Supplementation with Folic Acid or 5-Methyltetrahydrofolate and Prevention of Neural Tube Defects: An Evidence-Based Narrative Review
Abstract
1. Introduction
| Organisation | EFSA [15,16] | WHO [6,17] | IOM [18,19] | CDC [8] | Moreiras et al. [9] | SEGO [10] |
|---|---|---|---|---|---|---|
| Women of reproductive age | 330 DFE | 400 DFE | 400 DFE | 400 DFE | 400 DFE | No specific recommendations |
| Women planning pregnancy | FA supplementation: 400 ** | FA supplementation: 400 | FA supplementation: 400 | FA supplementation: 400 | No specific recommendations | FA supplementation: 400 * |
| Pregnant women | FA supplementation: 400 | FA supplementation: 400 | FA supplementation: 400 | FA supplementation: 400 | 600 DFE | FA supplementation: 400 * |
| Lactating women | 500 DFE | 500 DFE | 500 DFE | 500 DFE | 500 DFE | FA supplementation: 400 |
2. Objective
3. Materials and Methods
3.1. Search Methods
3.2. Selection Criteria and Eligibility
4. Results and Discussion
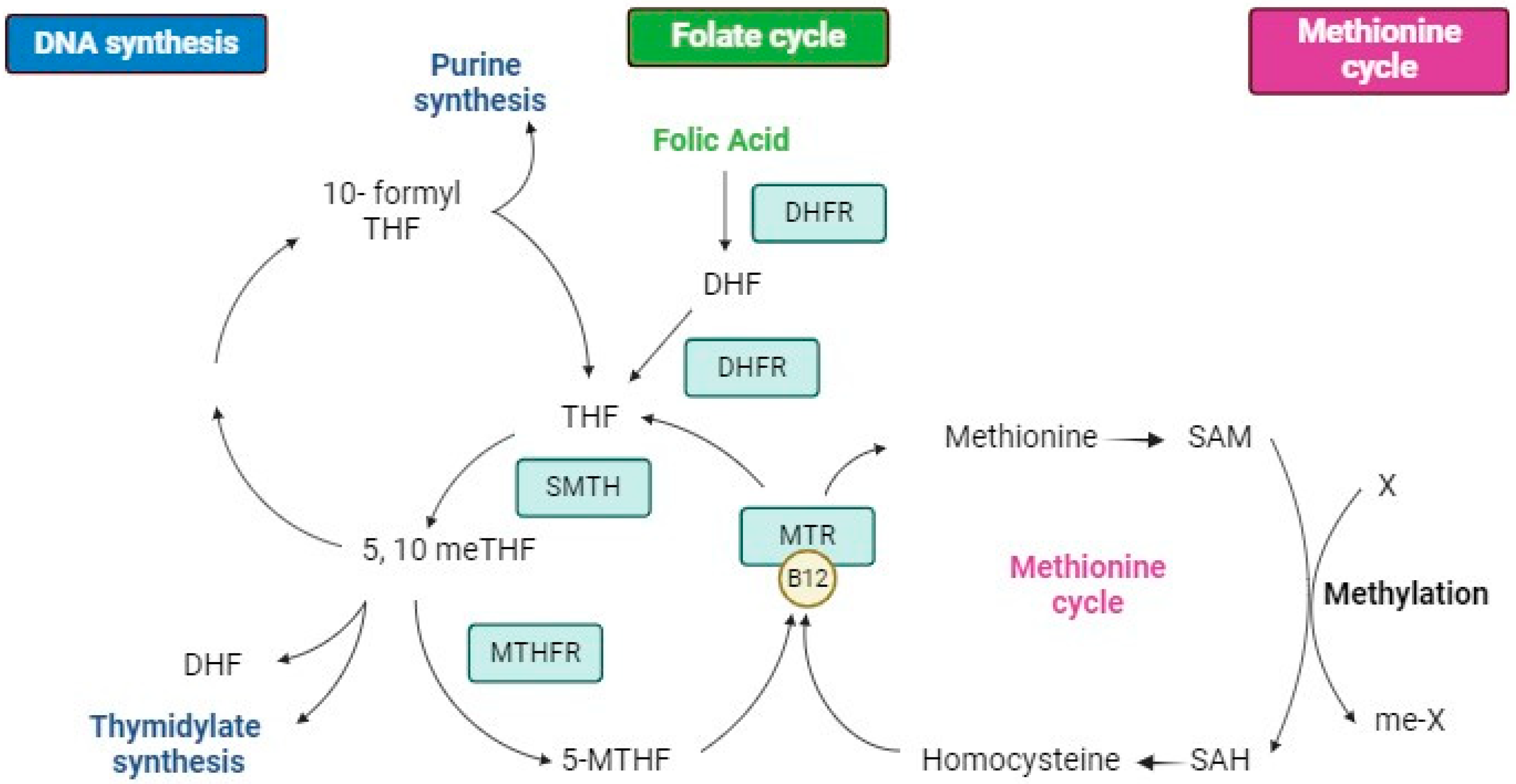
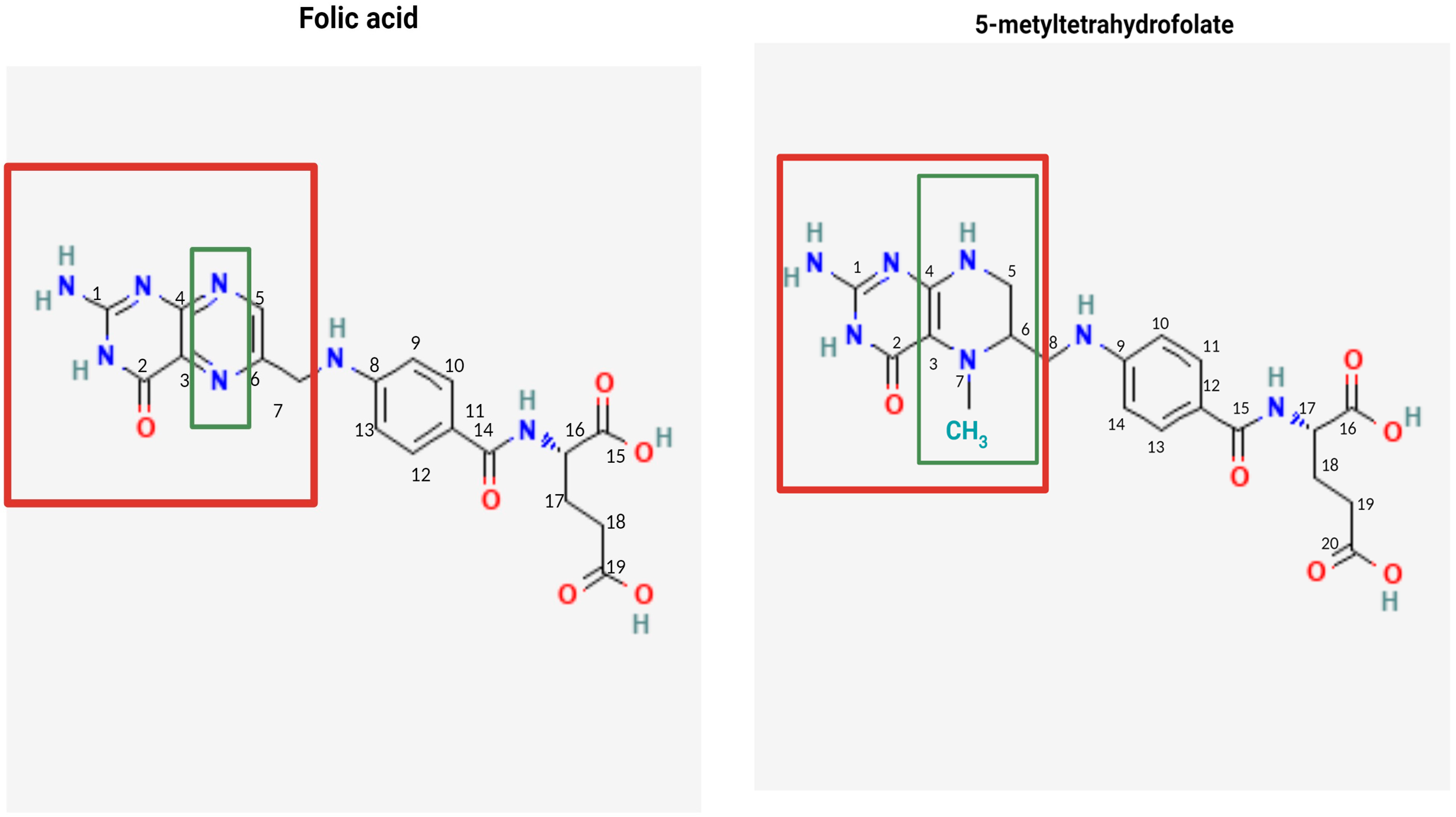
4.1. Structure, Metabolism, and Bioavailability of Folates
4.2. Bioavailability of FA and 5-MTHF
4.2.1. Pharmacokinetic Studies
4.2.2. Studies on Erythrocyte Folate Levels
4.2.3. Effect on Hcy Levels
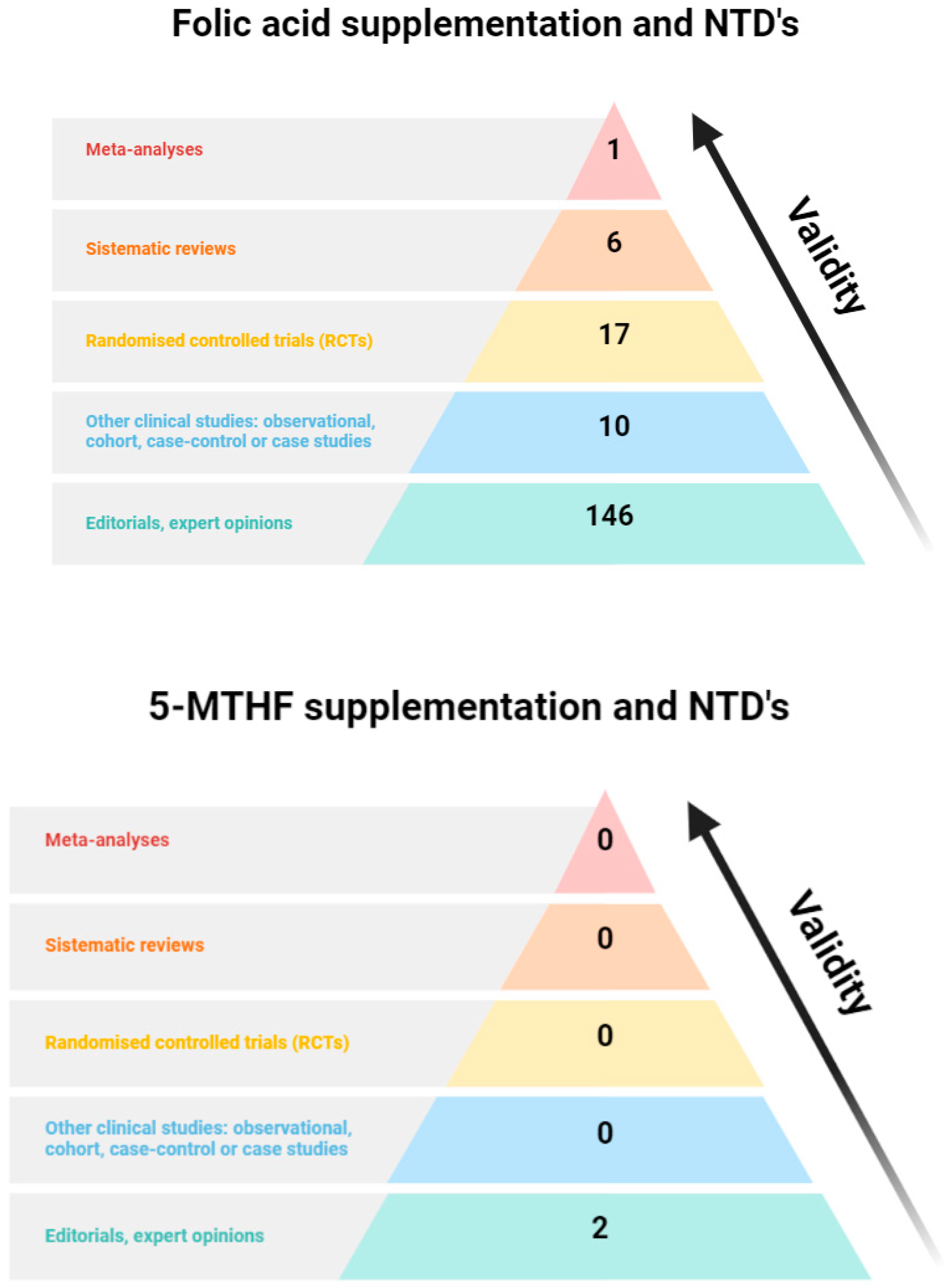
4.3. Clinical Evidence of Dietary Folates, 5-MTHF, and FA
4.4. Only FA Has Been Clinically Proven to Be Effective in Preventing NTDs
Far beyond NTDs: Clinical Evidence of FA on Other Maternal and Fetal Outcomes
4.5. Effect of Genetic Background on the Effectiveness of FA
4.6. Safety of Dietary Folates, 5-MTHF, and FA
Excessive FA Intake and Masking Vitamin-B12 Deficiency
4.7. Unmetabolised FA
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Crider, K.S.; Qi, Y.P.; Yeung, L.F.; Mai, C.T.; Zauche, L.H.; Wang, A.; Daniels, K.; Williams, J.L. Folic Acid and the Prevention of Birth Defects: 30 Years of Opportunity and Controversies. Annu. Rev. Nutr. 2022, 42, 423–452. [Google Scholar] [CrossRef]
- Blakley, R. The Biochemistry of Folic Acid and Related Pteridines; North-Holland Publishing Company: Amsterdam, The Netherlands, 1969; Volume 13. [Google Scholar]
- Obeid, R.; Oexle, K.; Rißmann, A.; Pietrzik, K.; Koletzko, B. Folate status and health: Challenges and opportunities. J. Perinat. Med. 2016, 44, 261–268. [Google Scholar] [CrossRef][Green Version]
- Group, M.V.S.R. Prevention of neural tube defects: Results of the Medical Research Council Vitamin Study. Lancet 1991, 338, 131–137. [Google Scholar]
- Authority, E.F.S. Dietary Reference Values for Nutrients Summary Report; Wiley Online Library: Hoboken, NJ, USA, 2017; ISSN 2397-8325. [Google Scholar]
- World Health Organization. Vitamin and Mineral Requirements in Human Nutrition, 2nd ed.; World Health Organization: Geneva, Switzerland, 2005; p. 341. [Google Scholar]
- Nutrition Board; Subcommittee on Upper Reference Levels of Nutrients; Standing Committee on the Scientific Evaluation of Dietary Reference Intakes its Panel on Folate, Other B Vitamins, and Choline. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. 2000. Available online: https://nap.nationalacademies.org/catalog/6015/dietary-reference-intakes-for-thiamin-riboflavin-niacin-vitamin-b6-folate-vitamin-b12-pantothenic-acid-biotin-and-choline (accessed on 24 January 2024).
- CDC MTHFR Gene Variant and Folic Acid Facts. 2024. Available online: https://www.cdc.gov/folic-acid/data-research/mthfr/index.html (accessed on 16 January 2024).
- Moreiras, O.; Carbajal, A.; Cabrera, L.; Cuadrado, C. Tablas de Composición de Alimentos. Guía de Prácticas, 20th ed.; Ediciones Pirámide (Grupo Anaya, SA): Madrid, Spain, 2022. [Google Scholar]
- SEGO. Control prenatal del embarazo normal. Prog. Obs. Ginecol. 2018, 61, 510–527. [Google Scholar]
- López Rodriguez, M.; Sánchez Méndez, J.I.; Sánchez Martínez, M.C.; Calderay Domínguez, M. Suplementos en embarazadas: Controversias, evidencias y recomendaciones. Inf. Ter. Sist. Nac. Salud 2010, 34, 117–128. [Google Scholar]
- Partearroyo, T.; Samaniego-Vaesken, M.d.L.; Ruiz, E.; Olza, J.; Aranceta-Bartrina, J.; Gil, Á.; González-Gross, M.; Ortega, R.M.; Serra-Majem, L.; Varela-Moreiras, G. Dietary sources and intakes of folates and vitamin B12 in the Spanish population: Findings from the ANIBES study. PLoS ONE 2017, 12, e0189230. [Google Scholar] [CrossRef]
- Brody, T.; Shane, B. Folic Acid. In Handbook of Vitamins, 3rd ed.; Rucker, R.S.J., McCormick, B., Maclin, J., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2001; pp. 427–462. [Google Scholar]
- Brouwer, I.A.; Steegers-Theunissen, R.P.M.; West, C.E.; van Dusseldorp, M. Bioavailability and bioefficacy of folate and folic acid in man. Nutr. Res. Rev. 2001, 14, 267–294. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products NaAN. Scientific Opinion on the substantiation of a health claim related toincreasing maternal folate status by supplemental folate intake and reducedrisk of neural tube defects pursuant to Article 14 of Regulation (EC) No1924/2006. EFSA J. 2013, 11, 3328. [Google Scholar]
- EFSA Panel on Dietetic Products NaAN. Scientific Opinion on Dietary Reference Values for folate. EFSA J. 2014, 12, 3893. [Google Scholar]
- World Health Organization. Periconceptional Folic Acid Supplementation to Prevent Neural Tube Defects. 2023. Available online: https://www.who.int/tools/elena/interventions/folate-periconceptional (accessed on 25 January 2024).
- Cho, E.; Zhang, X.; Townsend, M.K.; Selhub, J.; Paul, L.; Rosner, B.; Fuchs, C.S.; Willett, W.C.; Giovannucci, E.L. Unmetabolized folic acid in prediagnostic plasma and the risk for colorectal cancer. J. Natl. Cancer Inst. 2015, 107, djv260. [Google Scholar] [CrossRef][Green Version]
- Institute of Medicine. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B(6), Folate, Vitamin B(12), Pantothenic Acid, Biotin, and Choline; The National Academies Press: Washington, DC, USA, 1998. [Google Scholar] [CrossRef]
- Eichholzer, M.; Tönz, O.; Zimmermann, R. Folic acid: A public-health challenge. Lancet 2006, 367, 1352–1361. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Folic Acid Recommendations. Available online: https://www.cdc.gov/folic-acid/about/intake-and-sources.html#:~:text=contain%20folic%20acid.-,Overview,mcg)%20of%20folic%20acid%20daily (accessed on 6 February 2024).
- Williams, J.; Mai, C.T.; Mulinare, J.; Isenburg, J.; Flood, T.J.; Ethen, M.; Frohnert, B.; Kirby, R.S. Updated estimates of neural tube defects prevented by mandatory folic acid fortification—United States, 1995–2011. Morb. Mortal. Wkly. Rep. 2015, 64, 1. [Google Scholar]
- US Preventive Services Task Force. Folic Acid Supplementation to Prevent Neural Tube Defects: US Preventive Services Task Force Reaffirmation Recommendation Statement. JAMA 2023, 330, 454–459. [Google Scholar] [CrossRef]
- Cochrane, K.M.; Devlin, A.M.; Elango, R.; Hutcheon, J.A.; Karakochuk, C.D.; Mayer, C. Supplementation with (6S)-5-methyltetrahydrofolic acid appears as effective as folic acid in maintaining maternal folate status while reducing unmetabolised folic acid in maternal plasma: A randomised trial of pregnant women in Canada. Br. J. Nutr. 2024, 131, 92–102. [Google Scholar] [CrossRef]
- López-Camelo, J.S.; Orioli, I.M.; Dutra, M.d.G.; Nazer-Herrera, J.; Rivera, N.; Ojeda, M.E.; Canessa, A.; Wettig, E.; Fontannaz, A.M.; Mellado, C. Reduction of birth prevalence rates of neural tube defects after folic acid fortification in Chile. Am. J. Med. Genet. Part A 2005, 135, 120–125. [Google Scholar] [CrossRef]
- Maruvada, P.; Stover, P.J.; Mason, J.B.; Bailey, R.L.; Davis, C.D.; Field, M.S.; Finnell, R.H.; Garza, C.; Green, R.; Gueant, J.-L. Knowledge gaps in understanding the metabolic and clinical effects of excess folates/folic acid: A summary, and perspectives, from an NIH workshop. Am. J. Clin. Nutr. 2020, 112, 1390–1403. [Google Scholar] [CrossRef]
- Czeizel, A.E.; Dudás, I.; Vereczkey, A.; Bánhidy, F. Folate Deficiency and Folic Acid Supplementation: The Prevention of Neural-Tube Defects and Congenital Heart Defects. Nutrients 2013, 5, 4760–4775. [Google Scholar] [CrossRef]
- Viswanathan, M.; Urrutia, R.P.; Hudson, K.N.; Middleton, J.C.; Kahwati, L.C. Folic acid supplementation to prevent neural tube defects: Updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2023, 330, 460–466. [Google Scholar] [CrossRef]
- Hodgetts, V.; Morris, R.; Francis, A.; Gardosi, J.; Ismail, K. Effectiveness of folic acid supplementation in pregnancy on reducing the risk of small-for-gestational age neonates: A population study, systematic review and meta-analysis. BJOG Int. J. Obstet. Gynaecol. 2015, 122, 478–490. [Google Scholar] [CrossRef]
- Zhou, Q.; Dong, G.; Wang, Q.; Shen, H.; Zhang, Y.; Zhang, S.; Chen, J.; Li, X. Preconception folic acid supplementation for the prevention of birth defects: A prospective, population-based cohort study in mainland China. BMC Pregnancy Childbirth 2024, 24, 114. [Google Scholar] [CrossRef]
- Czeizel, A.E.; Dudas, I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N. Engl. J. Med. 1992, 327, 1832–1835. [Google Scholar] [CrossRef]
- Picciano, M.F. Pregnancy and Lactation: Physiological Adjustments, Nutritional Requirements and the Role of Dietary Supplements. J. Nutr. 2003, 133, 1997S–2002S. [Google Scholar] [CrossRef]
- Obeid, R.; Holzgreve, W.; Pietrzik, K. Is 5-methyltetrahydrofolate an alternative to folic acid for the prevention of neural tube defects? J. Perinat. Med. 2013, 41, 469–483. [Google Scholar] [CrossRef]
- Additives, E.P.o.F.; Food, N.S.a.t. Scientific Opinion on (6S)-5-methyltetrahydrofolic acid, glucosamine salt as a source of folate added for nutritional purposes to food supplements. EFSA J. 2013, 11, 3358. [Google Scholar]
- Froese, D.S.; Fowler, B.; Baumgartner, M.R. Vitamin B12, folate, and the methionine remethylation cycle—Biochemistry, pathways, and regulation. J. Inherit. Metab. Dis. 2019, 42, 673–685. [Google Scholar] [CrossRef]
- Zheng, Y.; Cantley, L.C. Toward a better understanding of folate metabolism in health and disease. J. Exp. Med. 2019, 216, 253–266. [Google Scholar] [CrossRef]
- Scaglione, F.; Panzavolta, G. Folate, folic acid and 5-methyltetrahydrofolate are not the same thing. Xenobiotica 2014, 44, 480–488. [Google Scholar] [CrossRef]
- Maynard, A.G.; Petrova, B.; Kanarek, N. Notes from the 2022 Folate, Vitamin B12, and One-Carbon Metabolism Conference. Metabolites 2023, 13, 486. [Google Scholar] [CrossRef]
- Blom, H.J.; Smulders, Y. Overview of homocysteine and folate metabolism. With special references to cardiovascular disease and neural tube defects. J. Inherit. Metab. Dis. 2011, 34, 75–81. [Google Scholar] [CrossRef]
- Ismail, S.; Eljazzar, S.; Ganji, V. Intended and Unintended Benefits of Folic Acid Fortification—A Narrative Review. Foods 2023, 12, 1612. [Google Scholar] [CrossRef] [PubMed]
- Shaw, E.; Baugh, C.M.; Krumdieck, C.L. The chemical degradation of folic acid. Photolysis of 2,4,7-trihydroxypteridine. J. Biol. Chem. 1966, 241, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Vora, A.; Riga, A.; Dollimore, D.; Kenneth, A. Thermal Stability of Folic Acid in the Solid-State. J. Therm. Anal. Calorim. 2004, 75, 709–717. [Google Scholar] [CrossRef]
- Yang, Y.; Li, J.; Gu, L.; Chang, C.; Su, Y.; Liu, Y.; Yang, Y.; Dong, S. Degradation of 5-methyltetrahydrofolate in model and egg yolk systems and strategies for its stabilization. J. Food Sci. Technol. 2021, 58, 3473–3481. [Google Scholar] [CrossRef]
- Ferrazzi, E.; Tiso, G.; Di Martino, D. Folic acid versus 5-methyl tetrahydrofolate supplementation in pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 253, 312–319. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 135398658, Folic Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Folic-Acid (accessed on 15 June 2024).
- Bailey, L.B.; Gregory III, J.F. Folate metabolism and requirements. J. Nutr. 1999, 129, 779–782. [Google Scholar] [CrossRef]
- Bhandari, S.D.; Gregory III, J.F. Folic acid, 5-methyl-tetrahydrofolate and 5-formyl-tetrahydrofolate exhibit equivalent intestinal absorption, metabolism and in vivo kinetics in rats. J. Nutr. 1992, 122, 1847–1854. [Google Scholar] [CrossRef]
- Said, H.M.; Ma, T.Y.; Ortiz, A.; Tapia, A.; Valerio, C.K. Intracellular regulation of intestinal folate uptake: Studies with cultured IEC-6 epithelial cells. Am. J. Physiol.-Cell Physiol. 1997, 272, C729–C736. [Google Scholar] [CrossRef]
- Venn, B.J.; Green, T.J.; Moser, R.; Mann, J.I. Comparison of the effect of low-dose supplementation with L-5-methyltetrahydrofolate or folic acid on plasma homocysteine: A randomized placebo-controlled study. Am. J. Clin. Nutr. 2003, 77, 658–662. [Google Scholar] [CrossRef]
- Pentieva, K.; McNulty, H.; Reichert, R.; Ward, M.; Strain, J.J.; McKillop, D.J.; Connolly, E.; McPartlin, J.M.; Molloy, A.; Krämer, K.; et al. The Short-Term Bioavailabilities of [6S]-5-Methyltetrahydrofolate and Folic Acid Are Equivalent in Men. J. Nutr. 2004, 134, 580–585. [Google Scholar] [PubMed]
- De Meer, K.; Smulders, Y.; Dainty, J.; Smith, D.; Kok, R.; Stehouwer, C.; Finglas, P.; Jakobs, C. [6S] 5-methyltetrahydrofolate or folic acid supplementation and absorption and initial elimination of folate in young and middle-aged adults. Eur. J. Clin. Nutr. 2005, 59, 1409–1416. [Google Scholar] [PubMed]
- Öhrvik, V.E.; Büttner, B.E.; Rychlik, M.; Lundin, E.; Witthöft, C.M. Folate bioavailability from breads and a meal assessed with a human stable-isotope area under the curve and ileostomy model. Am. J. Clin. Nutr. 2010, 92, 532–538. [Google Scholar] [CrossRef]
- Bailey, S.W.; Ayling, J.E. The pharmacokinetic advantage of 5-methyltetrahydrofolate for minimization of the risk for birth defects. Sci. Rep. 2018, 8, 4096. [Google Scholar] [CrossRef]
- Daly, L.E.; Kirke, P.N.; Molloy, A.; Weir, D.G.; Scott, J.M. Folate levels and neural tube defects: Implications for prevention. JAMA 1995, 274, 1698–1702. [Google Scholar] [CrossRef]
- Lamers, Y.; Prinz-Langenohl, R.; Bramswig, S.; Pietrzik, K. Red blood cell folate concentrations increase more after supplementation with [6S]-5-methyltetrahydrofolate than with folic acid in women of childbearing age. Am. J. Clin. Nutr. 2006, 84, 156–161. [Google Scholar] [CrossRef]
- Houghton, L.A.; Sherwood, K.L.; Pawlosky, R.; Ito, S.; O’Connor, D.L. [6S]-5-Methyltetrahydrofolate is at least as effective as folic acid in preventing a decline in blood folate concentrations during lactation. Am. J. Clin. Nutr. 2006, 83, 842–850. [Google Scholar]
- Venn, B.J.; Green, T.J.; Moser, R.; McKenzie, J.E.; Skeaff, C.M.; Mann, J. Increases in Blood Folate Indices Are Similar in Women of Childbearing Age Supplemented with [6S]-5-Methyltetrahydrofolate and Folic Acid. J. Nutr. 2002, 132, 3353–3355. [Google Scholar]
- Selhub, J. Homocysteine metabolism. Annu. Rev. Nutr. 1999, 19, 217–246. [Google Scholar] [CrossRef]
- Ho, P.I.; Ortiz, D.; Rogers, E.; Shea, T.B. Multiple aspects of homocysteine neurotoxicity: Glutamate excitotoxicity, kinase hyperactivation and DNA damage. J. Neurosci. Res. 2002, 70, 694–702. [Google Scholar] [CrossRef]
- McNulty, B.; McNulty, H.; Marshall, B.; Ward, M.; Molloy, A.M.; Scott, J.M.; Dornan, J.; Pentieva, K. Impact of continuing folic acid after the first trimester of pregnancy: Findings of a randomized trial of Folic Acid Supplementation in the Second and Third Trimesters. Am. J. Clin. Nutr. 2013, 98, 92–98. [Google Scholar] [CrossRef]
- Green, T.J.; Skeaff, C.M.; Rockell, J.E.; Venn, B.J. Folic acid fortified milk increases blood folate and lowers homocysteine concentration in women of childbearing age. Asia Pac. J. Clin. Nutr. 2005, 14, 173–178. [Google Scholar]
- Holmes, V.A.; Wallace, J.M.; Alexander, H.D.; Gilmore, W.S.; Bradbury, I.; Ward, M.; Scott, J.M.; McFaul, P.; McNulty, H. Homocysteine is lower in the third trimester of pregnancy in women with enhanced folate status from continued folic acid supplementation. Clin. Chem. 2005, 51, 629–634. [Google Scholar] [CrossRef]
- Ellison, J.; Clark, P.; Walker, I.D.; Greer, I.A. Effect of supplementation with folic acid throughout pregnancy on plasma homocysteine concentration. Thromb. Res. 2004, 114, 25–27. [Google Scholar] [CrossRef]
- Hekmati Azar Mehrabani, Z.; Ghorbanihaghjo, A.; Sayyah Melli, M.; Hamzeh-Mivehroud, M.; Fathi Maroufi, N.; Bargahi, N.; Bannazadeh Amirkhiz, M.; Rashtchizadeh, N. Effects of folic acid supplementation on serum homocysteine and lipoprotein (a) levels during pregnancy. Bioimpacts 2015, 5, 177–182. [Google Scholar] [CrossRef]
- Prinz-Langenohl, R.; Brämswig, S.; Tobolski, O.; Smulders, Y.; Smith, D.; Finglas, P.; Pietrzik, K. [6S]-5-methyltetrahydrofolate increases plasma folate more effectively than folic acid in women with the homozygous or wild-type 677C→T polymorphism of methylenetetrahydrofolate reductase. Br. J. Pharmacol. 2009, 158, 2014–2021. [Google Scholar]
- Clément, A.; Menezo, Y.; Cohen, M.; Cornet, D.; Clément, P. 5-Methyltetrahydrofolate reduces blood homocysteine level significantly in C677T methyltetrahydrofolate reductase single-nucleotide polymorphism carriers consulting for infertility. J. Gynecol. Obstet. Hum. Reprod. 2020, 49, 101622. [Google Scholar] [CrossRef]
- Lamers, Y.; Prinz-Langenohl, R.; Moser, R.; Pietrzik, K. Supplementation with [6S]-5-methyltetrahydrofolate or folic acid equally reduces plasma total homocysteine concentrations in healthy women. Am. J. Clin. Nutr. 2004, 79, 473–478. [Google Scholar]
- Zappacosta, B.; Mastroiacovo, P.; Persichilli, S.; Pounis, G.; Ruggeri, S.; Minucci, A.; Carnovale, E.; Andria, G.; Ricci, R.; Scala, I.; et al. Homocysteine lowering by folate-rich diet or pharmacological supplementations in subjects with moderate hyperhomocysteinemia. Nutrients 2013, 5, 1531–1543. [Google Scholar] [CrossRef]
- Padmanabhan, R. Etiology, pathogenesis and prevention of neural tube defects. Congenit. Anom. 2006, 46, 55–67. [Google Scholar] [CrossRef]
- Wallingford, J.B.; Niswander, L.A.; Shaw, G.M.; Finnell, R.H. The continuing challenge of understanding, preventing, and treating neural tube defects. Science 2013, 339, 1222002. [Google Scholar] [CrossRef]
- Ichi, S.; Costa, F.F.; Bischof, J.M.; Nakazaki, H.; Shen, Y.-W.; Boshnjaku, V.; Sharma, S.; Mania-Farnell, B.; McLone, D.G.; Tomita, T. Folic acid remodels chromatin on Hes1 and Neurog2 promoters during caudal neural tube development. J. Biol. Chem. 2010, 285, 36922–36932. [Google Scholar] [CrossRef]
- Adams, J.B.; Kirby, J.K.; Sorensen, J.C.; Pollard, E.L.; Audhya, T. Evidence based recommendations for an optimal prenatal supplement for women in the US: Vitamins and related nutrients. Matern. Health Neonatol. Perinatol. 2022, 8, 4. [Google Scholar] [CrossRef]
- De-Regil, L.M.; Peña-Rosas, J.P.; Fernández-Gaxiola, A.C.; Rayco-Solon, P. Effects and safety of periconceptional oral folate supplementation for preventing birth defects. Cochrane Database Syst. Rev. 2015, 2015, CD007950. [Google Scholar] [CrossRef]
- Bermejo Sanchez, E. Frecuencias de defectos congénitos al nacimiento en España y su comportamiento temporal y por comunidades autónomas. Causas de las variaciones de las frecuencias. Med. Fam. Semer. 2010, 36, 449–455. [Google Scholar] [CrossRef]
- CDC. Impact of Folic Acid Fortification. 2024. Available online: https://www.cdc.gov/folic-acid/php/communication-resources/impact-of-folic-acid.html (accessed on 15 May 2024).
- EFSA Panel on Dietetic Products NaAN. Scientific Opinion on the substantiation of health claims related to folate and blood formation (ID 79), homocysteine metabolism (ID 80), energy-yielding metabolism (ID 90), function of the immune system (ID 91), function of blood vessels (ID 94, 175, 192), cell division (ID 193), andmaternal tissue growth during pregnancy (ID 2882). EFSA J. 2009, 7, 1213. [Google Scholar]
- Lassi, Z.S.; Salam, R.A.; Haider, B.A.; Bhutta, Z.A. Folic acid supplementation during pregnancy for maternal health and pregnancy outcomes. Cochrane Database Syst. Rev. 2013, 2013, CD006896. [Google Scholar] [CrossRef]
- Ingrid Goh, Y.; Bollano, E.; Einarson, T.R.; Koren, G. Prenatal multivitamin supplementation and rates of congenital anomalies: A meta-analysis. J. Obs. Gynaecol. Can. 2006, 28, 680–689. [Google Scholar] [CrossRef]
- Gomes, F.; Askari, S.; Black, R.E.; Christian, P.; Dewey, K.G.; Mwangi, M.N.; Rana, Z.; Reed, S.; Shankar, A.H.; Smith, E.R.; et al. Antenatal multiple micronutrient supplements versus iron-folic acid supplements and birth outcomes: Analysis by gestational age assessment method. Matern. Child Nutr. 2023, 19, e13509. [Google Scholar] [CrossRef]
- Guo, H.; Mao, B.; Wang, M.; Liu, Q.; Yang, L.; Xie, Y.; Wang, Y.; He, X.; Cui, H.; Lin, X.; et al. Folic acid supplementation, dietary folate intake and risk of small for gestational age in China. Public Health Nutr. 2020, 23, 1965–1973. [Google Scholar] [CrossRef]
- Kaldygulova, L.; Ukybassova, T.; Aimagambetova, G.; Gaiday, A.; Tussupkaliyev, A. Biological Role of Folic Acid in Pregnancy and Possible Therapeutic Application for the Prevention of Preeclampsia. Biomedicines 2023, 11, 272. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; Zhang, J.; Guo, Y.; Shen, M.; Gaudet, L.; Janoudi, G.; Walker, M.; Wen, S.W. Effect of folic acid supplementation during pregnancy on gestational hypertension/preeclampsia: A systematic review and meta-analysis. Hypertens. Pregnancy 2016, 35, 447–460. [Google Scholar] [CrossRef] [PubMed]
- Caffrey, A.; McNulty, H.; Rollins, M.; Prasad, G.; Gaur, P.; Talcott, J.B.; Witton, C.; Cassidy, T.; Marshall, B.; Dornan, J.; et al. Effects of maternal folic acid supplementation during the second and third trimesters of pregnancy on neurocognitive development in the child: An 11-year follow-up from a randomised controlled trial. BMC Med. 2021, 19, 73. [Google Scholar] [CrossRef] [PubMed]
- McNulty, H.; Rollins, M.; Cassidy, T.; Caffrey, A.; Marshall, B.; Dornan, J.; McLaughlin, M.; McNulty, B.A.; Ward, M.; Strain, J.J.; et al. Effect of continued folic acid supplementation beyond the first trimester of pregnancy on cognitive performance in the child: A follow-up study from a randomized controlled trial (FASSTT Offspring Trial). BMC Med. 2019, 17, 196. [Google Scholar] [CrossRef] [PubMed]
- Saccone, G.; Sarno, L.; Roman, A.; Donadono, V.; Maruotti, G.M.; Martinelli, P. 5-Methyl-tetrahydrofolate in prevention of recurrent preeclampsia. J. Matern. Fetal Neonatal. Med. 2016, 29, 916–920. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Azaryah, H.; Verdejo-Roman, J.; Martin-Perez, C.; Garcia-Santos, J.A.; Martinez-Zaldivar, C.; Torres-Espinola, F.J.; Campos, D.; Koletzko, B.; Perez-Garcia, M.; Catena, A.; et al. Effects of Maternal Fish Oil and/or 5-Methyl-Tetrahydrofolate Supplementation during Pregnancy on Offspring Brain Resting-State at 10 Years Old: A Follow-Up Study from the NUHEAL Randomized Controlled Trial. Nutrients 2020, 12, 2701. [Google Scholar] [CrossRef]
- Molloy, A.M. Folate and homocysteine interrelationships including genetics of the relevant enzymes. Curr. Opin. Lipidol. 2004, 15, 49–57. [Google Scholar] [CrossRef]
- Hustad, S.; Midttun, Ø.; Schneede, J.; Vollset, S.E.; Grotmol, T.; Ueland, P.M. The methylenetetrahydrofolate reductase 677C→ T polymorphism as a modulator of a B vitamin network with major effects on homocysteine metabolism. Am. J. Hum. Genet. 2007, 80, 846–855. [Google Scholar] [CrossRef]
- Tsang, B.L.; Devine, O.J.; Cordero, A.M.; Marchetta, C.M.; Mulinare, J.; Mersereau, P.; Guo, J.; Qi, Y.P.; Berry, R.J.; Rosenthal, J.; et al. Assessing the association between the methylenetetrahydrofolate reductase (MTHFR) 677C>T polymorphism and blood folate concentrations: A systematic review and meta-analysis of trials and observational studies. Am. J. Clin. Nutr. 2015, 101, 1286–1294. [Google Scholar] [CrossRef]
- Hiraoka, M.; Kagawa, Y. Genetic polymorphisms and folate status. Congenit. Anom. 2017, 57, 142–149. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin No. 197: Inherited Thrombophilias in Pregnancy. Obstet. Gynaecol. 2018, 132, e18–e34. [Google Scholar] [CrossRef]
- Berry, R.J.; Li, Z.; Erickson, J.D.; Li, S.; Moore, C.A.; Wang, H.; Mulinare, J.; Zhao, P.; Wong, L.Y.; Gindler, J.; et al. Prevention of neural-tube defects with folic acid in China. China-U.S. Collaborative Project for Neural Tube Defect Prevention. N. Engl. J. Med. 1999, 341, 1485–1490. [Google Scholar] [CrossRef]
- Crider, K.S.; Zhu, J.-H.; Hao, L.; Yang, Q.-H.; Yang, T.P.; Gindler, J.; Maneval, D.R.; Quinlivan, E.P.; Li, Z.; Bailey, L.B. MTHFR 677C→ T genotype is associated with folate and homocysteine concentrations in a large, population-based, double-blind trial of folic acid supplementation. Am. J. Clin. Nutr. 2011, 93, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Hekmatdoost, A.; Vahid, F.; Yari, Z.; Sadeghi, M.; Eini-Zinab, H.; Lakpour, N.; Arefi, S. Methyltetrahydrofolate vs Folic Acid Supplementation in Idiopathic Recurrent Miscarriage with Respect to Methylenetetrahydrofolate Reductase C677T and A1298C Polymorphisms: A Randomized Controlled Trial. PLoS ONE 2015, 10, e0143569. [Google Scholar] [CrossRef] [PubMed]
- Selhub, J.; Rosenberg, I.H. Excessive folic acid intake and relation to adverse health outcome. Biochimie 2016, 126, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Boyles, A.L.; Yetley, E.A.; Thayer, K.A.; Coates, P.M. Safe use of high intakes of folic acid: Research challenges and paths forward. Nutr. Rev. 2016, 74, 469–474. [Google Scholar] [CrossRef]
- Spanish Agency of Medicines and Medical Devices. CIMA. Healthcare Professionals Browser. 2024. Available online: https://cima.aemps.es/cima/publico/buscadoravanzado.html (accessed on 15 May 2024).
- Folic Acid Safety, Interactions, and Health Outcomes. 15 May 2024. Available online: https://www.cdc.gov/folic-acid/about/safety.html#:~:text=Folic%20acid%20is%20safe%20and%20effective%20at%20recommended%20amounts&text=Each%20group%20has%20consistently%20concluded,been%20shown%20to%20cause%20harm (accessed on 18 July 2024).
- Scott, J.; Weir, D. The methyl folate trap: A physiological response in man to prevent methyl group deficiency in kwashiorkor (methionine deficiency) and an explanation for folic-acid-induced exacerbation of subacute combined degeneration in pernicious anaemia. Lancet 1981, 318, 337–340. [Google Scholar] [CrossRef]
- Reynolds, E. Vitamin B12, folic acid, and the nervous system. Lancet Neurol. 2006, 5, 949–960. [Google Scholar] [CrossRef]
- Hoffman, R.; Benz, E.J.; Silberstein, L.E.; Heslop, H.; Weitz, J.; Salama, M.E.; Abutalib, S.A. Hematology: Basic Principles and Practice; Elsevier Health Sciences: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Pfeiffer, C.M.; Sternberg, M.R.; Fazili, Z.; Yetley, E.A.; Lacher, D.A.; Bailey, R.L.; Johnson, C.L. Unmetabolized folic acid is detected in nearly all serum samples from US children, adolescents, and adults. J. Nutr. 2015, 145, 520–531. [Google Scholar] [CrossRef]
- Bailey, S.W.; Ayling, J.E. The extremely slow and variable activity of dihydrofolate reductase in human liver and its implications for high folic acid intake. Proc. Natl. Acad. Sci. USA 2009, 106, 15424–15429. [Google Scholar] [CrossRef]
- Menezo, Y.; Elder, K.; Clement, A.; Clement, P. Folic Acid, Folinic Acid, 5 Methyl TetraHydroFolate Supplementation for Mutations That Affect Epigenesis through the Folate and One-Carbon Cycles. Biomolecules 2022, 12, 197. [Google Scholar] [CrossRef]
- Menezo, Y.; Clement, P.; Elder, K. Are UMFA (un-metabolized folic acid) and endocrine disruptor chemicals (EDCs) co-responsible for sperm degradation? An epigenetic/methylation perspective. Andrologia 2022, 54, e14400. [Google Scholar] [CrossRef]
- Servy, E.J.; Jacquesson-Fournols, L.; Cohen, M.; Menezo, Y.J. MTHFR isoform carriers. 5-MTHF (5-methyl tetrahydrofolate) vs folic acid: A key to pregnancy outcome: A case series. J. Assist. Reprod. Genet. 2018, 35, 1431–1435. [Google Scholar] [CrossRef] [PubMed]
- Tam, C.; O’Connor, D.; Koren, G. Circulating unmetabolized folic acid: Relationship to folate status and effect of supplementation. Obstet. Gynecol. Int. 2010, 2012, 485179. [Google Scholar] [CrossRef] [PubMed]
- Troen, A.M.; Mitchell, B.; Sorensen, B.; Wener, M.H.; Johnston, A.; Wood, B.; Selhub, J.; McTiernan, A.; Yasui, Y.; Oral, E. Unmetabolized folic acid in plasma is associated with reduced natural killer cell cytotoxicity among postmenopausal women. J. Nutr. 2006, 136, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Paniz, C.; Bertinato, J.F.; Lucena, M.R.; De Carli, E.; da Silva Amorim, P.M.; Gomes, G.W.; Palchetti, C.Z.; Figueiredo, M.S.; Pfeiffer, C.M.; Fazili, Z. A daily dose of 5 mg folic acid for 90 days is associated with increased serum unmetabolized folic acid and reduced natural killer cell cytotoxicity in healthy Brazilian adults. J. Nutr. 2017, 147, 1677–1685. [Google Scholar] [CrossRef]
- Berry, R.J.; Bailey, L.; Mulinare, J.; Bower, C.; Dary, O. Fortification of flour with folic acid. Food Nutr. Bull. 2010, 31, S22–S35. [Google Scholar] [CrossRef]
- Gibson, T.M.; Weinstein, S.J.; Pfeiffer, R.M.; Hollenbeck, A.R.; Subar, A.F.; Schatzkin, A.; Mayne, S.T.; Stolzenberg-Solomon, R. Pre-and postfortification intake of folate and risk of colorectal cancer in a large prospective cohort study in the United States. Am. J. Clin. Nutr. 2011, 94, 1053–1062. [Google Scholar] [CrossRef]
- Oaks, B.M.; Dodd, K.W.; Meinhold, C.L.; Jiao, L.; Church, T.R.; Stolzenberg-Solomon, R.Z. Folate intake, post–folic acid grain fortification, and pancreatic cancer risk in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Am. J. Clin. Nutr. 2010, 91, 449–455. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samaniego-Vaesken, M.d.L.; Morais-Moreno, C.; Carretero-Krug, A.; Puga, A.M.; Montero-Bravo, A.M.; Partearroyo, T.; Gregorio, V.-M. Supplementation with Folic Acid or 5-Methyltetrahydrofolate and Prevention of Neural Tube Defects: An Evidence-Based Narrative Review. Nutrients 2024, 16, 3154. https://doi.org/10.3390/nu16183154
Samaniego-Vaesken MdL, Morais-Moreno C, Carretero-Krug A, Puga AM, Montero-Bravo AM, Partearroyo T, Gregorio V-M. Supplementation with Folic Acid or 5-Methyltetrahydrofolate and Prevention of Neural Tube Defects: An Evidence-Based Narrative Review. Nutrients. 2024; 16(18):3154. https://doi.org/10.3390/nu16183154
Chicago/Turabian StyleSamaniego-Vaesken, María de Lourdes, Carmen Morais-Moreno, Alejandra Carretero-Krug, Ana María Puga, Ana María Montero-Bravo, Teresa Partearroyo, and Varela-Moreiras Gregorio. 2024. "Supplementation with Folic Acid or 5-Methyltetrahydrofolate and Prevention of Neural Tube Defects: An Evidence-Based Narrative Review" Nutrients 16, no. 18: 3154. https://doi.org/10.3390/nu16183154
APA StyleSamaniego-Vaesken, M. d. L., Morais-Moreno, C., Carretero-Krug, A., Puga, A. M., Montero-Bravo, A. M., Partearroyo, T., & Gregorio, V.-M. (2024). Supplementation with Folic Acid or 5-Methyltetrahydrofolate and Prevention of Neural Tube Defects: An Evidence-Based Narrative Review. Nutrients, 16(18), 3154. https://doi.org/10.3390/nu16183154









