Abstract
Recently, there has been significant exploration into the utilization of food by-products as natural reservoirs of bioactive substances, particularly in the creation of functional foods naturally enriched with antioxidants. Citrus peels represent a viable option for formulating enhanced olive oils that contribute to a healthier diet, due to their bioactive compound content. This study aimed to (i) ascertain the compositional characteristics of Citrus reticulata olive oil (CrOO) and (ii) assess its nutraceutical properties in rats with metabolic disorder induced by 3 weeks of feeding with a high-fat diet (HFD). The results showed a peculiar phytochemical composition, thanks to the contribution of citrus peels, which are excellent bio-products. In addition, it demonstrated HFD-induced weight gain (18 ± 2% for HFD vs. 13 ± 0.9% for CrOO) and showed protective effects on fasting blood glucose levels (90.2 ± 3.8 mg/dL for HFD vs. 72.3 ± 2.6 for CrOO). Furthermore, a reduction in cardiovascular risk (total cholesterol/HDL cholesterol = 5.0 ± 0.3 for HFD vs. 3.8 ± 0.3 for CrOO) and an improvement in myocardial tissue function were observed, as well as a significant reduction in inflammatory mediators such as iNOS, COX-2, and mPGES-1 in aortic vessel tissues, thus preserving endothelial function at the vascular level.
1. Introduction
Citrus by-products are valuable sources of essential bioactive compounds with potential applications in animal feed, processed foods, and healthcare [1].
Citrus fruits are a rich source of bioactive secondary metabolites, which are primarily produced by plants as a defense strategy against insects and microbial pathogens [2,3,4]. Their phytochemical content imparts noteworthy health protection and disease prevention properties. Citrus fruits are rich in flavonoids, carotenoids, and limonoids, with approximately 95% of flavonoids being flavanones, such as naringenin, hesperetin, and eriodictyol [5,6,7].
Epidemiological, clinical, and preclinical evidence highlights the nutraceutical benefits of Citrus fruits on the cardiovascular system, demonstrating vasorelaxing and cardioprotective effects [8]. Naringenin and hesperetin have also shown SIRT1-mediated anti-aging properties in nematode and yeast models [6,9]. Studies suggest that citrus fruits contribute to cardiovascular health by improving the cardiometabolic profile, reducing plasma levels of total and low-density lipoprotein (LDL) cholesterol and triglycerides, and limiting the increase in weight caused by the high-fat diet [10].
Moreover, citrus fruits are rich in carotenoids, offering at least 110 different carotenes and xanthophylls. These pigments play a crucial role in photosynthesis and help prevent photooxidation. Carotenoids provide numerous health benefits, reducing the risk of diseases such as tumors and heart conditions and ophthalmological issues [11,12].
Limonoids represent another relevant class of biologically active molecules in Citrus, and exhibit anticancer effects and possess pharmacological properties like antimicrobial, antioxidant, antidiabetic, and insecticidal effects [13,14]. Given the growing interest in bioactive compounds in foods, there is a considerable focus on formulating combined nutraceutical compounds in healthy functional foods to prevent and contrast chronic diseases [15].
Fortified food (FF) emerges as an effective tool to enhance nutritional intake, as it is widely accessible, socially acceptable, quickly introduced, and generally perceived as safe and cost-effective [16,17]. In this context, innovatively produced fortified olive oils were obtained using an innovative method, incorporating cryomacerated citrus peels or leaves (C. aurantium or C. lemon) during the olive oil extraction process [10]. This approach resulted in oils enriched with the nutraceutical properties typical of citrus fruits, enhancing the organoleptic profile in terms of smell complexity and hedonic response [10]. Based on the previous evaluation regarding the addition of citrus by-products during olive oil extraction, an innovative product was developed, using Citrus reticulata peels, according to the previously described system [10]. The present research aimed to determine the chemical profile of Citrus reticulata olive oil (CrOO) and evaluate its nutraceutical qualities in rats with metabolic disorders associated with a high-fat diet.
2. Materials and Methods
2.1. Plant Material
Citrus reticulata fruits were harvested at full maturity during the 2020/2021 crop season, following the protocol described by Ascrizzi et al. [18]. Olives (Moraiolo cultivar) were collected during the 2021/2022 crop season and supplied by an organic farm in Tuscany (Azienda Agricola Val Di Lama, Pontedera (PI), Italy), and characterized according to the method outlined by Macaluso et al. [19].
2.2. Citrus Peel Cryomaceration and Citrus Olive Oil Extraction
Citrus fruits were prepared and cryomacerated following the methods described in previous studies [10]. After cryomaceration, the citrus peels were directly added to the olives at a ratio of 25% by weight before milling to produce Citrus-enriched Olive Oil (CrOO). The extraction of olive oil was carried out using a micro oil mill (Spremioliva mod. C30, Mori-TEM srl, Barberino Tavarnelle (FI), Italy) according to the extraction protocol detailed in a previous publication [19].
2.3. Citrus Olive Oil Chemical Analyses
2.3.1. Chemical Quality Standards
Free acidity, peroxide value, and spectrophotometric indices were determined following the official analytical methods detailed in the European legal standards [20].
2.3.2. Total Phenols
Total phenols (TP) were extracted using a liquid–liquid extraction method with a methanol solution (80:20 v/v), following the procedure outlined in a previous study [19].
2.3.3. Free-Radical Scavenging Capacity (FRSC)
The free-radical scavenging capacity (FRSC) was assessed using two methods: the DPPH (2,2-diphenyl-1-picrylhydrazyl) assay, referred to as FRSCDPPH [10], and the ABTS (2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)) assay (FRSCABTS). The radical solutions were prepared according to the procedure previously described [10].
2.3.4. Intensity of Bitterness (IB)
The intensity of bitterness was assessed using the method of Gutiérrez Rosales et al. [21], without modifications.
2.3.5. Carotenoids and Chlorophylls
Total carotenoids and chlorophylls were measured according to the procedure outlined by Minguez Mosquera, without any modifications [22].
2.3.6. Analysis of Hydroxytyrosol and Tyrosol
The analysis of hydroxytyrosol and tyrosol was conducted using reverse-phase high-performance liquid chromatography (RP-HPLC) under the chromatographic conditions previously reported [23].
2.3.7. Extraction and Detection of Tocopherols (Vitamin E)
As reported [10], tocopherols were extracted in the dark. Specifically, α-, β-, γ-, and δ-tocopherol isoforms were analyzed by isocratic reverse-phase high-performance liquid chromatography (RP-HPLC) using a Shimadzu LC-20AD apparatus (Shimadzu Europa GmbH, Duisburg, Germany) with an electrochemical detector (Metrohm model 791, Varese, Italy) and a glassy carbon electrode.
2.3.8. Headspace-Solid Phase Microextraction Analysis
The volatile emissions of both the control and the C. reticulata olive oil were analyzed by headspace-solid phase microextraction (HS-SPME) following the procedure previously described [18].
2.3.9. Gas Chromatography–Mass Spectrometry Analyses
The gas chromatography–electron ionization mass spectrometry (GC-EIMS) analysis was carried out using an Agilent 7890B gas chromatograph (Agilent Technologies Inc., Santa Clara, CA, USA), equipped with an Agilent HP-5MS capillary column (30 m × 0.25 mm, 0.25 µm film thickness) and an Agilent 5977B single quadrupole mass detector. The analytical conditions included an oven temperature program that increased from 60 °C to 240 °C at a rate of 3 °C/min, with the injector and transfer line temperatures set at 250 °C and 240 °C, respectively. Helium was used as the carrier gas, at a flow rate of 1 mL/min. Mass spectra were acquired in full scan mode over a range of 30–300 m/z with a scan time of 1.0 s. Peak identification was performed by comparing retention times with those of reference compounds and evaluating linear retention indices relative to a series of n-alkanes (C8–C27). Further identification was supported by matching spectra with commercial mass spectral libraries (NIST 14 and ADAMS 2007) and a custom library of pure substances, essential oil components, and relevant literature data [24].
2.4. In Vivo Evaluation of the Nutraceutical Properties of Enriched Extra Virgin Olive Oil
In vivo experimentation was performed according to European (EEC Directive 2010/63) and Italian (D.L. 4 March 2014 n.26) legislation. The project protocol was discussed at the Animal Ethics Committee of the University of Pisa and received approval from the Committee of the Italian Ministry of Health (number protocol 144/2019-PR, 18 February 2019). Moreover, ARRIVE guidelines were followed. The purpose of ARRIVE guidelines is to improve the quality and reproducibility of in vivo research [25]. Animals were caged with free access to food and water and with a 12 h dark/light cycle. The experiments were carried out on adult male Wistar rats (10 weeks old, ENVIGO, Nanakramguda Village, Hyderabad, India) weighing between 305 and 360 g; each treatment group was arranged in order to present the same average weight at the beginning of the treatment. Experiments were performed only on male rats in order to avoid hormonal interferences. According to the literature [26], animals were divided into 4 groups (n = 5/group) and treated for 21 days. The first group received a standard diet (STD, ENVIGO; for composition see Table 1; this pellet was polyphenol-free), the second group received a high-fat diet (HFD, U8220P version 0151, SAFE; composition reported in Table 1; ingredients: casein, lard, sucrose, maltodextrin, pregelatinized cornstarch, crude cellulose, soybean oil, potassium citrate, dicalcium phosphate, pre-mixture of minerals PM AIN 93M/G 3.5%, pre-mixture of vitamins PV AIN 93M/G 1%, calcium carbonate, L-cysteine, choline bitartrate); and the third and fourth groups received, respectively, HFD + extra virgin olive oil (CEOO) 2% p/p and HFD + CrOO 2% p/p. The amount of CEOO was calculated based on the regulatory authorities’ guidelines regarding the daily intake of polyphenols [27]. All 4 groups ate the same amount and the same calories, and water intake, food intake, and body weight were evaluated three times a week for each animal, contemporaneously with the administration of supplementations. At the end of the treatment, after 21 days, animals were starved overnight and at the end of the starvation period they were anesthetized with isoflurane and a blood glucose test was performed using blood from the caudal vein; subsequently, animals were sacrificed by cervical dislocation. EDTA tubes (BD Vacutainer, Franklin Lakes, NJ, USA) were used to collect intracardiac blood, and organs (heart, epididymal white adipose tissue, and intrascapular brown adipose tissue and liver) were recovered, weighed, and stored for further analysis. The lipid panel (high-density lipoproteins (HDL) and low-density lipoproteins (LDL), cholesterol and triglycerides) was evaluated rapidly using intracardiac blood with a Cobas b101 instrument (Roche Diagnostics, Rotkreuz, Switzerland). The remaining blood was centrifugated at 3200 RPM for 10 min in order to obtain plasma that was stored for further investigations.

Table 1.
Macro- and micro-nutrient composition of standard diet (STD) and high-fat diet (HFD).
2.4.1. Functional Analysis of Cardiac Mitochondrial Membrane Potential
Harvested hearts were finely minced into (2–3 mm3) pieces in isolation buffer (composition: Sucrose 250 mM, Tris 5 mM, EGTA 1 mM; pH 7.4); the whole procedure was carried out in an ice bath. Heart pieces then received three cycles of homogenization of about 20 s each. To obtain functional mitochondria, the homogenates were then centrifuged at 1090× g for 3 min at 4 °C, the pellets were removed and discarded, and the supernatants were further centrifuged at 11,970× g for 10 min at 4 °C. The pellet was preserved and resuspended in ice-cold isolation buffer without EGTA and centrifuged again at the same conditions. The mitochondrial fraction was represented by a pellet that was immediately resuspended in 400 μL of buffer and transferred in a tube. The amount of protein in the mitochondrial fraction was assessed following the Bradford assay (Bio-Rad, Hercules, CA, USA) with a microplate reader (EnSpire, PerkinElmer, Waltham, MA, USA).
To assess the mitochondrial membrane potential (ΔΨm) of the isolated mitochondria, a potentiometric method was employed. Tetraphenylphosphonium (TPP+), a lipophilic cation, was detected using a TPP+-sensitive mini-electrode (WPI, TipTPP, Sarasota, FL, USA) in conjunction with a reference electrode (WPI, Sarasota, FL, USA) and software for data collection (Biopac Inc., Goleta, CA, USA). Mitochondria (1 mg protein/mL) were rapidly added to an incubation buffer with the following composition: KCl 120 mM, K2HPO4 5 mM, Hepes 10 mM, succinic acid 10 mM, MgCl2 2 mM, TPP+Cl− 10 μM, and pH 7.4. The mixture was maintained in suspension with continuous magnetic stirring. The membrane potential value was calculated according to the following experimental equation, derived from the Nernst Equation (1), and following the protocol previously reported by Flori et al. (see reference [10]):
The mitochondrial membrane potential was measured for all the animals in the different groups, and data were expressed as mean ± SEM.
2.4.2. Western Blot
Protein analysis was performed on tissue samples following established protocols [10], with all experiments repeated at least three times. Densitometric analysis of the immunoblots was performed using NIH Image J 1.48v software. The data, presented as arbitrary density units (ADU) ± standard deviation (SD), were normalized against β-actin, which served as the loading control [28].
2.4.3. Immunohistochemistry
Immunohistochemical analysis was performed using seven μm thick cryostat sections obtained from tissue samples. The tissue sections were initially fixed in 4% paraformaldehyde (PFA) for 20 min, followed by treatment with 3% H2O2 for 10 min. Afterwards, they were rinsed three times in PBS for 5 min each and blocked for non-specific binding using 5% goat serum. The sections were then incubated at 4 °C for 18 h with a rabbit monoclonal anti-CD68 antibody and diluted 1:100 in PBS containing 0.05% goat serum. Following three additional 5 min washes in PBS with 0.05% goat serum, the sections were treated with biotinylated goat anti-rabbit IgG for 60 min. After washing again (three times for 5 min each in PBS with 0.05% goat serum), the sections were exposed to streptavidin-conjugated HRP for 10 min. To visualize the antibody binding, 3,3-diaminobenzidine tetrahydrochloride (DAB) was applied for 8 min, resulting in a brown color. The sections were then counterstained with hematoxylin and mounted using Eukitt® Quick-hardening mounting medium (Merck KGaA, Darmstadt, Germany) [10].
2.4.4. Hematoxylin and Eosin Staining
The sections (7 μm thick) were stained with hematoxylin for 3 min and then washed under running water for 5 min. Subsequently, eosin was added for 6 min, followed by a 5 min wash with running water. This was followed by dehydration in increasingly graded alcohols up to xylene. Finally, the sections were mounted using Eukitt® Quick-hardening mounting medium.
2.5. Statistical Analysis
The data are presented as the mean values from three independent experiments. Statistical differences between group means were analyzed using one-way ANOVA (CoStat, Version 6.451, CoHort Software, Pacific Grove, CA, USA). Post hoc comparisons were conducted using either Tukey’s or Bonferroni’s test, with a significance threshold of p < 0.05. UV and MS data analyses were conducted using Xcalibur 3.1 software.
3. Results and Discussion
3.1. Oil Chemical Characterization
The characterization of Citrus-enriched Olive Oil (CrOO), produced by adding mandarin peels to olives during extraction, was performed in comparison to the control Cold-Extracted Virgin Olive Oil (CEVOO). This evaluation considered not only the quality criteria specified in trade standards but also the phytochemical characteristics, including the bioactive compounds detected in the CrOO.
3.1.1. Legal Quality Parameters
The quality parameters outlined in the trade standard are designed to classify oils into various categories. As indicated in Table 2, despite the olives being relatively ripe, with a maturity index of 3.8 on a scale of 7.0, the olive oil samples met the legal quality criteria. Key parameters, such as free acidity, peroxide value, and spectrophotometric indices (K232, K270, and ΔK), were all below the maximum limits established by EU regulations for EVOO.

Table 2.
Olive fruit characterization. Data are expressed as mean ± confidence interval (n = 3) at p = 0.05.
Table 3 presents analytical results for the different types of olive oils, specifically CEVOO and CrOO. The significance level indicates the statistical significance of the differences observed.

Table 3.
Chemical characterization of CEVOO, CrOO, and EVOO legal limit regulations.
CEVOO and CrOO both exhibit significantly lower free acidity (0.44), meeting the specified limit. The differences among the three types were not statistically significant.
Both CEVOO and CrOO demonstrate low peroxide levels (8.90 and 8.80, respectively), well below the specified limit. The differences among the three types were not statistically significant. CEVOO (1.98) fell within the specified limit, while CrOO (2.10) was slightly higher, but both CEVOO (0.14) and CrOO (0.18) were still within the specified limit.
3.1.2. Phytochemical Composition
Chlorophylls and carotenoids play a crucial role in determining the color of olive oil, a quality factor that can significantly impact consumer preferences. Additionally, the levels of these pigments are associated with factors such as olive variety, processing methods, and storage conditions. Beyond their influence on visual appeal, both chlorophylls and carotenoids are noteworthy for their potential positive effects on human health. The obtained results indicated significant variations in the total carotenoid content between the two oils, as outlined in Table 4. This divergence in carotenoid levels suggested distinct compositions or concentrations of these pigments in the analyzed oils. The differences observed may be attributed to factors such as the specific olive varieties used, variations in processing techniques, or differences in storage conditions affecting the stability of these pigments. Understanding and monitoring the levels of chlorophylls and carotenoids not only contributes to the assessment of olive oil quality but also provide insights into the potential health benefits associated with these bioactive compounds. The observed variations underscore the need for further investigation into the specific factors influencing pigment content, as this knowledge can guide producers in optimizing processing methods and storage conditions to maintain both visual appeal and potential health-promoting properties in olive oil. The choice of qualitative markers can also help to standardize production by selecting an optimal harvest period in order to obtain an ideal quantity of bioactive compounds which will then characterize this type of product.

Table 4.
Phytochemical characterization of control (CEVOO) and Citrus reticulata olive oil (CrOO).
As expected, CrOO displayed the highest carotenoid content among the studied oils. Moving on to the broader category of oil pigments and total chlorophyll, significant differences were noted, with CEVOO exhibiting the highest concentration of chlorophylls. The health-promoting attributes of olive oil stem from its unique composition, encompassing a well-balanced ratio of unsaturated fatty acids alongside minor components such as phenolic compounds and tocopherols. These components not only contribute to the nutritional quality of the product but also influence its shelf life.
The tocopherol profile and phenolic content of the Citrus-enriched Olive Oil (CrOO) were assessed. Under the experimental conditions, the addition of cryomacerated citrus peels did not significantly alter the oil samples regarding total tocopherols or their isoforms (alpha, gamma, and delta). Similarly, the total phenol content showed no substantial differences among the samples, with values around 135 ppm of gallic acid. However, CrOO exhibited lower concentrations of hydroxytyrosol and tyrosol compared to Cold-Extracted Virgin Olive Oil (CEVOO). These observations were supported by the free-radical scavenging capacity (FRSC) results, which indicated that citrus olive oils had lower antioxidant activity than the control in both analytical methods. Additionally, the study evaluated compounds related to olive oil bitterness, using the Intensity of Bitterness (IB) as a parameter.
3.1.3. Volatile Composition
The complete composition of the volatile emission of CrOO, reported in Table 5, consisted of 11 compounds, representing 100% of the aroma profile, almost all belonging to the class of monoterpene hydrocarbons. Within this class, limonene was undoubtedly the chief component, accounting for 90.4% of the headspace composition; however, non-negligible amounts of myrcene, α-pinene, and sabinene were also revealed, even though they did not exceed the 5%. The occurrence of those major chemical compounds in the volatile emission of the CrOO was most likely determined by mandarin peels [29]. Conversely, the presence of non-terpene derivative, albeit found in very low percentages, was probably attributed to the olive oil. In particular, (E)-2-hexenal, accounting for 0.3% in the C. reticulata product, represented the major component detected in the volatile emissions of the extra virgin olive oil produced using the same olive fruits (CEVOO) and the same technological process employed to obtain the mandarin olive oil.

Table 5.
Volatile composition of CrOO and CEVOO.
3.2. Effects of CEVOO and CrOO Supplementation on the Cardiometabolic Profile of HFD-Fed Rats
According to the literature [10,30,31], the high-fat diet determined a significant increase in body weight gain (18 ± 2%) compared to the standard chow (10 ± 1%). Animals that received supplementation with CEVOO showed a reduced gain in body weight (12 ± 0.6%). Likewise, at the end of the protocol, animals fed with HFD + CrOO showed a significant containment of body weight gain (13 ± 0.9%), superimposable with that observed in animals fed with STD and with the CEVOO supplementation (Figure 1).
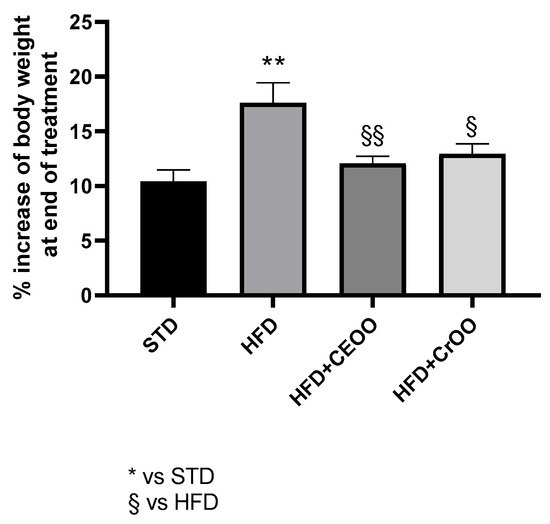
Figure 1.
Time course weight increase (%) during the treatment period (n = 5). * indicates a statistically significant difference between the HFD group and the STD group. § indicates statistically significant difference vs. HFD. Single symbol corresponds to p ≤ 0.05. Double symbol corresponds to p ≤ 0.01.
A high-fat dietary regimen is a widely used and recognized experimental model to induce significant metabolic alterations in rodents. In fact, together with the increase in body weight, this diet determines a metabolic dysfunction, characterized by altered glycemic and lipidic homeostasis, as well as cellular modifications prodromic to increased cardiovascular risk.
Indeed, animals fed with HFD for three weeks showed, at the end of the period, higher levels of blood glucose and cholesterol, triglycerides, and an increase in cardiovascular risk value (Total Cholesterol/HDL-cholesterol) compared with STD, as reported in Table 6.

Table 6.
Summary of data on lipid panel and glycemic profile at the end of treatment.
Of note, blood glucose and triglycerides levels were markedly reduced in animals that received CEVOO supplementation compared to the HFD group; conversely, the levels of total cholesterol, LDL, HDL, and non-HDL-cholesterol were not modified. However, the cardiovascular risk was markedly reduced. The CrOO-supplementation presented a superimposable profile to the group fed with HFD + CEOO; in particular, the blood glycemia and triglycerides were significantly reduced, and the cardiovascular risk was also markedly contained compared to the HFD group (Table 6).
Consistently, LDL levels presented a reducing trend, while HDL values were slightly increased (Table 6). The liver showed the presence of numerous fat deposits, reflecting a significant increase in liver weight. On the other hand, treatment with oils did not significantly alter this parameter. For other organs, such as the heart, HFD did not caused any significant change in weight.
HFD induced a significant increase in liver weight; nevertheless, CEVOO supplementation was not able to reduce this parameter. HFD also determined a significant increase in the weight of white adipose tissue and a significative reduction in brown adipose tissue weight, and the supplementation with CEVOO showed a trend, even if not significative, in improving these parameters, while CrOO ameliorated only the weight of the brown adipose tissue (Figure 2).
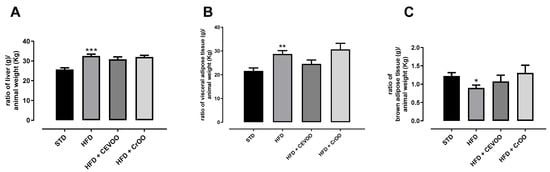
Figure 2.
(A) Ratio of liver weight (g) to animal weight (kg); (B) ratio of epididymal white adipose tissue weight (g) to animal weight (kg); and (C) ratio of intrascapular brown adipose tissue weight (g) to animal weight (kg) at the end of the treatments. * Indicates a statistically significant difference between the HFD group and the STD group. * Corresponds to p < 0.05, ** corresponds to p < 0.01 and *** p < 0.001 (n = 5).
Lastly, mitochondrial isolation was performed to assess mitochondrial membrane potential. This value is an important indicator of the health of heart mitochondria and their ability to produce ATP despite the stress induced with HFD. In a healthy animal, physiological values of ΔΨ were −191 ± 3.9 mV and HFD determined a depolarization, increasing ΔΨ at −178.5 ± 3.8 mV. The supplementation with CEVOO and CrOO showed a trend of restoring the mitochondrial membrane potential (−185 ± 4 and −186 ± 3 mV, respectively) (Figure 3).
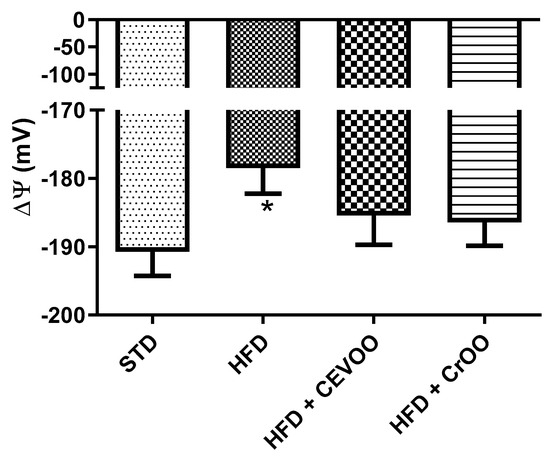
Figure 3.
Changes in mitochondrial membrane potential between the different treatment groups (n = 5). * Indicates a statistically significant difference between the HFD group and the STD group. * Corresponds to p < 0.05.
On the basis of these results and in accordance with the literature, the HFD regimen effectively induced profound metabolic alterations, determining an increase in total cholesterol, triglycerides, HDL and LDL, and blood glucose levels, typical of the metabolic disorders [10,19].
Animals that received CEVOO or CrOO supplementation presented a significant reduction in blood glucose levels, presenting values like the ones observed with the standard diet, demonstrating the hypoglycemic and protective properties of these supplementations. The whole lipid profile (total cholesterol, triglycerides, HDL and LDL-cholesterol) was significantly increased with the administration of the HFD, while supplementation with either CEVOO or CrOO significantly reduced the cardiovascular risk. Regarding triglyceride levels, the administration of the CEVOO was also able to restore levels similar to the ones observed with STD, while CrOO induced a reduction in terms of triglyceride levels. Regarding the evaluation of the health of the heart mitochondria, both CEVOO and CrOO partially rescued mitochondrial membrane potential, leading to the conclusion that this transforming strategy does not impair the well-known nutraceutical profile of the extra virgin olive oil.
3.3. Effects of CEVOO and CrOO Supplementation on Levels of Pro-Inflammatory Markers in Rat Aortic Vessels
Systemic and local inflammation play pivotal roles in the progression of cardiovascular disease [32], and the high expression of inflammatory mediators is a risk factor for arterial disease [33]. In rat aortic tissues, we assessed the expression of COX-2 and mPGES-1, inducible enzymes involved in the arachidonic acid cascade, leading to the production of PGE2, as well as inducible nitric oxide synthase (iNOS). Our results demonstrated that an HFD significantly increased the protein expression of COX-2 and mPGES-1 compared to an STD (Figure 4A,C,D). This upregulation highlights the pro-inflammatory effects of an HFD, which could contribute to the development and progression of cardiovascular disease. Interestingly, supplementation with CrOO was effective in reducing the inflammatory tone, as evidenced by the decreased expression of both COX-2 and mPGES-1 (Figure 4A,C,D). This suggests that CrOO has potent anti-inflammatory properties that can counteract the harmful effects of an HFD. Conversely, CEVOO alone did not reduce the HFD-induced overexpression of mPGES-1. Additionally, CrOO proved to be as effective as CEVOO alone in reducing the overexpression of iNOS induced by HFD (Figure 4B). Anti-inflammatory and antioxidant effects of virgin olive oil on vascular tissue were mainly associated with the levels of hydroxytyrosol and tyrosol [34,35,36]. Although CrOO showed lower concentrations of hydroxytyrosol and tyrosol than CEVOO, both olive oils were shown to reduce vascular inflammation, and CrOO did so with a larger effect, suggesting that its efficacy could be attributed to the vitamin content of carotenoids [37] and tocopherols [38,39], widely reported as potential antioxidants and anti-inflammatory agents.
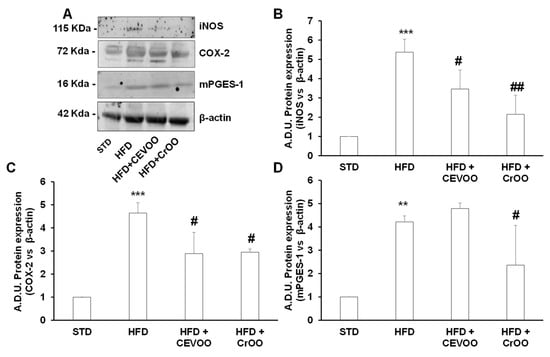
Figure 4.
Activity of CEVOO and CrOO supplementation on inflammatory markers in aortic tissues of rats fed a high-fat diet. (A) Representative blots of inflammatory factors (iNOS, COX-2, and mPGES-1) in aortic vessel tissues. Each lane was loaded with 50 μg of total protein. (B) Quantification of iNOS. (C) Quantification of COX-2. (D) Quantification of mPGES-1. Data (ratio of A.D.U. normalized on β-actin) are reported as fold change vs. STD, assigned a value of 1 (n = 3/group). ** p < 0.01 and *** p < 0.001 vs. STD; # p < 0.05 and ## p < 0.01 vs. HFD. Comparisons among means in (B–D) were performed with the Bonferroni’s post hoc test.
Further morphological assessment of the aortic tissues through hematoxylin and eosin staining revealed no significant variations in tissue morphology across the different diet and supplementation groups (Figure 5A). This duration is not sufficient to induce morphological alterations in the aortic structure, as widely reported in the literature, where some studies extend the treatment to 8–12 weeks [40,41]. This suggests that the observed biochemical changes did not translate into gross morphological alterations within the timeframe of our study. Subsequent CD56 staining was performed to evaluate immune cell infiltration in the aortic vascular wall. Our analysis showed that CD56 expression was absent in the STD, CEVOO, and CrOO groups, while positive CD56 staining was observed in the HFD group (Figure 5B). This indicates that an HFD promotes immune cell infiltration into the aortic wall, a process that is mitigated by CrOO supplementation. The absence of CD56 staining in the CEVOO and CrOO groups further supports the anti-inflammatory effects of these supplements.
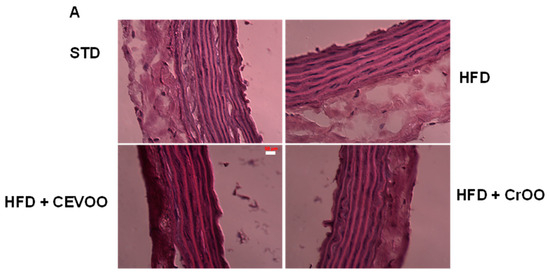
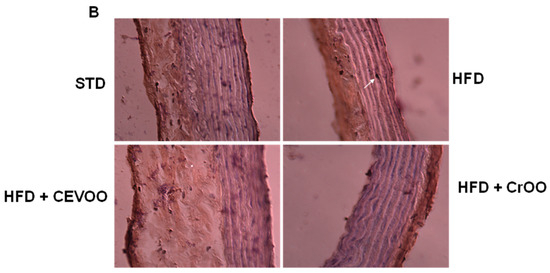
Figure 5.
Morphology and CEVOO and CrOO supplementation activity on aortic vascular wall inflammation from rats fed with/without HFD. Magnification ×40. Scale bar 50 µm for all panels. (A) Morphology of aortic tissue under hematoxylin and eosin staining. (B) Evaluation of aortic sections by immunohistochemistry. Arrow in HFD panel shows CD56 staining. Representative section of three rats/experimental group.
Altogether, our study highlights the detrimental impact of a high-fat diet on inflammation within the aortic tissues, as evidenced by the upregulation of COX-2, mPGES-1, and iNOS, and increased immune cell infiltration. CrOO supplementation emerged as a potent anti-inflammatory intervention, effectively reducing the expression of inflammatory markers and preventing immune cell infiltration. While CEVOO and CrOO showed some efficacy in modulating specific inflammatory pathways, CrOO demonstrated a broader protective effect. These findings suggest that dietary interventions, particularly with CrOO, could be a valuable strategy in mitigating inflammation and potentially reducing the risk of cardiovascular disease associated with high-fat diets.
4. Conclusions
Our findings revealed that, despite the high maturity index, the olive oil samples used in this study met the stringent quality standards for extra virgin olive oil (EVOO). Both CEVOO and CrOO exhibited significantly low free acidity and peroxide levels, with no significant differences among the oil types. The high carotenoid content in CrOO and high chlorophyll concentration in CEVOO were notable, influencing both visual appeal and potential health benefits. Despite these differences, both oils demonstrated robust profiles in maintaining the quality and health-promoting attributes of olive oil, including their balanced fatty acid composition and the presence of minor compounds like phenolics and tocopherols. While Citrus cryomacerated peels did not significantly alter tocopherol profiles or total phenolic content, CrOO had lower hydroxytyrosol and tyrosol concentrations compared to CEVOO, correlating with its lower free-radical scavenging capacity. Furthermore, CrOO showed high levels of limonene, a volatile compound peculiar to citrus fruits and endowed with renowned beneficial effects, that was almost absent in the CEVOO.
Despite the short duration of the treatment (3 weeks), which could—at least in part—lead to underestimation of the real nutraceutical impact of CEVOO as well as CrOO on obesogenic dietary regimen, in in vivo studies, CEVOO showed potential in reducing body weight gain, blood glucose, and triglycerides, while CrOO showed similar effects, especially in containing cardiovascular risk. The HFD significantly impacted metabolic parameters, increasing total cholesterol, triglycerides, HDL, LDL, and blood glucose levels. Supplementation with CEVOO or CrOO mitigated these effects, highlighting their protective properties. Both oils helped to restore mitochondrial membrane potential, suggesting maintained nutraceutical profiles. Additionally, CrOO reduced inflammatory markers like COX-2, mPGES-1, and iNOS in aortic tissues, further supporting its anti-inflammatory efficacy.
In summary, our study underscores the negative impact of a high-fat diet on cardiovascular health and inflammation, confirming the benefits of CEVOO supplementation and highlighting that the technological strategy implemented to exploit citrus by-products does not compromise the well-known nutraceutical value on the cardiovascular system of extra virgin olive oil, suggesting, rather, its potential as a dietary intervention to combat the negative effects of high-fat diets.
Author Contributions
Conceptualization, A.Z., S.D. and L.T.; methodology, V.C. (Valerio Ciccone), G.F., Y.P., J.S. and M.M.; formal analysis, V.C. (Valerio Ciccone), J.S., Y.P. and M.M.; investigation, J.S., V.C. (Valerio Ciccone), Y.P. and M.M.; data curation, J.S., V.C. (Valerio Ciccone), Y.P. and M.M.; writing—original draft preparation, M.M., L.T., S.D., J.S. and Y.P.; writing—review and editing, V.C. (Vincenzo Calderone), M.M., G.F., L.T., S.D., J.S. and Y.P.; supervision, A.Z., S.D., G.F. and L.T.; project administration and funding acquisition, A.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FERS-TOSCANA 2014–2020, LONG LIFE OIL, Bando 1: Progetti strategici di ricerca e sviluppo; TEQEVO, PSR 2014–2020 Tuscany region, 16.2, Bando GAL F.A.R Maremma.
Institutional Review Board Statement
The project protocol was discussed at the Animal Ethics Committee of the University of Pisa and received the approval by the Committee of the Italian Ministry of Health (number protocol 144/2019-PR, 18 February 2019).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Maqbool, Z.; Khalid, W.; Atiq, H.T.; Koraqi, H.; Javaid, Z.; Alhag, S.K.; Al-Shuraym, L.A.; Bader, D.M.D.; Almarzuq, M.; Afifi, M.; et al. Citrus Waste as Source of Bioactive Compounds: Extraction and Utilization in Health and Food Industry. Molecules 2023, 28, 1636. [Google Scholar] [CrossRef] [PubMed]
- Addi, M.; Elbouzidi, A.; Abid, M.; Tungmunnithum, D.; Elamrani, A.; Hano, C. An Overview of Bioactive Flavonoids from Citrus Fruits. Appl. Sci. 2022, 12, 29. [Google Scholar] [CrossRef]
- Lv, X.; Zhao, S.; Ning, Z.; Zeng, H.; Shu, Y.; Tao, O.; Xiao, C.; Lu, C.; Liu, Y. Citrus fruits as a treasure trove of active natural metabolites that potentially provide benefits for human health. Chem. Cent. J. 2015, 9, 68. [Google Scholar] [CrossRef] [PubMed]
- Gualdani, R.; Cavalluzzi, M.M.; Lentini, G.; Habtemariam, S. The Chemistry and Pharmacology of Citrus Limonoids. Molecules 2016, 21, 1530. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Lou, Y.; Li, Y.; Zhang, J.; Li, P.; Yang, B.; Gu, Q. Review of phytochemical and nutritional characteristics and food applications of Citrus L. fruits. Front. Nutr. 2022, 9, 968604. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.Q.; Zeng, Y.; Xu, J.; Xu, X. Le Naringenin alleviates nonalcoholic steatohepatitis in middle-aged Apoe−/− mice: Role of SIRT1. Phytomedicine 2021, 81, 153412. [Google Scholar] [CrossRef]
- Li, H.; Liu, L.; Cao, Z.; Li, W.; Liu, R.; Chen, Y.; Li, C.; Song, Y.; Liu, G.; Hu, J.; et al. Naringenin ameliorates homocysteine induced endothelial damage via the AMPKα/Sirt1 pathway. J. Adv. Res. 2021, 34, 137–147. [Google Scholar] [CrossRef]
- Testai, L.; Calderone, V. Nutraceutical value of citrus flavanones and their implications in cardiovascular disease. Nutrients 2017, 9, 502. [Google Scholar] [CrossRef]
- Idrees, M.; Kumar, V.; Khan, A.M.; Joo, M.D.; Uddin, Z.; Lee, K.W.; Kong, I.K. Hesperetin activated SIRT1 neutralizes cadmium effects on the early bovine embryo development. Theriogenology 2022, 189, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Flori, L.; Macaluso, M.; Taglieri, I.; Sanmartin, C.; Sgherri, C.; De Leo, M.; Ciccone, V.; Donnini, S.; Venturi, F.; Pistelli, L.; et al. Development of Fortified Citrus Olive Oils: From Their Production to Their Nutraceutical Properties on the Cardiovascular System. Nutrients 2020, 12, 1557. [Google Scholar] [CrossRef]
- Bohn, T.; Bonet, M.L.; Borel, P.; Keijer, J.; Landrier, J.F.; Milisav, I.; Ribot, J.; Riso, P.; Winklhofer-Roob, B.; Sharoni, Y.; et al. Mechanistic aspects of carotenoid health benefits—Where are we now? Nutr. Res. Rev. 2021, 34, 276–302. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, N.; Re, R.; Yang, M.; Rice-Evans, C. Screening of dietary carotenoids and carotenoid-rich fruit extracts for antioxidant activities applying 2,2’-azinobis(3-ethylenebenzothiazoline-6- sulfonic acid radical cation decolorization assay. Methods Enzymol. 1998, 299, 379–389. [Google Scholar] [CrossRef]
- Saini, R.K.; Ranjit, A.; Sharma, K.; Prasad, P.; Shang, X.; Gowda, K.G.M.; Keum, Y.S. Bioactive compounds of citrus fruits: A review of the composition and health benefits of carotenoids, flavonoids, limonoids, and terpenes. Antioxidants 2022, 11, 239. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, S.; Ejaz, A.; Matsuda, K.; Chae, W.L. Limonoids as cancer chemopreventive agents. J. Sci. Food Agric. 2006, 86, 339–345. [Google Scholar] [CrossRef]
- Teodoro, A.J. Bioactive compounds of food: Their role in the prevention and treatment of diseases. Oxid. Med. Cell. Longev. 2019, 2019, 4–7. [Google Scholar] [CrossRef]
- Ofori, K.F.; Antoniello, S.; English, M.M.; Aryee, A.N.A. Improving nutrition through biofortification—A systematic review. Front. Nutr. 2022, 9, 1–20. [Google Scholar] [CrossRef]
- Olson, R.; Gavin-Smith, B.; Ferraboschi, C.; Kraemer, K. Food fortification: The advantages, disadvantages and lessons from sight and life programs. Nutrients 2021, 13, 1118. [Google Scholar] [CrossRef]
- Ascrizzi, R.; Taglieri, I.; Sgherri, C.; Flamini, G.; Macaluso, M.; Sanmartin, C.; Venturi, F.; Quartacci, M.F.; Pistelli, L.; Zinnai, A. Nutraceutical oils produced by olives and citrus peel of Tuscany varieties as sources of functional ingredients. Molecules 2019, 24, 65. [Google Scholar] [CrossRef]
- Macaluso, M.; Taglieri, I.; Venturi, F.; Sanmartin, C.; Bianchi, A.; De Leo, M.; Braca, A.; Quartacci, M.F.; Zinnai, A. Influence of the Atmosphere Composition during Malaxation and Storage on the Shelf Life of an Unfiltered Extra Virgin Olive Oil: Preliminary Results. Eur. J. Lipid Sci. Technol. 2021, 123, 2000122. [Google Scholar] [CrossRef]
- EU Commission. European Union Commission Implementing Regulation (EU) No 1348/2013 amending Regulation (EEC) No 2568/91. Off. J. Eur. Union 2013, 139, 31–67. [Google Scholar]
- Gutierrez Rosales, F.G.; Perdiguero, S.; Gutierrez, R.; Olias, J.M. Evaluation of the Bitter Taste in Virgin Olive Oil. J. Am. Oil Chem. Soc. 1992, 69, 394–395. [Google Scholar] [CrossRef]
- Mínguez-Mosquera, M.I.; Gandul-Rojas, B.; Gallardo-Guerrero, M.L. Rapid Method of Quantification of Chlorophylls and Carotenoids in Virgin Olive Oil by High-Performance Liquid Chromatography. J. Agric. Food Chem. 1992, 40, 60–63. [Google Scholar] [CrossRef]
- Sgherri, C.; Pérez-López, U.; Micaelli, F.; Miranda-Apodaca, J.; Mena-Petite, A.; Muñoz-Rueda, A.; Quartacci, M.F. Elevated CO2 and salinity are responsible for phenolics-enrichment in two differently pigmented lettuces. Plant Physiol. Biochem. 2017, 115, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Flamini, G.; Pistelli, L.; Nardoni, S.; Ebani, V.V.; Zinnai, A.; Mancianti, F.; Ascrizzi, R.; Pistelli, L. Essential oil composition and biological activity of “Pompia”, a Sardinian citrus ecotype. Molecules 2019, 24, 908. [Google Scholar] [CrossRef] [PubMed]
- Leung, V.; Rousseau-Blass, F.; Beauchamp, G.; Pang, D.S.J. Arrive has not arrived: Support for the arrive (animal research: Reporting of in vivo experiments) guidelines does not improve the reporting quality of papers in animal welfare, analgesia or anesthesia. PLoS ONE 2018, 13, e0197882. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Shah, A.; Waiz, M.; Chaturvedi, C.P.; Alvi, S.S.; Khan, M.S. Organosulfur Compounds, S-Allyl-L-Cysteine and S-Ethyl-L-Cysteine, Target PCSK-9/LDL-R-Axis to Ameliorate Cardiovascular, Hepatic, and Metabolic Changes in High Carbohydrate and High Fat Diet-Induced Metabolic Syndrome in Rats. Phyther. Res. 2024, 1–21. [Google Scholar] [CrossRef]
- Bellumori, M.; Cecchi, L.; Innocenti, M.; Clodoveo, M.L.; Corbo, F.; Mulinacci, N. The EFSA health claim on olive oil polyphenols: Acid hydrolysis validation and total hydroxytyrosol and tyrosol determination in Italian virgin olive oils. Molecules 2019, 24, 2179. [Google Scholar] [CrossRef]
- Ciccone, V.; Terzuoli, E.; Ristori, E.; Filippelli, A.; Ziche, M.; Morbidelli, L.; Donnini, S. ALDH1A1 overexpression in melanoma cells promotes tumor angiogenesis by activating the IL-8/Notch signaling cascade. Int. J. Mol. Med. 2022, 50, 99. [Google Scholar] [CrossRef]
- Pieracci, Y.; Pistelli, L.; Cecchi, M.; Pistelli, L.; De Leo, M. Phytochemical Characterization of Citrus-Based Products Supporting Their Antioxidant Effect and Sensory Quality. Foods 2022, 11, 1550. [Google Scholar] [CrossRef]
- Hariri, N.; Thibault, L. High-fat diet-induced obesity in animal models. Nutr. Res. Rev. 2010, 23, 270–299. [Google Scholar] [CrossRef]
- Macaluso, M.; Bianchi, A.; Sanmartin, C.; Taglieri, I.; Venturi, F.; Testai, L.; Flori, L.; Calderone, V.; De Leo, M.; Braca, A.; et al. By-products from winemaking and olive mill value chains for the enrichment of refined olive oil: Technological challenges and nutraceutical features. Foods 2020, 9, 1390. [Google Scholar] [CrossRef] [PubMed]
- Henein, M.Y.; Vancheri, S.; Longo, G.; Vancheri, F. The Role of Inflammation in Cardiovascular Disease. Int. J. Mol. Sci. 2022, 23, 2906. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.; Yang, C.; Ballew, S.H.; Kalbaugh, C.A.; McEvoy, J.W.; Salameh, M.; Aguilar, D.; Hoogeveen, R.C.; Nambi, V.; Selvin, E.; et al. Fibrosis and Inflammatory Markers and Long-Term Risk of Peripheral Artery Disease The ARIC Study. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2322–2331. [Google Scholar] [CrossRef] [PubMed]
- Terzuoli, E.; Nannelli, G.; Giachetti, A.; Morbidelli, L.; Ziche, M.; Donnini, S. Targeting endothelial-to-mesenchymal transition: The protective role of hydroxytyrosol sulfate metabolite. Eur. J. Nutr. 2020, 59, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Pastor, R.; Bouzas, C.; Tur, J.A. Beneficial effects of dietary supplementation with olive oil, oleic acid, or hydroxytyrosol in metabolic syndrome: Systematic review and meta-analysis. Free Radic. Biol. Med. 2021, 172, 372–385. [Google Scholar] [CrossRef]
- de Pablos, R.M.; Espinosa-Oliva, A.M.; Hornedo-Ortega, R.; Cano, M.; Arguelles, S. Hydroxytyrosol protects from aging process via AMPK and autophagy; a review of its effects on cancer, metabolic syndrome, osteoporosis, immune-mediated and neurodegenerative diseases. Pharmacol. Res. 2019, 143, 58–72. [Google Scholar] [CrossRef]
- Sluijs, I.; Beulens, J.W.J.; Grobbee, D.E.; Van Der Schouw, Y.T. Dietary carotenoid intake is associated with lower prevalence of metabolic syndrome in middle-aged and elderly men. J. Nutr. 2009, 139, 987–992. [Google Scholar] [CrossRef]
- Pokala, A.; Quarles, W.R.; Ortega-Anaya, J.; Jimenez-Flores, R.; Cao, S.; Zeng, M.; Hodges, J.K.; Bruno, R.S. Milk-Fat-Globule-Membrane-Enriched Dairy Milk Compared with a Soy-Lecithin-Enriched Beverage Did Not Adversely Affect Endotoxemia or Biomarkers of Gut Barrier Function and Cardiometabolic Risk in Adults with Metabolic Syndrome: A Randomized Controlled Cro. Nutrients 2023, 15, 3259. [Google Scholar] [CrossRef]
- Wong, S.K.; Chin, K.Y.; Suhaimi, F.H.; Ahmad, F.; Ima-Nirwana, S. Vitamin E as a potential interventional treatment for metabolic syndrome: Evidence from animal and human studies. Front. Pharmacol. 2017, 8, 444. [Google Scholar] [CrossRef]
- Logvinov, S.V.; Naryzhnaya, N.V.; Kurbatov, B.K.; Gorbunov, A.S.; Birulina, Y.G.; Maslov, L.L.; Oeltgen, P.R. High carbohydrate high fat diet causes arterial hypertension and histological changes in the aortic wall in aged rats: The involvement of connective tissue growth factors and fibronectin. Exp. Gerontol. 2021, 154, 111543. [Google Scholar] [CrossRef]
- Henson, G.D.; Walker, A.E.; Reihl, K.D.; Donato, A.J.; Lesniewski, L.A. Dichotomous mechanisms of aortic stiffening in high-fat diet fed young and old B6D2F1 mice. Physiol. Rep. 2014, 2, e00268. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).