observational and interventional studies have shown its ability to prevent cytoskeletal outcomes [,,]. However, measuring 25(OH)D has significant limitations. First, some studies have shown that individuals with low 25(OH)D levels may not have the expected clinical signs of deficiency, such as bone mineralization defects or secondary hyperparathyroidism (elevated parathyroid hormone (PTH) levels) [], suggesting that 25(OH)D is not a complete measure of vitamin D sufficiency. Second, 25(OH)D levels are differentially associated with bone mineral density, depending on factors such as ethnicity, geographical location and season. For example, black Americans, although having less 25(OH)D compared to white Americans, have a higher bone mineral density and a lower risk of fragility fractures [,]. In addition, although a reference method exists, some commercial methods have not been standardized yet. This situation increases the analytical variation between assays and the discrepancies between reported results [].
Given these limitations, there has been increasing interest in alternative biomarkers based on other vitamin D forms that may provide a more comprehensive assessment of vitamin D status. One promising approach is the measurement of 24,25-dihydroxyvitamin D [24,25(OH)2D], the major catabolic product of 25(OH)D, together with the vitamin D metabolite ratio (VMR), defined as the ratio of 24,25(OH)2D to 25(OH)D multiplied by 100 []. This approach has several advantages over traditional 25(OH)D measurements. Indeed, we have previously shown in a large population of children that, for the same 25(OH)D concentration, some of them had already started to catabolize 25(OH)D and presented quantifiable 24.25(OH)2-vitamin D concentrations, whereas some other did not, revealing the potential personalization of an individual threshold for vitamin D []. Additional research has shown that VMR provides better information about vitamin D status and bone health than 25(OH)D levels alone. For example, individuals with a low VMR (<4%) often have higher PTH levels and markers of bone turnover, indicating functional vitamin D deficiency regardless of serum 25(OH)D concentrations []. This suggests that VMR may better identify individuals at risk of bone-related complications and other health problems associated with vitamin D deficiency than 25(OH)D alone. Furthermore, despite significantly lower 25(OH)D levels in African Americans, Berg et al. showed that the average VMR and bone health metrics were comparable to those of white participants []. In the context of population screening and prevention of outcomes, Herrmann et al. has found that a low VMR was associated with higher all-cause mortality, independent of 25(OH)D levels []. This supports the notion that VMR captures critical aspects of vitamin D metabolism that are not reflected by 25(OH)D measurements alone.
This study aims to confirm the utility of the VMR as a superior biomarker for the assessment of functional vitamin D deficiency in the SarcoPhAge cohort of community-dwelling older adults in Belgium []. Specifically, this study aims to (1) assess if VMR correlates better with PTH levels than 25(OH)D and 24,25(OH)2D and (2) assess the predictive value of VMR for long-term mortality compared to 25(OH)D and 24,25(OH)2D.
2. Methods
2.1. Population
The SarcoPhAge study is a prospective longitudinal cohort study designed to assess the health and functional consequences of sarcopenia in community-dwelling older adults []. The study was approved by the Ethics Committee of the University Teaching Hospital of Liège (number 2012/277) and all participants gave informed consent. Participants were recruited between June 2013 and June 2014 from various outpatient clinics in Liège, Belgium, including osteoporosis, geriatric, rheumatology, and rehabilitation centers, and through press advertisements. The inclusion criteria was community-dwelling individuals aged 65 years or older. A total of 534 subjects were enrolled, with a mean age of 73.5 ± 6.16 years, of whom 60.5% were women. Participants were followed up for up to nine years. Data collected included sociodemographic variables (age, sex, and BMI), clinical variables, and several health-related measures including mortality. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement was followed for this research [].
2.2. Laboratory Measurements
Blood samples were taken from participants at the second-year follow-up visit. The samples were processed and stored under standardized conditions at −80 °C to ensure the accuracy and reliability of subsequent laboratory analyses.
The primary biomarkers measured in this study were 25(OH)D and 24,25(OH)2D. These measurements were made using a previously published liquid chromatography–tandem mass spectrometry (LC–MS/MS) method []. This method is certificated for the measurement of 25(OH)D by the Centers for Disease Control and Prevention (CDC) for its precision and accuracy based on the Vitamin D Standardization and Certification Program (VDSCP).
The VMR was calculated to assess the functional status of vitamin D metabolism in the participants. The VMR was calculated using the formula: VMR = .
2.3. Assessment of Outcomes
The primary outcome of this study was PTH levels, which were measured to assess the relationship between VMR and PTH, as elevated PTH is a known indicator of functional vitamin D deficiency. Blood samples for PTH measurement were taken at the same time as vitamin D status and analyzed using a Liaison 1-84 PTH immunoassay on Liaison XL analyzer (Diasorin, Italy). The association between PTH levels and different vitamin D metabolites, including VMR, 25(OH)D, and 24,25(OH)2D, was assessed.
Secondary outcomes included assessment of bone turnover markers (BTMs) and mortality. C-telopeptide collagen type 1 (CTXS) and propeptide N-terminal procollagen type 1 (PINP) were selected to evaluate bone turnover and both were measured on an Isys analyzer (IDS, England) according to the manufacturer’s instruction. Mortality was tracked over the follow-up period, with data collected through periodic interviews, medical records, and national death registries []. In case of confirmed death but unspecified date of death, death was arbitrarily set at the 1st of January 2024.
2.4. Statistical Analysis
Descriptive statistics were used to summarize baseline characteristics, including the distribution of vitamin D metabolites (25(OH)D, 24,25(OH)2D, and VMR) and outcomes (PTH, BTMs levels, and mortality). The prevalence of vitamin D deficiency was assessed using previously proposed cut-offs: 25(OH)D < 20 ng/mL [], 24,25(OH)2D < 1.2 ng/mL, and VMR < 4% (arbitrarily derived from the DESIRE cohort) []. Statistical differences between normal profile and low profile groups were assessed with the independent Mann–Whitney test for continuous variables and with the Chi-squared test for dichotomous variables.
Spearman’s rank correlation coefficients were used to assess the associations between the three vitamin D markers (25(OH)D, 24,25(OH)2D, and VMR) and PTH levels. Participants were divided into quartiles according to their levels of 25(OH)D, 24,25(OH)2D, and VMR. The Kruskal–Wallis test was used to assess differences in PTH levels between these quartiles.
Cox proportional hazards regression models were used to assess the association between vitamin D markers and nine-year mortality. Categorical forms of vitamin D markers were analyzed. Kaplan–Meier survival curves and log-rank tests were used to compare mortality rates between groups with low and adequate vitamin D levels based on established cut-offs.
All statistical analyses were performed on Medcalc° version 22.021 (Medcalc software Ltd., Ostend, Belgium). Statistical significance was set at p < 0.05 for all tests.
3. Results
A total of 204 patients with complete assessment of 25(OH)D, 24,25(OH)2D, and PTH levels as well as mortality data were available at the second follow-up. A total of 49.5% of patients were male with a median age of 74.0 years old (IQR: 8.9) and a median body mass index (BMI) of 27.0 (IQR: 5.3). A total of 68.1% of the subjects were self-reporting oral vitamin D supplementation. During the 9-year follow-up period, 43 subjects died (21.1%) in relation to all-cause mortality. The median concentration was 30.9 ng/mL (IQR: 12.4) for 25(OH)D and 2.49 ng/mL (IQR: 1.85) for 24,25(OH)2D. A total of 5 subjects, one of whom died, had 24,25(OH)2D below our limit of quantification, disabling VMR calculation for these patients, and thus establishing the number of patients at 199 subjects with available VMR, among which 42 died.
According to cut-offs for vitamin D deficiency proposed by Hermann et al. (cut-offs that were arbitrarily fixed from the DESIRE cohort) [], 35 individuals (17.2%) had 25(OH)D levels below 20 ng/mL, 40 individuals (19.6%) had 24,25(OH)2D levels below 1.2 ng/mL, and 14 individuals (7.0%) had VMR values below 4%. Of note, 5 individuals, in which 24.25(OH)2D was below the limit of quantification, were excluded from the VMR analysis since we were unable to calculate the ratio. Men were more likely to have low 25(OH)D or low 24,25(OH)2D (Table 1). No difference in terms of PTH or BTMs (PINP and CTXS) was observed between the normal or low profile groups, no matter which vitamin D parameter was assessed (Table 1).

Table 1.
Description of the cohort according to various cut-offs for functional vitamin D deficiency. Results are expressed as median (IQR). The statistical difference between the normal profile and low profile participants were assessed with the independent Mann–Whitney test for continuous variables and with the Chi-squared test for dichotomous variables. Statistically different results are highlighted in bold.
VMR showed the strongest correlation with PTH levels (rho = −0.292; p < 0.0001). 24,25(OH)2D also had a significant correlation with PTH levels (rho = −0.254; p = 0.0003), while 25(OH)D had a weaker but still significant correlation with PTH levels (rho = −0.139; p = 0.0471) (Figure 1). However, CTXS and PINP BTMs, were not associated with VMR, 24,25(OH)2D, or 25(OH)D. The Kruskal–Wallis test revealed significant increases in PTH levels across quartiles for VMR (p < 0.0001) and 24,25(OH)2D (p = 0.002). However, no significant changes in PTH levels were observed across quartiles for 25(OH)D (Figure 2).
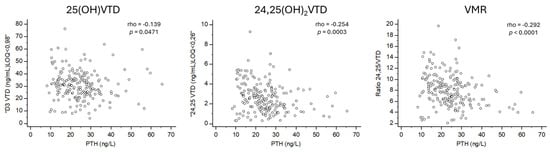
Figure 1.
Correlation between PTH and vitamin D biomarkers.
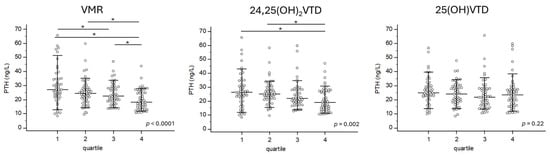
Figure 2.
PTH is differentially expressed according to the VMR quartile but not according to the 25(OH)VTD quartile. The Kruskal–Wallis test was used to assess differences in PTH levels between these quartiles. Each subject is represented by a dot. * represent groups that are statistically different.
Survival analysis was performed to assess the predictive value of the vitamin D markers for nine-year mortality. Patients with a low VMR profile had the highest rate of all-cause mortality (42.9%) (Table 1). According to the Cox proportional hazards regression with age, sex, and BMI as other variables of the model, the hazard ratio was 3.74 for VMR (1.50–9.31, 95% confidence interval (CI)) and 2.41 (1.19–4.89, 95% CI) (Table 2). In addition, Kaplan–Meier survival curves indicated significant differences in mortality rates for both low VMR and low 25(OH)D profiles (Figure 3).

Table 2.
Cox proportional-hazards regression. Statistically different results are highlighted in bold in tables.
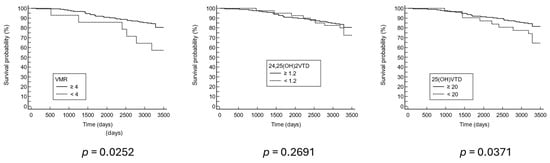
Figure 3.
Nine-year Kaplan–Meier survival curve.
4. Discussion
This study suggests that the VMR may be a more effective marker of functional vitamin D deficiency than traditional markers such as 25(OH)D and 24,25(OH)2D. Indeed, VMR showed the strongest correlation with PTH levels, with significant increases in PTH across VMR quartiles. Furthermore, the low VMR profile participants had the highest rate of mortality, and more additional associations were discovered between 9-year all-cause mortality and low VMR or low 25(OH)D profiles. These findings suggest that VMR has potential as a more accurate biomarker for assessing vitamin D deficiency and its associated health risks.
Our results are consistent with the conclusions of previous studies that support a functional assessment of vitamin D status using the VMR and 24,25(OH)2D, suggesting that this approach provides superior diagnostic information compared to serum 25(OH)D levels alone. In the study by Herrmann et al. (2023), which included the DESIRE and LURIC cohorts, the authors evaluated the utility of a low vitamin D metabolite profile defined by 24,25(OH)2D < 1.2 ng/mL and VMR < 4% []. They found that this profile was associated with significantly higher parathyroid hormone (PTH) levels, accelerated bone turnover, and increased all-cause mortality, independent of serum 25(OH)D concentrations. These results support our findings that VMR is more strongly correlated with PTH levels than 25(OH)D and is a better predictor of long-term health outcomes, including mortality. Similar to our study, Herrmann et al. showed that individuals with low VMR had higher PTH levels, suggesting a more functional vitamin D deficiency, despite having 25(OH)D levels that would not traditionally be classified as deficient. Of note, both Herrmann’s and our LC–MS/MS method have shown very close results when compared together [].
Additional strengths of VMR have been reported in other studies []. First, VMR remains stable despite significant fluctuations in vitamin D binding protein (VDBP) levels [,]. This stability underlines the potential of VMR as a reliable indicator of vitamin D status, unaffected by VDBP variability, which can confound vitamin D metabolites measurements. Furthermore, Ginsberg et al. found that lower VMR was significantly associated with a rapid decline in bone mineral density (BMD) and an increased fracture risk in older adults [,]. In contrast, 25(OH)D levels did not show a significant association with these outcomes. This supports the potential of VMR as a more sensitive marker of bone health, reflecting the metabolic balance of vitamin D more accurately than 25(OH)D alone.
The relationship between VMR and mortality, as demonstrated in both our study and Herrmann et al. [], highlights the broader implications of VMR as an indicator of health outcomes beyond bone health. Although the direct link between VMR and PTH does not extend to mortality in our data, we propose that VMR captures critical aspects of vitamin D metabolism that are not solely reflected in bone-related biomarkers. Mortality is a multifactorial outcome, and the fact that low VMR was associated with increased all-cause mortality supports its potential utility as a global indicator of vitamin D deficiency []. The traditional reliance on 25(OH)D levels as the sole indicator of vitamin D status may be inadequate, as it does not take into account functional deficiencies that can affect bone health and all-cause mortality. The VMR may provide a more reliable diagnostic tool by more accurately reflecting vitamin D metabolism and its physiological effects [,,]. Implementation of VMR in clinical practice could improve the identification of individuals at risk of vitamin D deficiency-related complications, allowing for more targeted and effective interventions. This approach could potentially lead to better patient outcomes through personalized treatment plans based on a comprehensive understanding of an individual’s vitamin D status []. However, further research is needed to establish more direct links between VMR and clinical outcomes, including bone fractures and disease-specific mortality. Interventional studies will be particularly important to validate whether targeting VMR can improve health outcomes more effectively than traditional vitamin D measures such as 25(OH)D. Additionally, the cost-effectiveness of this approach needs to be further investigated.
This study has several strengths, including the use of a well-defined cohort of community-dwelling older adults, the application of rigorous measurement techniques using liquid chromatography–tandem mass spectrometry (LC–MS/MS), and a long follow-up period of up to nine years. However, there are limitations. The study population, although well characterized, is limited to a relatively small number of older adults and to a specific European region, which may affect the generalizability of the findings to other populations. Potential confounders, such as variations in diet or vitamin D supplementation, physical activity, and sunlight exposure, were not considered. Furthermore, categorization of the population regarding vitamin D is based on arbitrary cut-offs previously proposed based on a single cohort. These cut-offs might bias a part of the results since the low VMR group is very reduced. The observational nature of the study precludes the establishment of causality between VMR and health outcomes, and additional interventional studies are required to properly validate cut-offs for functional vitamin D deficiency.
Based on the findings of this study and others, future research should focus on validating the clinical utility of VMR in larger and more diverse populations and especially in a population without vitamin D supplementation. In particular, randomized controlled trials are needed to assess whether interventions based on VMR measurements can lead to better health outcomes than traditional approaches using 25(OH)D levels alone, as these kinds of approaches have led so far to conflicting data [,,,]. In particular, criteria for functional vitamin D deficiency failed to identify subjects under vitamin D supplementation in a randomized controlled trial of hypertensive patients []. In addition, research into the biological mechanisms underlying the relationship between VMR, vitamin D metabolism, and health outcomes could provide further insights into the role of vitamin D in different physiological processes [].
5. Conclusions
In conclusion, the VMR appears to be a more accurate and reliable marker for assessing functional vitamin D deficiency than traditional markers such as 25(OH)D and 24,25(OH)2D. The results of this study, supported by previous research, suggest that VMR better reflects vitamin D status and its associated health risks, including bone health and mortality. The implementation of VMR in clinical practice could improve the diagnosis and management of vitamin D deficiency, leading to improved patient outcomes. Further research is needed to validate these findings and explore the wider clinical applications of VMR.
Author Contributions
Conceptualization, A.L., O.B. and E.C.; methodology, A.L., C.D., O.B. and E.C.; formal analysis, A.-S.G. and S.K.; investigation, A.L.; resources, S.P., C.L.G. and E.C.; data curation, C.D. and C.B.; writing—original draft preparation, A.L., O.B. and E.C.; writing—review and editing, A.L., C.D., C.B., S.P., M.S.A., N.M.A.-D., C.L.G., J.-Y.R., O.B. and E.C.; supervision, J.-Y.R., O.B. and E.C.; funding acquisition, J.-Y.R., M.S.A. and N.M.A.-D. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported the Distinguished Scientists Fellowship Programme of the King Saud University, Riyadh, Kingdom of Saudi Arabia.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by Ethical committee of the CHU de Liège (2012/277l, 8 January 2013).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The raw data can be obtained on request from the corresponding author.
Conflicts of Interest
OB has received consulting or lecture fees from Amgen, Aptissen, Biophytis, IBSA, Mylan, Novartis, Nutricia, Orifarm, Sanofi, UCB and Viatris, outside the submitted work. All the other authors declare no conflict of interest.
References
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Tmava Berisha, A.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D Deficiency 2.0: An Update on the Current Status Worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef] [PubMed]
- Pittas, A.G.; Dawson-Hughes, B.; Sheehan, P.; Ware, J.H.; Knowler, W.C.; Aroda, V.R.; Brodsky, I.; Ceglia, L.; Chadha, C.; Chatterjee, R.; et al. Vitamin D Supplementation and Prevention of Type 2 Diabetes. N. Engl. J. Med. 2019, 381, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.E.; Cook, N.R.; Lee, I.-M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Gordon, D.; Copeland, T.; D’Agostino, D.; et al. Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease. N. Engl. J. Med. 2019, 380, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Cui, A.; Zhang, T.; Xiao, P.; Fan, Z.; Wang, H.; Zhuang, Y. Global and Regional Prevalence of Vitamin D Deficiency in Population-Based Studies from 2000 to 2022: A Pooled Analysis of 7.9 Million Participants. Front. Nutr. 2023, 10, 1070808. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, S.; Barbieri, V.; Di Pierro, A.M.; Rossi, F.; Widmann, T.; Lucchiari, M.; Pusceddu, I.; Pilz, S.; Obermayer-Pietsch, B.; Herrmann, M. LC–MS/MS Based 25(OH)D Status in a Large Southern European Outpatient Cohort: Gender- and Age-Specific Differences. Eur. J. Nutr. 2019, 58, 2511–2520. [Google Scholar] [CrossRef]
- Cashman, K.D. Vitamin D Deficiency: Defining, Prevalence, Causes, and Strategies of Addressing. Calcif. Tissue Int. 2020, 106, 14–29. [Google Scholar] [CrossRef]
- Cashman, K.D.; Dowling, K.G.; Škrabáková, Z.; Gonzalez-Gross, M.; Valtueña, J.; De Henauw, S.; Moreno, L.; Damsgaard, C.T.; Michaelsen, K.F.; Mølgaard, C.; et al. Vitamin D Deficiency in Europe: Pandemic? Am. J. Clin. Nutr. 2016, 103, 1033–1044. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Demay, M.B.; Pittas, A.G.; Bikle, D.D.; Diab, D.L.; Kiely, M.E.; Lazaretti-Castro, M.; Lips, P.; Mitchell, D.M.; Murad, M.H.; Powers, S.; et al. Vitamin D for the Prevention of Disease: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2024, 109, 1907–1947. [Google Scholar] [CrossRef]
- Giustina, A.; Bilezikian, J.P.; Adler, R.A.; Banfi, G.; Bikle, D.D.; Binkley, N.C.; Bollerslev, J.; Bouillon, R.; Brandi, M.L.; Casanueva, F.F.; et al. Consensus Statement on Vitamin D Status Assessment and Supplementation: Whys, Whens, and Hows. Endocr. Rev. 2024, 45, bnae009. [Google Scholar] [CrossRef]
- Yao, P.; Bennett, D.; Mafham, M.; Lin, X.; Chen, Z.; Armitage, J.; Clarke, R. Vitamin D and Calcium for the Prevention of Fracture: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2019, 2, e1917789. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, D.P. Effect of Four Monthly Oral Vitamin D3 (Cholecalciferol) Supplementation on Fractures and Mortality in Men and Women Living in the Community: Randomised Double Blind Controlled Trial. BMJ 2003, 326, 469. [Google Scholar] [CrossRef] [PubMed]
- Priemel, M.; Von Domarus, C.; Klatte, T.O.; Kessler, S.; Schlie, J.; Meier, S.; Proksch, N.; Pastor, F.; Netter, C.; Streichert, T.; et al. Bone Mineralization Defects and Vitamin D Deficiency: Histomorphometric Analysis of Iliac Crest Bone Biopsies and Circulating 25-Hydroxyvitamin D in 675 Patients. J. Bone Miner. Res. 2010, 25, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, O.M.; Farwell, W.R.; Kermah, D.; Taylor, E.N. Racial Differences in the Relationship between Vitamin D, Bone Mineral Density, and Parathyroid Hormone in the National Health and Nutrition Examination Survey. Osteoporos. Int. 2011, 22, 1745–1753. [Google Scholar] [CrossRef] [PubMed]
- Powe, C.E.; Evans, M.K.; Wenger, J.; Zonderman, A.B.; Berg, A.H.; Nalls, M.; Tamez, H.; Zhang, D.; Bhan, I.; Karumanchi, S.A.; et al. Vitamin D–Binding Protein and Vitamin D Status of Black Americans and White Americans. N. Engl. J. Med. 2013, 369, 1991–2000. [Google Scholar] [CrossRef]
- Máčová, L.; Bičíková, M. Vitamin D: Current Challenges between the Laboratory and Clinical Practice. Nutrients 2021, 13, 1758. [Google Scholar] [CrossRef]
- Alonso, N.; Zelzer, S.; Eibinger, G.; Herrmann, M. Vitamin D Metabolites: Analytical Challenges and Clinical Relevance. Calcif. Tissue Int. 2022, 112, 158–177. [Google Scholar] [CrossRef]
- Cavalier, E.; Huyghebaert, L.; Rousselle, O.; Bekaert, A.-C.; Kovacs, S.; Vranken, L.; Peeters, S.; Goff, C.L.; Ladang, A. Simultaneous Measurement of 25(OH)-Vitamin D and 24,25(OH)2-Vitamin D to Define Cut-Offs for CYP24A1 Mutation and Vitamin D Deficiency in a Population of 1200 Young Subjects. Clin. Chem. Lab. Med. CCLM 2020, 58, 197–201. [Google Scholar] [CrossRef]
- Herrmann, M.; Zelzer, S.; Cavalier, E.; Kleber, M.; Drexler-Helmberg, C.; Schlenke, P.; Curcic, P.; Keppel, M.H.; Enko, D.; Scharnagl, H.; et al. Functional Assessment of Vitamin D Status by a Novel Metabolic Approach: The Low Vitamin D Profile Concept. Clin. Chem. 2023, 69, 1307–1316. [Google Scholar] [CrossRef]
- Berg, A.H.; Powe, C.E.; Evans, M.K.; Wenger, J.; Ortiz, G.; Zonderman, A.B.; Suntharalingam, P.; Lucchesi, K.; Powe, N.R.; Karumanchi, S.A.; et al. 24,25-Dihydroxyvitamin D3 and Vitamin D Status of Community-Dwelling Black and White Americans. Clin. Chem. 2015, 61, 877–884. [Google Scholar] [CrossRef]
- Beaudart, C.; Reginster, J.Y.; Petermans, J.; Gillain, S.; Quabron, A.; Locquet, M.; Slomian, J.; Buckinx, F.; Bruyère, O. Quality of Life and Physical Components Linked to Sarcopenia: The SarcoPhAge Study. Exp. Gerontol. 2015, 69, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Fabregat-Cabello, N.; Farre-Segura, J.; Huyghebaert, L.; Peeters, S.; Le Goff, C.; Souberbielle, J.-C.; Cavalier, É. A Fast and Simple Method for Simultaneous Measurements of 25(OH)D, 24,25(OH)2D and the Vitamin D Metabolite Ratio (VMR) in Serum Samples by LC-MS/MS. Clin. Chim. Acta Int. J. Clin. Chem. 2017, 473, 116–123. [Google Scholar] [CrossRef]
- Sanchez-Rodriguez, D.; Demonceau, C.; Bruyère, O.; Cavalier, E.; Reginster, J.-Y.; Beaudart, C. Intrinsic Capacity and Risk of Death: Focus on the Impact of Using Different Diagnostic Criteria for the Nutritional Domain. Maturitas 2023, 176, 107817. [Google Scholar] [CrossRef] [PubMed]
- Zelzer, S.; Le Goff, C.; Peeters, S.; Calaprice, C.; Meinitzer, A.; Enko, D.; Goessler, W.; Herrmann, M.; Cavalier, E. Comparison of Two LC-MS/MS Methods for the Quantification of 24,25-Dihydroxyvitamin D3 in Patients and External Quality Assurance Samples. Clin. Chem. Lab. Med. 2022, 60, 74–81. [Google Scholar] [CrossRef]
- Dugar, A.; Hoofnagle, A.N.; Sanchez, A.P.; Ward, D.M.; Corey-Bloom, J.; Cheng, J.H.; Ix, J.H.; Ginsberg, C. The Vitamin D Metabolite Ratio (VMR) Is a Biomarker of Vitamin D Status That Is Not Affected by Acute Changes in Vitamin D Binding Protein. Clin. Chem. 2023, 69, 718–723. [Google Scholar] [CrossRef]
- Ginsberg, C.; Hoofnagle, A.N.; Katz, R.; Becker, J.O.; Kritchevsky, S.B.; Shlipak, M.G.; Sarnak, M.J.; Ix, J.H. The Vitamin D Metabolite Ratio Is Independent of Vitamin D Binding Protein Concentration. Clin. Chem. 2021, 67, 385–393. [Google Scholar] [CrossRef]
- Ginsberg, C.; Katz, R.; De Boer, I.H.; Kestenbaum, B.R.; Chonchol, M.; Shlipak, M.G.; Sarnak, M.J.; Hoofnagle, A.N.; Rifkin, D.E.; Garimella, P.S.; et al. The 24,25 to 25-Hydroxyvitamin D Ratio and Fracture Risk in Older Adults: The Cardiovascular Health Study. Bone 2018, 107, 124–130. [Google Scholar] [CrossRef]
- Ginsberg, C.; Hoofnagle, A.N.; Katz, R.; Hughes-Austin, J.; Miller, L.M.; Becker, J.O.; Kritchevsky, S.B.; Shlipak, M.G.; Sarnak, M.J.; Ix, J.H. The Vitamin D Metabolite Ratio Is Associated With Changes in Bone Density and Fracture Risk in Older Adults. J. Bone Miner. Res. 2020, 36, 2343–2350. [Google Scholar] [CrossRef]
- Bansal, N.; Katz, R.; Appel, L.; Denburg, M.; Feldman, H.; Go, A.S.; He, J.; Hoofnagle, A.; Isakova, T.; Kestenbaum, B.; et al. Vitamin D Metabolic Ratio and Risks of Death and CKD Progression. Kidney Int. Rep. 2019, 4, 1598–1607. [Google Scholar] [CrossRef]
- Toribio, M.J.; Priego-Capote, F.; Pérez-Gómez, B.; Fernández De Larrea-Baz, N.; Ruiz-Moreno, E.; Castelló, A.; Lucas, P.; Sierra, M.Á.; Pino, M.N.; Martínez-Cortés, M.; et al. Factors Associated with Serum Vitamin D Metabolites and Vitamin D Metabolite Ratios in Premenopausal Women. Nutrients 2021, 13, 3747. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, L.H.M.; Butler, A.E.; Dargham, S.R.; Latif, A.; Chidiac, O.M.; Atkin, S.L.; Abi Khalil, C. Vitamin D3 Metabolite Ratio as an Indicator of Vitamin D Status and Its Association with Diabetes Complications. BMC Endocr. Disord. 2020, 20, 161. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Chung, H.J.; Jung, S.; Jang, H.N.; Chang, S.-H.; Kim, H.-J.; Cho, M.-C. 24,25-Dihydroxy Vitamin D and Vitamin D Metabolite Ratio as Biomarkers of Vitamin D in Chronic Kidney Disease. Nutrients 2023, 15, 578. [Google Scholar] [CrossRef] [PubMed]
- Wagner, D.; Hanwell, H.E.; Schnabl, K.; Yazdanpanah, M.; Kimball, S.; Fu, L.; Sidhom, G.; Rousseau, D.; Cole, D.E.C.; Vieth, R. The Ratio of Serum 24,25-Dihydroxyvitamin D3 to 25-Hydroxyvitamin D3 Is Predictive of 25-Hydroxyvitamin D3 Response to Vitamin D3 Supplementation. J. Steroid Biochem. Mol. Biol. 2011, 126, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Francic, V.; Ursem, S.R.; Dirks, N.F.; Keppel, M.H.; Theiler-Schwetz, V.; Trummer, C.; Pandis, M.; Borzan, V.; Grübler, M.R.; Verheyen, N.D.; et al. The Effect of Vitamin D Supplementation on Its Metabolism and the Vitamin D Metabolite Ratio. Nutrients 2019, 11, 2539. [Google Scholar] [CrossRef]
- Lehmann, U.; Riedel, A.; Hirche, F.; Brandsch, C.; Girndt, M.; Ulrich, C.; Seibert, E.; Henning, C.; Glomb, M.A.; Dierkes, J.; et al. Vitamin D3 Supplementation: Response and Predictors of Vitamin D3 Metabolites—A Randomized Controlled Trial. Clin. Nutr. 2016, 35, 351–358. [Google Scholar] [CrossRef]
- Aloia, J.; Fazzari, M.; Shieh, A.; Dhaliwal, R.; Mikhail, M.; Hoofnagle, A.N.; Ragolia, L. The Vitamin D Metabolite Ratio (VMR) as a Predictor of Functional Biomarkers of Bone Health. Clin. Endocrinol. 2017, 86, 674–679. [Google Scholar] [CrossRef]
- Zelzer, S.; Meinitzer, A.; Enko, D.; Keppel, M.H.; Herrmann, M.; Theiler-Schwetz, V.; Trummer, C.; Schmitt, L.; Tomaschitz, A.; Sadoghi, P.; et al. Classification of Vitamin D Status Based on Vitamin D Metabolism: A Randomized Controlled Trial in Hypertensive Patients. Nutrients 2024, 16, 839. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).