Euglena gracilis Enhances Innate and Adaptive Immunity through Specific Expression of Dectin-1 in CP-Induced Immunosuppressed Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Preparation
2.2. Cell Culture and Treatment
2.3. CCK-8 Assay
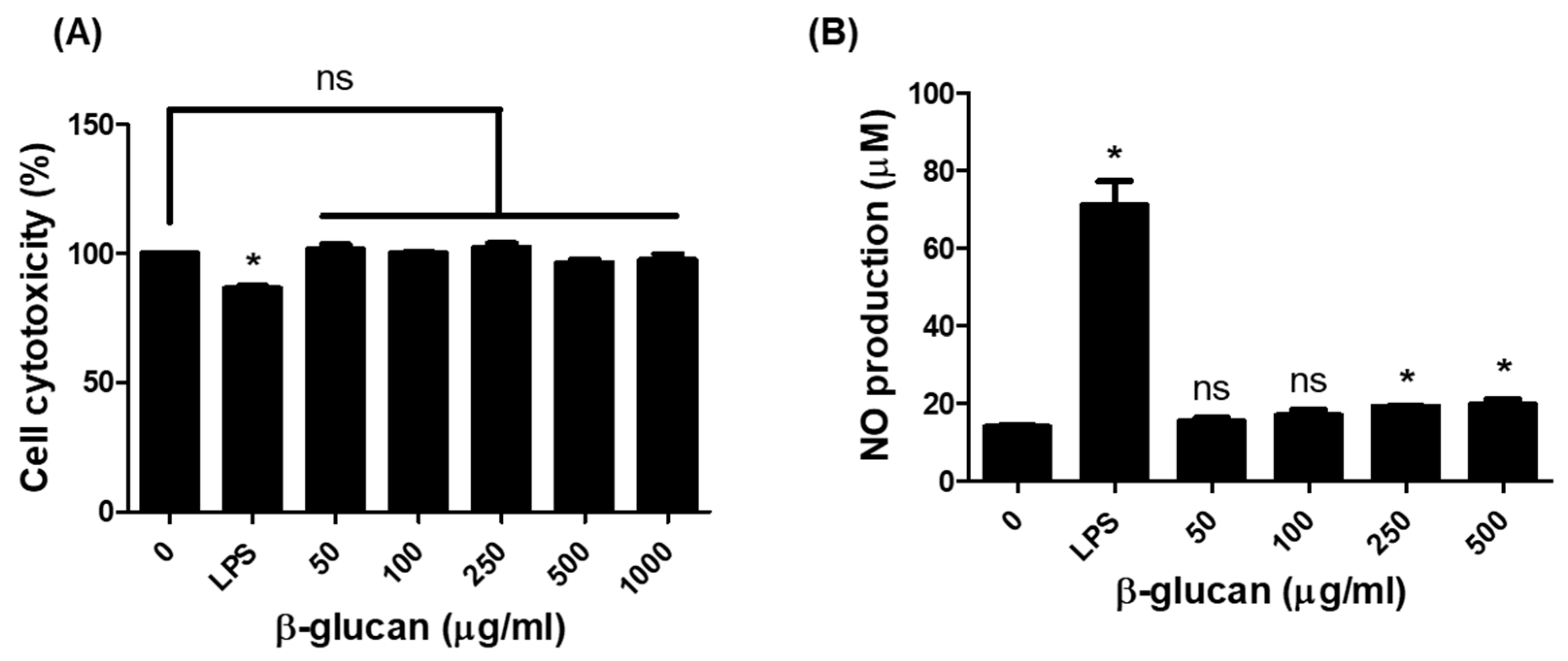
2.4. Nitric Oxide (NO) Assay
2.5. Cytokine Analysis
2.6. Western Blot Analysis
2.7. In Vivo Experiments Using Immunosuppressed C57BL/6 Mice
2.8. Complete Blood Cell Count (CBC) Analysis
2.9. Antibodies (IgM, IgG) Analysis
2.10. Splenocyte Proliferation Assay
2.11. Cell Surface Antigens Analysis by Flow Cytometry
2.12. Immunohistochemistry (IHC) Staining
2.13. Statistics
3. Results
3.1. Production of Nitric Oxide (NO) in RAW264.7 Cells by β-1,3-Glucan
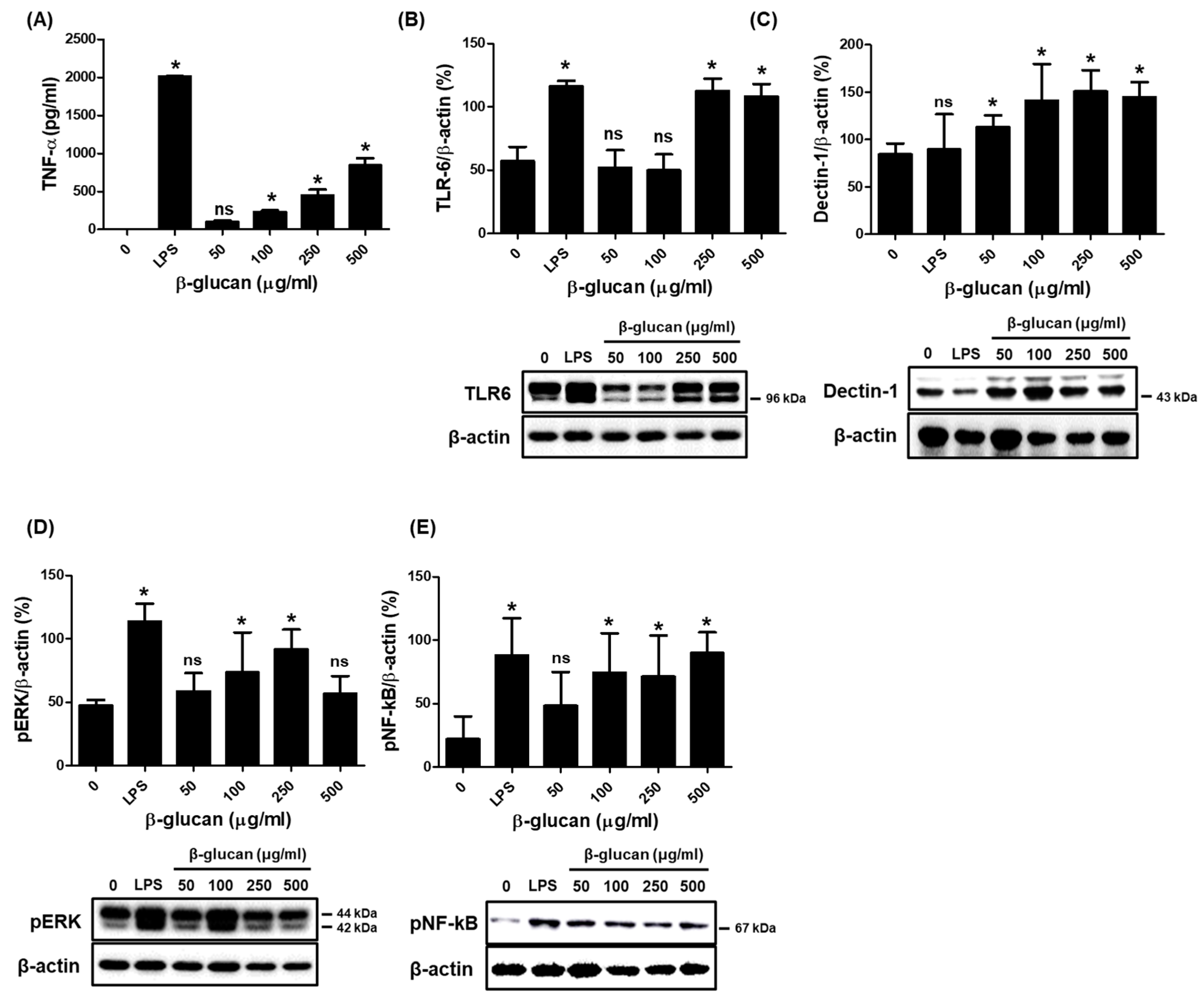
3.2. Specific Production of Pro-Inflammatory Cytokine TNF-α via a Stimulation of TLR-6 and Dectin-1 Proteins on a Surface of RAW264.7 Cells by β-1,3-Glucan
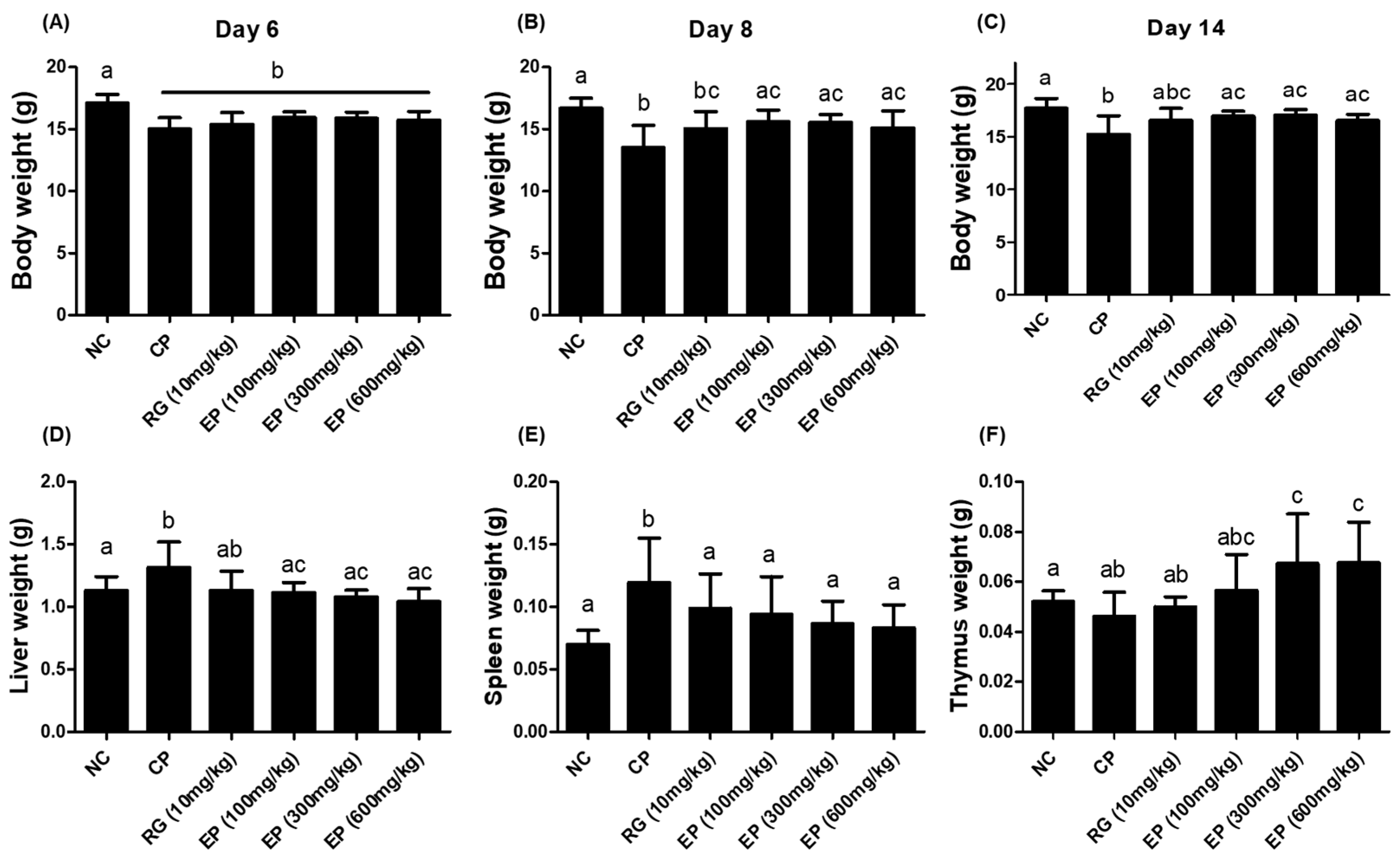
3.3. Effect of EP on the Weight of Body and Organs in the Immunosuppressed Mice
3.4. CBC Analysis of Whole Blood from the Mice Treated with EP
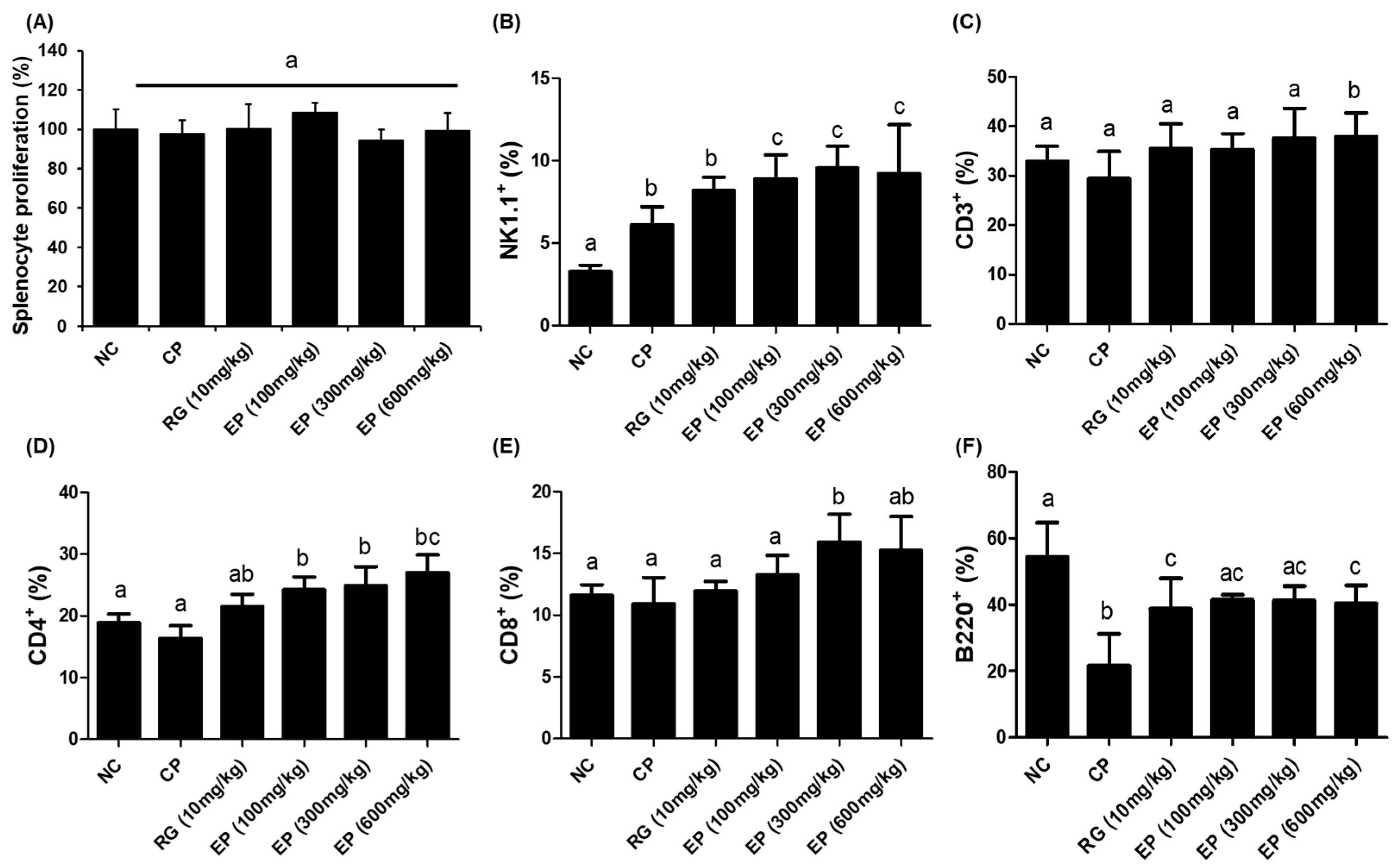
3.5. Increased Frequency of Lymphoid Cells in Mouse Spleen by EP
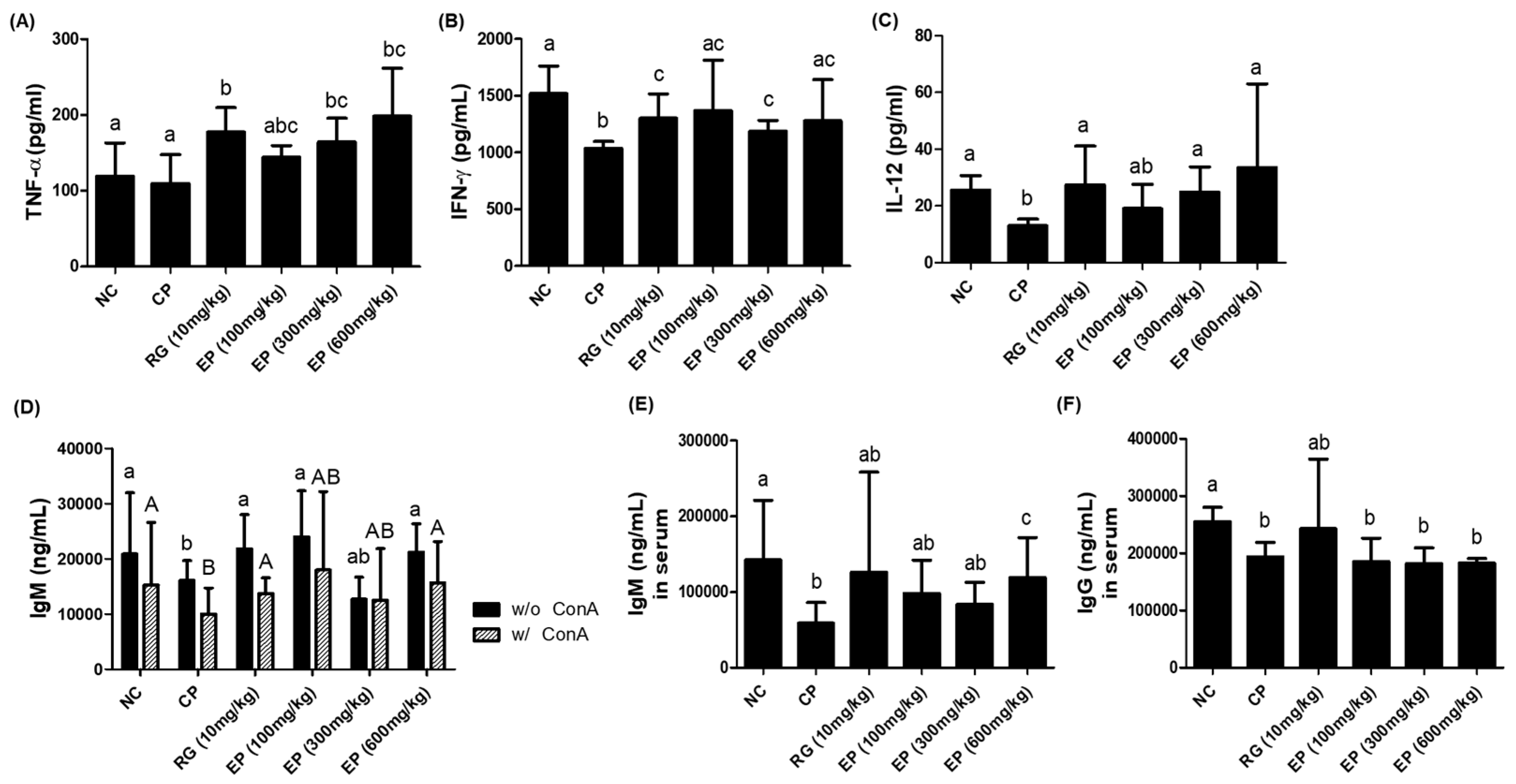
3.6. Increased Production of Cytokines (TNF-α, IFN-γ, IL-12) and IgM Antibody in Immunosuppressed Mice by EP
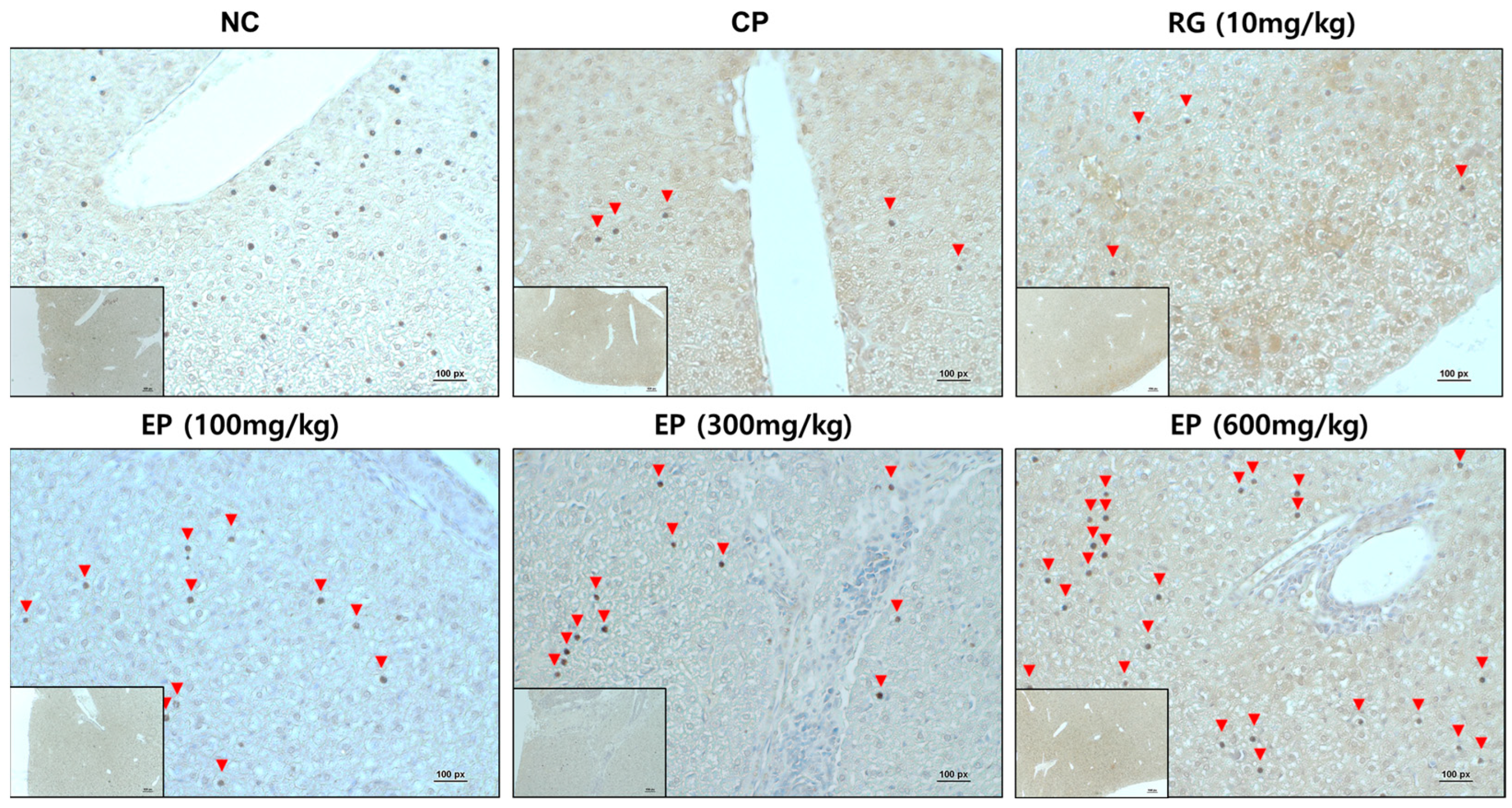
3.7. Increased Expression of Dectin-1 in Liver Tissue of Immunosuppressed Mice by EP
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tubo, N.J.; Jenkins, M.K. CD4+ T Cells: Guardians of the phagosome. Clin. Microbiol. Rev. 2014, 27, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Grégoire, C.; Chasson, L.; Luci, C.; Tomasello, E.; Geissmann, F.; Vivier, E.; Walzer, T. The trafficking of natural killer cells. Immunol. Rev. 2007, 220, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Lostao, L.; Anel, A.; Pardo, J. How Do Cytotoxic Lymphocytes Kill Cancer Cells? Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2015, 21, 5047–5056. [Google Scholar] [CrossRef] [PubMed]
- Fauriat, C.; Long, E.O.; Ljunggren, H.G.; Bryceson, Y.T. Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood 2010, 115, 2167–2176. [Google Scholar] [CrossRef]
- Janeway, C.A., Jr.; Travers, P.; Walport, M.; Shlomchik, M. Immunobiology: The Immune System in Health and Disease. In Principles of Innate and Adaptive Immunity, 5th ed.; Garland Science: New York, NY, USA, 2001. [Google Scholar]
- Palacios-Pedrero, M.; Osterhaus, A.; Becker, T.; Elbahesh, H.; Rimmelzwaan, G.F.; Saletti, G. Aging and Options to Halt Declining Immunity to Virus Infections. Front. Immunol. 2021, 12, 681449. [Google Scholar] [CrossRef]
- Chae, S.R.; Hwang, E.J.; Shin, H.S. Single cell protein production of Euglena gracilis and carbon dioxide fixation in an innovative photo-bioreactor. Bioresour. Technol. 2006, 97, 322–329. [Google Scholar] [CrossRef]
- Ivušić, F.; Rezić, T.; Šantek, B. Heterotrophic Cultivation of Euglena gracilis in Stirred Tank Bioreactor: A Promising Bioprocess for Sustainable Paramylon Production. Molecules 2022, 27, 5866. [Google Scholar] [CrossRef]
- Takeyama, H.; Kanamaru, A.; Yoshino, Y.; Kakuta, H.; Kawamura, Y.; Matsunaga, T. Production of antioxidant vitamins, β-carotene, vitamin C, and vitamin E, by two-step culture of Euglena gracilis Z. Biotechnol. Bioeng. 1997, 53, 185–190. [Google Scholar] [CrossRef]
- Rodríguez Zavala, J.S.; Ortiz Cruz, M.A.; Mendoza Hernández, G.; Moreno Sánchez, R. Increased synthesis of α-tocopherol, paramylon and tyrosine by Euglena gracilis under conditions of high biomass production. J. Appl. Microbiol. 2010, 109, 2160–2172. [Google Scholar] [CrossRef]
- Pollak, D.W.; Bostick, M.W.; Yoon, H.; Wang, J.; Hollerbach, D.H.; He, H.; Damude, H.G.; Zhang, H.; Yadav, N.S.; Hong, S.-P.; et al. Isolation of a Δ5 Desaturase Gene from Euglena gracilis and Functional Dissection of Its HPGG and HDASH Motifs. Lipids 2012, 47, 913–926. [Google Scholar] [CrossRef]
- Barsanti, L.; Vismara, R.; Passarelli, V.; Gualtieri, P. Paramylon (β-1,3-glucan) content in wild type and WZSL mutant of Euglena gracilis. Effects of growth conditions. J. Appl. Phycol. 2001, 13, 59–65. [Google Scholar] [CrossRef]
- Russo, R.; Barsanti, L.; Evangelista, V.; Frassanito, A.M.; Longo, V.; Pucci, L.; Penno, G.; Gualtieri, P. Euglena gracilis paramylon activates human lymphocytes by upregulating pro-inflammatory factors. Food Sci. Nutr. 2017, 5, 205–214. [Google Scholar] [CrossRef]
- C.Ooi, E.V.; Liu, F. Immunomodulation and Anti-Cancer Activity of Polysaccharide-Protein Complexes. Curr. Med. Chem. 2000, 7, 715–729. [Google Scholar] [CrossRef]
- Gissibl, A.; Care, A.; Parker, L.M.; Iqbal, S.; Hobba, G.; Nevalainen, H.; Sunna, A. Microwave pretreatment of paramylon enhances the enzymatic production of soluble β-1,3-glucans with immunostimulatory activity. Carbohydr. Polym. 2018, 196, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Patidar, A.; Mahanty, T.; Raybarman, C.; Sarode, A.Y.; Basak, S.; Saha, B.; Bhattacharjee, S. Barley beta-Glucan and Zymosan induce Dectin-1 and Toll-like receptor 2 co-localization and anti-leishmanial immune response in Leishmania donovani-infected BALB/c mice. Scand. J. Immunol. 2020, 92, e12952. [Google Scholar] [CrossRef]
- Brown, G.D. Dectin-1: A signalling non-TLR pattern-recognition receptor. Nat. Rev. Immunol. 2006, 6, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Herre, J.; Williams, D.L.; Willment, J.A.; Marshall, A.S.; Gordon, S. Dectin-1 mediates the biological effects of beta-glucans. J. Exp. Med. 2003, 197, 1119–1124. [Google Scholar] [CrossRef]
- Underhill, D.M.; Rossnagle, E.; Lowell, C.A.; Simmons, R.M. Dectin-1 activates Syk tyrosine kinase in a dynamic subset of macrophages for reactive oxygen production. Blood 2005, 106, 2543–2550. [Google Scholar] [CrossRef]
- Galluzzi, L.; Buqué, A.; Kepp, O.; Zitvogel, L.; Kroemer, G. Immunological Effects of Conventional Chemotherapy and Targeted Anticancer Agents. Cancer Cell 2015, 28, 690–714. [Google Scholar] [CrossRef]
- Bao, L.; Hao, C.; Wang, J.; Wang, D.; Zhao, Y.; Li, Y.; Yao, W. High-Dose Cyclophosphamide Administration Orchestrates Phenotypic and Functional Alterations of Immature Dendritic Cells and Regulates Th Cell Polarization. Front. Pharmacol. 2020, 11, 00775. [Google Scholar] [CrossRef]
- Mills, K.A.; Chess-Williams, R.; McDermott, C. Novel insights into the mechanism of cyclophosphamide-induced bladder toxicity: Chloroacetaldehyde’s contribution to urothelial dysfunction in vitro. Arch. Toxicol. 2019, 93, 3291–3303. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.H.; Cho, Y.; Kim, G.H.; Cho, H. Undaria pinnatifida Fucoidan-Rich Extract Recovers Immunity of Immunosuppressed Mice. J. Microbiol. Biotechnol. 2020, 30, 439–447. [Google Scholar] [CrossRef]
- Kim, S.; Lee, H.H.; Kang, C.H.; Kang, H.; Cho, H. Immune-Enhancing Effects of Limosilactobacillus fermentum in BALB/c Mice Immunosuppressed by Cyclophosphamide. Nutrients 2023, 15, 1038. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, A.; Yamada, K.; Iwata, O.; Sugimoto, R.; Atsuji, K.; Ogawa, T.; Ishibashi-Ohgo, N.; Suzuki, K. β-Glucan in Foods and Its Physiological Functions. J. Nutr. Sci. Vitaminol. 2018, 64, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Isoda, N.; Eguchi, Y.; Nukaya, H.; Hosho, K.; Suga, Y.; Suga, T.; Nakazawa, S.; Sugano, K. Clinical efficacy of superfine dispersed lentinan (beta-1,3-glucan) in patients with hepatocellular carcinoma. Hepato-Gastroenterology 2009, 56, 437–441. [Google Scholar]
- Rahar, S.; Swami, G.; Nagpal, N.; Nagpal, M.A.; Singh, G.S. Preparation, characterization, and biological properties of β-glucans. J. Adv. Pharm. Technol. Res. 2011, 2, 94–103. [Google Scholar] [CrossRef]
- Gissibl, A.; Sun, A.; Care, A.; Nevalainen, H.; Sunna, A. Bioproducts From Euglena gracilis: Synthesis and Applications. Front. Bioeng. Biotechnol. 2019, 7, 108. [Google Scholar] [CrossRef]
- Wink, D.A.; Hines, H.B.; Cheng, R.Y.; Switzer, C.H.; Flores-Santana, W.; Vitek, M.P.; Ridnour, L.A.; Colton, C.A. Nitric oxide and redox mechanisms in the immune response. J. Leukoc. Biol. 2011, 89, 873–891. [Google Scholar] [CrossRef]
- Sonck, E.; Stuyven, E.; Goddeeris, B.; Cox, E. The effect of beta-glucans on porcine leukocytes. Vet. Immunol. Immunopathol. 2010, 135, 199–207. [Google Scholar] [CrossRef]
- Turpaev, K.; Glatigny, A.; Bignon, J.; Delacroix, H.; Drapier, J.C. Variation in gene expression profiles of human monocytic U937 cells exposed to various fluxes of nitric oxide. Free Radic. Biol. Med. 2010, 48, 298–305. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Guo, Q.; Bi, D.; Wu, M.; Yu, B.; Hu, L.; Liu, C.; Gu, L.; Zhu, H.; Lei, A.; Xu, X.; et al. Immune activation of murine RAW264.7 macrophages by sonicated and alkalized paramylon from Euglena gracilis. BMC Microbiol. 2020, 20, 171. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, A.; Suzuki, K.; Asayama, Y.; Konno, M.; Saito, K.; Yamazaki, N.; Takimoto, H. Oral administration of Euglena gracilis Z and its carbohydrate storage substance provides survival protection against influenza virus infection in mice. Biochem. Biophys. Res. Commun. 2017, 494, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Mazgaeen, L.; Gurung, P. Recent Advances in Lipopolysaccharide Recognition Systems. Int. J. Mol. Sci. 2020, 21, 379. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Schorey, J.S. The beta-glucan receptor dectin-1 functions together with TLR2 to mediate macrophage activation by mycobacteria. Blood 2006, 108, 3168–3175. [Google Scholar] [CrossRef]
- Dennehy, K.M.; Ferwerda, G.; Faro-Trindade, I.; Pyz, E.; Willment, J.A.; Taylor, P.R.; Kerrigan, A.; Tsoni, S.V.; Gordon, S.; Meyer-Wentrup, F.; et al. Syk kinase is required for collaborative cytokine production induced through Dectin-1 and Toll-like receptors. Eur. J. Immunol. 2008, 38, 500–506. [Google Scholar] [CrossRef]
- Choi, E.Y.; Lee, S.S.; Hyeon, J.Y.; Choe, S.H.; Keum, B.R.; Lim, J.M.; Park, D.C.; Choi, I.S.; Cho, K.K. Effects of β-Glucan on the Release of Nitric Oxide by Macrophages Stimulated with Lipopolysaccharide. Asian-Australas. J. Anim. Sci. 2016, 29, 1664–1674. [Google Scholar] [CrossRef] [PubMed]
- Batbayar, S.; Lee, D.H.; Kim, H.W. Immunomodulation of Fungal β-Glucan in Host Defense Signaling by Dectin-1. Biomol. Ther. 2012, 20, 433–445. [Google Scholar] [CrossRef]
- Feng, L.; Huang, Q.; Huang, Z.; Li, H.; Qi, X.; Wang, Y.; Liu, Z.; Liu, X.; Lu, L. Optimized Animal Model of Cyclophosphamide-induced Bone Marrow Suppression. Basic Clin. Pharmacol. Toxicol. 2016, 119, 428–435. [Google Scholar] [CrossRef]
- Hyun, S.H.; Ahn, H.Y.; Kim, H.J.; Kim, S.W.; So, S.H.; In, G.; Park, C.K.; Han, C.K. Immuno-enhancement effects of Korean Red Ginseng in healthy adults: A randomized, double-blind, placebo-controlled trial. J. Ginseng Res. 2021, 45, 191–198. [Google Scholar] [CrossRef]
- Zhao, N.; Wang, L.; Mou, H.Y.; Liang, M.; Yue, W. Synergism and attenuation effects of taurine on cyclophosphamide. Ai Zheng = Aizheng = Chin. J. Cancer 2009, 28, 244–248. [Google Scholar]
- Arango-Prado, M.D.C.; Villegas-Valverde, C.A.; Torres-López, G.; Soto-Pardeiro, P.; Suárez-Reyes, A.; Faxas-García, M.E.; Diéguez-Rodríguez, V.; Gracia-Medina, E.; Esperón-Noa, R.; Del Castillo-Bahi, R.; et al. Lymphocyte Subsets in Defense Against New Pathogens in Patients with Cancer. MEDICC Rev. 2022, 24, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Lodoen, M.B.; Lanier, L.L. Natural killer cells as an initial defense against pathogens. Curr. Opin. Immunol. 2006, 18, 391–398. [Google Scholar] [CrossRef]
- Osińska, I.; Popko, K.; Demkow, U. Perforin: An important player in immune response. Cent.-Eur. J. Immunol. 2014, 39, 109–115. [Google Scholar] [CrossRef]
- Loh, J.; Chu, D.T.; O’Guin, A.K.; Yokoyama, W.M.; Virgin, H.W.T. Natural killer cells utilize both perforin and gamma interferon to regulate murine cytomegalovirus infection in the spleen and liver. J. Virol. 2005, 79, 661–667. [Google Scholar] [CrossRef]
- Park, S.-y.; Kim, K.J.; Jo, S.M.; Jeon, J.-Y.; Kim, B.-R.; Hwang, J.E.; Kim, J.Y. Euglena gracilis (Euglena) powder supplementation enhanced immune function through natural killer cell activity in apparently healthy participants: A randomized, double-blind, placebo-controlled trial. Nutr. Res. 2023, 119, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Sauls, R.S.; McCausland, C.; Taylor, B.N. Histology, T-Cell Lymphocyte. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Wang, J.; Dong, S.; Liu, C.; Wang, W.; Sun, S.; Gu, J.; Wang, Y.; Boraschi, D.; Qu, D. β-Glucan oligosaccharide enhances CD8+ T cells immune response induced by a DNA vaccine encoding hepatitis B virus core antigen. J. Biomed. Biotechnol. 2010, 2010, 645213. [Google Scholar] [CrossRef]
- Jo, K.A.; Kim, K.J.; Park, S.Y.; Jeon, J.Y.; Hwang, J.E.; Kim, J.Y. Evaluation of the Effects of Euglena gracilis on Enhancing Immune Responses in RAW264.7 Cells and a Cyclophosphamide-Induced Mouse Model. J. Microbiol. Biotechnol. 2023, 33, 493–499. [Google Scholar] [CrossRef]
- Lee, G.R. Molecular Mechanisms of T Helper Cell Differentiation and Functional Specialization. Immune Netw. 2023, 23, e4. [Google Scholar] [CrossRef]
- Bandilla, K.K.; McDuffie, F.C.; Gleich, G.J. Immunoglobulin classes of antibodies produced in the primary and secondary responses in man. Clin. Exp. Immunol. 1969, 5, 627–641. [Google Scholar]
- de Carvalho, R.H.; Callegari, M.A.; Dias, C.P.; Kirwan, S.; da Costa, M.C.R.; da Silva, C.A. Euglena gracilis β-Glucans (1,3): Enriching Colostrum of Sow for Enhanced Piglet Immunity. Animals 2023, 13, 3490. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Gordon, S. A new receptor for β-glucans. Nature 2001, 413, 36–37. [Google Scholar] [CrossRef] [PubMed]
- Barsanti, L.; Gualtieri, P. Paramylon, a Potent Immunomodulator from WZSL Mutant of Euglena gracilis. Molecules 2019, 24, 3114. [Google Scholar] [CrossRef] [PubMed]
- Herre, J.; Gordon, S.; Brown, G.D. Dectin-1 and its role in the recognition of β-glucans by macrophages. Mol. Immunol. 2004, 40, 869–876. [Google Scholar] [CrossRef]
- Yang, H.; Choi, K.; Kim, K.J.; Park, S.Y.; Jeon, J.Y.; Kim, B.G.; Kim, J.Y. Immunoenhancing Effects of Euglena gracilis on a Cyclophosphamide-Induced Immunosuppressive Mouse Model. J. Microbiol. Biotechnol. 2022, 32, 228–237. [Google Scholar] [CrossRef]

| Hematological Index 1 | Group NC | CP | RG (10 mg/kg) | EP (100 mg/kg) | EP (300 mg/kg) | EP (600 mg/kg) |
|---|---|---|---|---|---|---|
| WBC (×103 cells/μL) | 3.40 ± 0.68 a | 1.68 ± 0.30 b | 1.48 ± 0.31 b | 1.12 ± 0.30 b | 1.34 ± 0.27 b | 1.52 ± 0.47 b |
| NEU (%) | 14.95 ± 2.21 a | 41.40 ± 17.51 b | 37.29 ± 8.35 b | 31.99 ± 7.32 b | 33.10 ± 6.41 b | 36.53 ± 10.23 b |
| LYM (%) | 75.90 ± 2.18 a | 37.20 ± 15.88 b | 53.55 ± 3.64 c | 56.75 ± 7.83 c | 54.00 ± 4.96 c | 55.23 ± 5.54 c |
| MONO (%) | 5.97 ± 0.37 a | 11.17 ± 5.44 a | 10.08 ± 2.16 a | 10.36 ± 5.17 a | 7.84 ± 3.30 a | 7.96 ± 1.97 a |
| EOS (%) | 3.26 ± 1.65 a | 5.43 ± 5.23 a | 4.00 ± 3.16 a | 6.28 ± 2.63 a | 6.27 ± 4.77 a | 4.70 ± 3.54 a |
| BASO (%) | 0.10 ± 0.00 a | 0.20 ± 0.15 a | 0.14 ± 0.07 a | 0.11 ± 0.04 a | 0.13 ± 0.05 a | 0.14 ± 0.07 a |
| RBC (×106 cells/μL) | 8.59 ± 0.49 a | 7.84 ± 0.44 b | 8.09 ± 0.48 ab | 8.17 ± 0.46 ab | 8.26 ± 0.46 ab | 8.27 ± 0.40 ab |
| HGB (g/dL) | 12.83 ± 0.80 a | 11.41 ± 0.85 a | 11.76 ± 0.94 a | 12.24 ± 0.77 a | 12.04 ± 1.01 a | 12.03 ± 0.63 a |
| HCT (%) | 40.78 ± 3.32 a | 36.39 ± 3.19 a | 38.36 ± 1.80 a | 39.15 ± 1.10 a | 38.83 ± 1.47 a | 38.56 ± 1.21 a |
| MCV (fL) | 47.46 ± 1.87 a | 46.44 ± 3.28 a | 47.55 ± 2.40 a | 48.05 ± 2.91 a | 47.08 ± 1.76 a | 46.78 ± 1.21 a |
| MCH (pg) | 14.96 ± 0.1 a 4 | 14.56 ± 0.78 a | 14.55 ± 0.42 a | 15.00 ± 0.33 a | 14.59 ± 0.48 a | 14.56 ± 0.51 a |
| MCHC (g/dL) | 31.56 ± 1.13 a | 31.43 ± 1.21 a | 30.64 ± 1.62 a | 31.29 ± 1.61 a | 31.04 ± 1.77 a | 31.18 ± 1.41 a |
| RDW (%) | 15.74 ± 0.63 a | 20.13 ± 3.14 b | 19.05 ± 1.43 ab | 19.03 ± 1.61 ab | 17.48 ± 2.87 ab | 17.72 ± 2.41 ab |
| MPV (fL) | 5.48 ± 0.31 a | 5.66 ± 0.59 a | 5.74 ± 0.57 a | 5.83 ± 0.51 a | 5.65 ± 0.44 a | 5.61 ± 0.49 a |
| PLT (×103 cells/μL) | 734.5 ± 169.08 a | 1013.85 ± 177.21 b | 993.62 ± 161.95 bc | 845.12 ± 156.75 abc | 970.25 ± 185.14 bc | 1073.12 ± 194.64 bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.H.; Seong, J.-Y.; Kang, H.; Cho, H. Euglena gracilis Enhances Innate and Adaptive Immunity through Specific Expression of Dectin-1 in CP-Induced Immunosuppressed Mice. Nutrients 2024, 16, 3158. https://doi.org/10.3390/nu16183158
Lee HH, Seong J-Y, Kang H, Cho H. Euglena gracilis Enhances Innate and Adaptive Immunity through Specific Expression of Dectin-1 in CP-Induced Immunosuppressed Mice. Nutrients. 2024; 16(18):3158. https://doi.org/10.3390/nu16183158
Chicago/Turabian StyleLee, Hwan Hee, Ji-Yeon Seong, Hyojeung Kang, and Hyosun Cho. 2024. "Euglena gracilis Enhances Innate and Adaptive Immunity through Specific Expression of Dectin-1 in CP-Induced Immunosuppressed Mice" Nutrients 16, no. 18: 3158. https://doi.org/10.3390/nu16183158
APA StyleLee, H. H., Seong, J.-Y., Kang, H., & Cho, H. (2024). Euglena gracilis Enhances Innate and Adaptive Immunity through Specific Expression of Dectin-1 in CP-Induced Immunosuppressed Mice. Nutrients, 16(18), 3158. https://doi.org/10.3390/nu16183158






