Mediterranean Diet Modulation of Neuroinflammation-Related Genes in Elderly Adults at High Cardiovascular Risk
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population Recruitment
2.2. Blood Chemistry Analysis
2.3. Cardiovascular and Lifestyle Factors
2.4. RNA Extraction, Reverse Transcription, and Gene Expression Analysis
2.5. Selection of Gene Targets
2.6. Reference Genes and Relative Quantification
2.7. Statistical Analyses
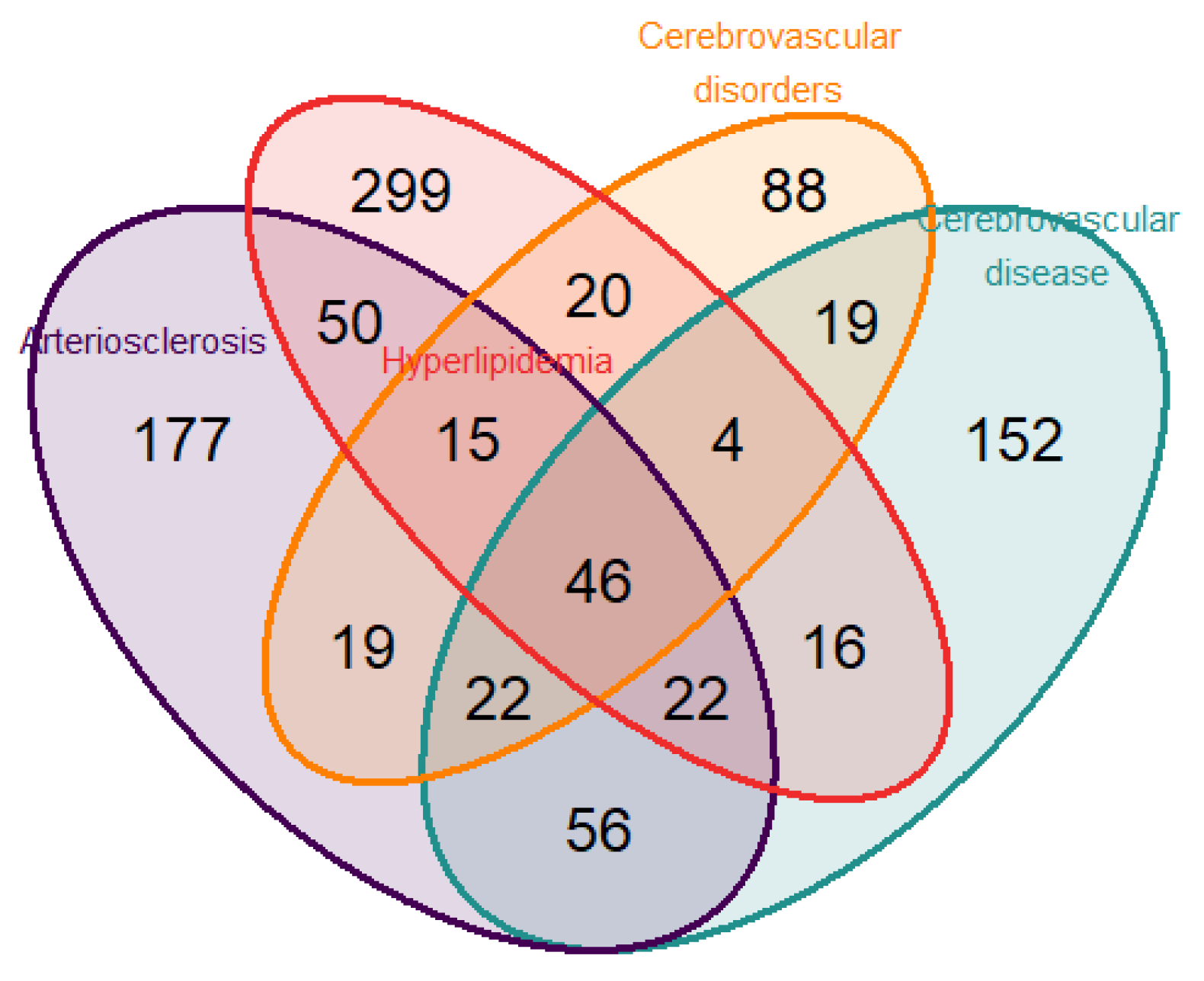
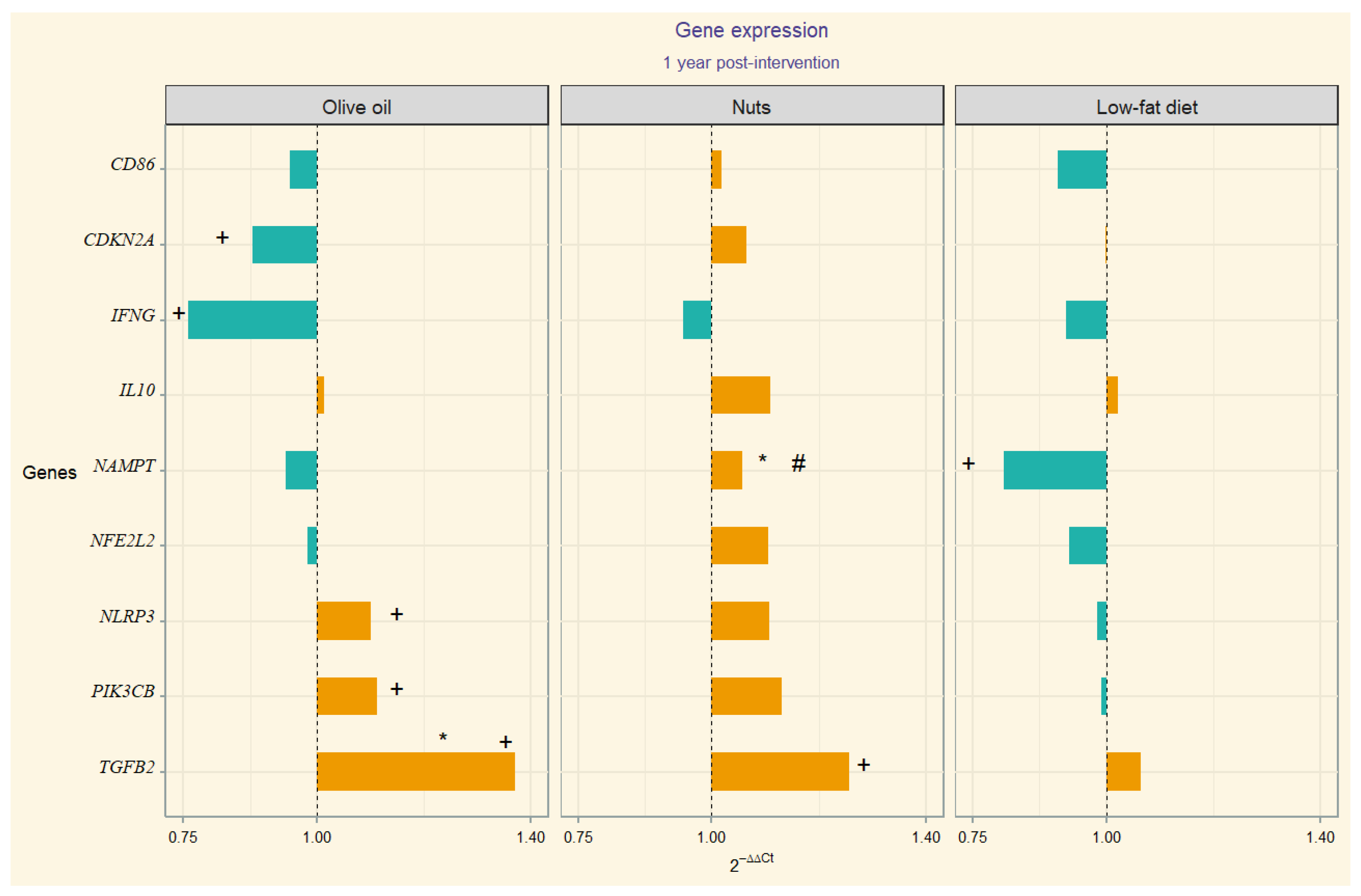
3. Results
Gene Expression
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, L.Y.; Ou, Y.N.; Yang, Y.X.; Wang, Z.T.; Tan, L.; Yu, J.T. Associations of cardiovascular risk factors and lifestyle behaviors with neurodegenerative disease: A Mendelian randomization study. Transl. Psychiatry 2023, 13, 267. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflammaging: An Evolutionary Perspective on Immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Van Dyken, P.; Lacoste, B. Impact of Metabolic Syndrome on Neuroinflammation and the Blood–Brain Barrier. Front. Neurosci. 2018, 12, 930. [Google Scholar] [CrossRef] [PubMed]
- Giridharan, V.V.; Generoso, J.S.; Collodel, A.; Sayana, P.; Barichello, T. The Impact of Systemic Inflammation on Neuroinflammation. In Translational Neuroimmunology; Elsevier: Amsterdam, The Netherlands, 2023; Volume 7, pp. 169–188. Available online: https://linkinghub.elsevier.com/retrieve/pii/B9780323858410000109 (accessed on 6 August 2024).
- Saeed, A.; Lopez, O.; Cohen, A.; Reis, S.E. Cardiovascular Disease and Alzheimer’s Disease: The Heart–Brain Axis. J. Am. Heart Assoc. 2023, 12, e030780. [Google Scholar] [CrossRef]
- Chen, W.W.; Zhang, X.; Huang, W.J. Role of neuroinflammation in neurodegenerative diseases (Review). Mol. Med. Rep. 2016, 13, 3391–3396. [Google Scholar] [CrossRef]
- Brain, J.; Greene, L.; Tang, E.Y.H.; Louise, J.; Salter, A.; Beach, S.; Turnbull, D.; Siervo, M.; Stephan, B.C.; Tully, P.J. Cardiovascular disease, associated risk factors, and risk of dementia: An umbrella review of meta-analyses. Front. Epidemiol. 2023, 3, 1095236. [Google Scholar] [CrossRef]
- DiSabato, D.J.; Quan, N.; Godbout, J.P. Neuroinflammation: The devil is in the details. J. Neurochem. 2016, 139, 136–153. [Google Scholar] [CrossRef]
- Lyman, M.; Lloyd, D.G.; Ji, X.; Vizcaychipi, M.P.; Ma, D. Neuroinflammation: The role and consequences. Neurosci. Res. 2014, 79, 1–12. [Google Scholar] [CrossRef]
- Traub, J.; Frey, A.; Störk, S. Chronic Neuroinflammation and Cognitive Decline in Patients with Cardiac Disease: Evidence, Relevance, and Therapeutic Implications. Life 2023, 13, 329. [Google Scholar] [CrossRef]
- Simon, E.; Obst, J.; Gomez-Nicola, D. The Evolving Dialogue of Microglia and Neurons in Alzheimer’s Disease: Microglia as Necessary Transducers of Pathology. Neuroscience 2019, 405, 24–34. [Google Scholar] [CrossRef]
- Kip, E.; Parr-Brownlie, L.C. Healthy lifestyles and wellbeing reduce neuroinflammation and prevent neurodegenerative and psychiatric disorders. Front. Neurosci. 2023, 17, 1092537. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.C.; Lockwood, A.H.; Sonawane, B.R. Neurodegenerative Diseases: An Overview of Environmental Risk Factors. Environ. Health Perspect. 2005, 113, 1250–1256. [Google Scholar] [CrossRef]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef] [PubMed]
- Petersson, S.D.; Philippou, E. Mediterranean diet, cognitive function, and dementia: A systematic review of the evidence. Adv. Nutr. 2016, 7, 889–904. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Torrisi, S.A.; Mogavero, M.P.; Currenti, W.; Castellano, S.; Godos, J.; Godos, J.; Ferri, R.; Galvano, F.; Leggio, G.M.; et al. Polyphenols and neuroprotection: Therapeutic implications for cognitive decline. Pharmacol. Ther. 2022, 232, 108013. [Google Scholar] [CrossRef] [PubMed]
- Novakovic, M.; Rout, A.; Kingsley, T.; Kirchoff, R.; Singh, A.; Verma, V.; Kant, R.; Chaudhary, R. Role of gut microbiota in cardiovascular diseases. World J. Cardiol. 2020, 12, 110–122. [Google Scholar] [CrossRef]
- Liu, G.; Yao, L.; Liu, J.; Jiang, Y.; Ma, G.; Chen, Z.; Zhao, B.; Li, K. Cardiovascular disease contributes to Alzheimer’s disease: Evidence from large-scale genome-wide association studies. Neurobiol. Aging 2014, 35, 786–792. [Google Scholar] [CrossRef]
- Lambert, J.C.; Ibrahim-Verbaas, C.A.; Harold, D.; Naj, A.C.; Sims, R.; Bellenguez, C.; Jun, G.; DeStefano, A.L.; Bis, J.C.; Beecham, G.W.; et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat. Genet. 2013, 45, 1452–1458. [Google Scholar] [CrossRef]
- ENGAGE Consortium; Surakka, I.; Horikoshi, M.; Mägi, R.; Sarin, A.-P.; Mahajan, A.; Lagou, V.; Marullo, L.; Ferreira, T.; Miraglio, B.; et al. The impact of low-frequency and rare variants on lipid levels. Nat. Genet. 2015, 47, 589–597. [Google Scholar] [CrossRef]
- Koh, W.; Pan, W.; Gawad, C.; Fan, H.C.; Kerchner, G.A.; Wyss-Coray, T.; Blumenfeld, Y.J.; El-Sayed, Y.Y.; Quake, S.R. Noninvasive in vivo monitoring of tissue-specific global gene expression in humans. Proc. Natl. Acad. Sci. USA 2014, 111, 7361–7366. [Google Scholar] [CrossRef]
- Toden, S.; Zhuang, J.; Acosta, A.D.; Karns, A.P.; Salathia, N.S.; Brewer, J.B.; Wilcock, D.M.; Aballi, J.; Nerenberg, M.; Quake, S.R.; et al. Noninvasive characterization of Alzheimer’s disease by circulating, cell-free messenger RNA next-generation sequencing. Sci. Adv. 2020, 6, eabb1654. [Google Scholar] [CrossRef]
- Martínez-González, M.Á.; Corella, D.; Salas-Salvadó, J.; Ros, E.; Covas, M.I.; Fiol, M.; Wärnberg, J.; Arós, F.; Ruíz-Gutiérrez, V.; Lamuela-Raventós, R.M.; et al. Cohort Profile: Design and methods of the PREDIMED study. Int. J. Epidemiol. 2012, 41, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Nigg, C.R.; Burbank, P.M.; Padula, C.; Dufresne, R.; Rossi, J.S.; Velicer, W.F.; Laforge, R.G.; Prochaska, J.O. Stages of Change across Ten Health Risk Behaviors for Older Adults; The Cerontologist: Washington, DC, USA, 1999; Volume 39, pp. 473–482. Available online: http://gerontologist.oxfordjournals.org/ (accessed on 17 June 2024).
- Schröder, H.; Fitó, M.; Estruch, R.; Martínez-González, M.A. A Short Screener Is Valid for Assessing Mediterranean Diet Adherence among OlderSpanish Men and Women. J. Nutr. 2011, 141, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Elosua, R.; Marrugat, J.; Molina, L.; Pons, S.; Pujol, E. Validation of the Minnesota leisure time physical activity questionnaire in Spanish men. The MARATHOM Investigators. Am. J. Epidemiol. 1994, 139, 1197–1209. [Google Scholar] [CrossRef] [PubMed]
- Elosua, R.; Garcia, M.; Aguilar, A.; Molina, L.; Covas, M.I.; Marrugat, J. Validation of the Minnesota Leisure Time Physical Activity Questionnaire in Spanish Women. Med. Sci. Sports Exerc. 2000, 32, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Piñero, J.; Ramírez-Anguita, J.M.; Saüch-Pitarch, J.; Ronzano, F.; Centeno, E.; Sanz, F.; I Furlong, L. The DisGeNET knowledge platform for disease genomics: 2019 update. Nucleic Acids Res. 2019, 48, D845–D855. [Google Scholar] [CrossRef]
- Baron, J.A.; Johnson, C.S.-B.; A Schor, M.; Olley, D.; Nickel, L.; Felix, V.; Munro, J.B.; Bello, S.M.; Bearer, C.; Lichenstein, R.; et al. The DO-KB Knowledgebase: A 20-year journey developing the disease open science ecosystem. Nucleic Acids Res. 2024, 52, D1305–D1314. [Google Scholar] [CrossRef]
- Gene Expression Assay Performance Guaranteed with the TaqMan ® Gene Expression Assays QPCR Guarantee Program; Life Technologies Corporation: Carlsbad, MA, USA, 2010.
- Gene Expression Assay Performance Guaranteed with the TaqMan Assays QPCR Guarantee Program; Themo Fisher Scientific: Waltham, MA, USA, 2015.
- Publish with Confidence Using TaqMan Assays; Thermo Fisher Scientific: Waltham, MA, USA, 2020.
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]
- Kozera, B.; Rapacz, M. Reference genes in real-time PCR. J. Appl. Genet. 2013, 54, 391–406. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.M.; Pinheiro, J.C.; Bates, D.M. Linear and nonlinear mixed-effects models. In Proceedings of the Conference on Applied Statistics in Agriculture, Manhattan, KS, USA, 26–28 April 1998. [Google Scholar]
- Lee, K.S.; Chung, J.H.; Lee, K.H.; Shin, M.-J.; Oh, B.H.; Hong, C.H. Bioplex analysis of plasma cytokines in Alzheimer’s disease and mild cognitive impairment. Immunol. Lett. 2008, 121, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.-N.; Niu, L.-D.; Wang, Y.-J.; Cao, X.-P.; Liu, Q.; Tan, L.; Zhang, C.; Yu, J.-T. Inflammatory markers in Alzheimer’s disease and mild cognitive impairment: A meta-analysis and systematic review of 170 studies. J. Neurol. Neurosurg. Psychiatry 2019, 90, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.S.P.; Liu, C.S.; Rau, A.; Lanctôt, K.L.; Köhler, C.A.; Pakosh, M.; Carvalho, A.F.; Herrmann, N. Peripheral inflammatory markers in Alzheimer’s disease: A systematic review and meta-analysis of 175 studies. J. Neurol. Neurosurg. Psychiatry 2017, 88, 876–882. [Google Scholar] [CrossRef] [PubMed]
- von Bernhardi, R.; Cornejo, F.; Parada, G.E.; Eugenín, J. Role of TGFβ signaling in the pathogenesis of Alzheimer’s disease. Front Cell Neurosci. 2015, 9, 426. [Google Scholar] [CrossRef]
- Kashima, R.; Hata, A. The role of TGF-β superfamily signaling in neurological disorders. Acta Biochim. Biophys. Sin. 2018, 50, 106–120. [Google Scholar] [CrossRef]
- Unsicker, K.; Krieglstein, K. TGF-betas and their roles in the regulation of neuron survival. Adv. Exp. Med. Biol. 2002, 513, 353–374. [Google Scholar]
- Dobolyi, A.; Vincze, C.; Pál, G.; Lovas, G. The Neuroprotective Functions of Transforming Growth Factor Beta Proteins. Int. J. Mol. Sci. 2012, 13, 8219–8258. [Google Scholar] [CrossRef]
- Mocali, A.; Cedrola, S.; Della Malva, N.; Bontempelli, M.; Mitidieri, V.; Bavazzano, A.; Comolli, R.; Paoletti, F.; La Porta, C. Increased plasma levels of soluble CD40, together with the decrease of TGFβ1, as possible differential markers of Alzheimer disease. Exp. Gerontol. 2004, 39, 1555–1561. [Google Scholar] [CrossRef]
- Perez-Grijalba, V.; Romero, J.; Pesini, P.; Sarasa, L.; Monleon, I.; San-Jose, I.; Arbizu, J.; Martinez-Lage, P.; Munuera, J.; Ruiz, A.; et al. Plasma Aβ42/40 Ratio Detects Early Stages of Alzheimer’s Disease and Correlates with CSF and Neuroimaging Biomarkers in the AB255 Study. J. Prev. Alzheimers Dis. 2018, 6, 34–41. [Google Scholar]
- Shaw, L.M.; Vanderstichele, H.; Knapik-Czajka, M.; Clark, C.M.; Aisen, P.S.; Petersen, R.C.; Blennow, K.; Soares, H.; Simon, A.; Lewczuk, P.; et al. Cerebrospinal fluid biomarker sig-nature in Alzheimer’s disease neuroimaging initiative subjects. Ann. Neurol. 2009, 65, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Fessel, J. Ineffective levels of transforming growth factors and their receptor account for old age being a risk factor for Alz-heimer’s disease. Alzheimers Dement. Transl. Res. Clin. Interv. 2019, 5, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.R.; Chai, Y.L.; Lee, J.H.; Howlett, D.; Attems, J.; Ballard, C.G.; Aarsland, D.; Francis, P.T.; Chen, C.P.; Lai, M.K.P. Increased Transforming Growth Factor β2 in the Neocortex of Alzheimer’s Disease and Dementia with Lewy Bodies is Correlated with Disease Severity and Soluble Aβ42 Load. J. Alz-heimers Dis. 2017, 56, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Flanders, K.C.; Lippa, C.F.; Smith, T.W.; Pollen, D.A.; Sporn, M.B. Altered expression of transforming growth factor-beta in Alzheimer’s disease. Neurology 1995, 45, 1561–1569. [Google Scholar] [CrossRef]
- Lippa, C.; Flanders, K.; Kim, E.; Croul, S. TGF-β receptors-I and -II immunoexpression in Alzheimer’s disease: A comparison with aging and progressive supranuclear palsy. Neurobiol. Aging 1998, 19, 527–533. [Google Scholar] [CrossRef]
- Noguchi, A.; Nawa, M.; Aiso, S.; Okamoto, K.; Matsuoka, M. Transforming Growth Factor β2 Level is Elevated in Neurons of Alzheimer’s Disease Brains. Int. J. Neurosci. 2010, 120, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Tesseur, I.; Zou, K.; Esposito, L.; Bard, F.; Berber, E.; Van Can, J.; Lin, A.H.; Crews, L.; Tremblay, P.; Mathews, P.; et al. Deficiency in neuronal TGF-β signaling promotes neurodegen-eration and Alzheimer’s pathology. J. Clin. Investig. 2006, 116, 3060–3069. [Google Scholar] [CrossRef] [PubMed]
- Ben-Lulu, S.; Pollak, Y.; Mogilner, J.; Bejar, J.; G Coran, A.; Sukhotnik, I. Dietary Transforming Growth Factor-Beta 2 (TGF-β2) Supplementation Reduces Methotrexate-Induced Intestinal Mucosal Injury in a Rat. PLoS ONE 2012, 7, e45221. [Google Scholar] [CrossRef]
- Tschopp, J.; Schroder, K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat. Rev. Immunol. 2010, 10, 210–215. [Google Scholar] [CrossRef]
- Bellenguez, C.; Kucukali, F.; Jansen, I.E.; Kleineidam, L.; Moreno-Grau, S.; Amin, N.; Naj, A.C.; Campos-Martin, R.; Grenier-Boley, B.; Andrade, V.; et al. New insights into the genetic etiology of Alzheimer’s disease and related dementias. Nat. Genet. 2022, 54, 412–436. [Google Scholar] [CrossRef]
- Konstantinidou, V.; Covas, M.; Muñoz-Aguayo, D.; Khymenets, O.; de la Torre, R.; Saez, G.; Tormos, M.D.C.; Toledo, E.; Marti, A.; Ruiz-Gutiérrez, V.; et al. In vivo nutrigenomic effects of virgin olive oil polyphenols within the frame of the Mediterranean diet: A randomized controlled trial. FASEB J. 2010, 24, 2546–2557. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidou, V.; Khymenets, O.; Fito, M.; De La Torre, R.; Anglada, R.; Dopazo, A.; Covas, M.I. Characterization of Human Gene Ex-pression Changes after Olive Oil Ingestion: An Exploratory Approach. 2009. Available online: https://pubmed.ncbi.nlm.nih.gov/19545487/ (accessed on 13 June 2022).
- Llorente-Cortés, V.; Estruch, R.; Mena, M.P.; Ros, E.; González, M.A.M.; Fitó, M.; Lamuela-Raventós, R.M.; Badimon, L. Effect of Mediterranean diet on the expression of pro-atherogenic genes in a population at high cardiovascular risk. Atherosclerosis 2010, 208, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Urpi-Sarda, M.; Casas, R.; Sacanella, E.; Corella, D.; Andrés-Lacueva, C.; Llorach, R.; Garrabou, G.; Cardellach, F.; Sala-Vila, A.; Ros, E.; et al. The 3-year effect of the Mediterranean diet intervention on inflammatory biomarkers related to cardiovascular disease. Biomedicines 2021, 9, 862. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P. Hormesis defined. Ageing Res. Rev. 2008, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hayes, D.P. Nutritional hormesis. Eur. J. Clin. Nutr. 2007, 61, 147–159. [Google Scholar] [CrossRef]
- Hooper, P.L.; Tytell, M.; Vígh, L. Xenohormesis: Health benefits from an eon of plant stress response evolution. Cell Stress Chaperon 2010, 15, 761–770. [Google Scholar] [CrossRef]
- Surh, Y. Xenohormesis mechanisms underlying chemopreventive effects of some dietary phytochemicals. Ann. N. Y. Acad. Sci. 2011, 1229, 1–6. [Google Scholar] [CrossRef]
- Testa, G.; Biasi, F.; Poli, G.; Chiarpotto, E. Calorie Restriction and Dietary Restriction Mimetics: A Strategy for Improving Healthy Aging and Longevity. Curr. Pharm. Des. 2014, 20, 2950–2977. [Google Scholar] [CrossRef]
- Calabrese, V.; Wenzel, U.; Piccoli, T.; Jacob, U.M.; Nicolosi, L.; Fazzolari, G.; Failla, G.; Fritsch, T.; Osakabe, N.; Calabrese, E.J. Investigating hormesis, aging, and neuro-degeneration: From bench to clinics. Open Med. 2024, 19, 20240986. [Google Scholar] [CrossRef]
- Murakami, A. Impact of hormesis to deepen our understanding of the mechanisms underlying the bioactivities of polyphenols. Curr. Opin. Biotechnol. 2024, 86, 103074. [Google Scholar] [CrossRef]
- Fitó, M.; Konstantinidou, V. Nutritional Genomics and the Mediterranean Diet’s Effects on Human Cardiovascular Health. Nutrients 2016, 8, 218. [Google Scholar] [CrossRef] [PubMed]
- Dahl, T.B.; Holm, S.; Aukrust, P.; Halvorsen, B. Visfatin/NAMPT: A Multifaceted Molecule with Diverse Roles in Physiology and Pathophysiology. Annu. Rev. Nutr. 2012, 32, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Dakroub, A.; A Nasser, S.; Younis, N.; Bhagani, H.; Al-Dhaheri, Y.; Pintus, G.; Eid, A.A.; El-Yazbi, A.F.; Eid, A.H. Visfatin: A Possible Role in Cardiovasculo-Metabolic Disorders. Cells 2020, 9, 2444. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Ahluwalia, N.; Brouns, F.; Buetler, T.; Clement, K.; Cunningham, K.; Esposito, K.; Jönsson, L.S.; Kolb, H.; Lansink, M.; et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br. J. Nutr. 2011, 106 (Suppl. S3), S5–S78. [Google Scholar] [CrossRef] [PubMed]
- Audrito, V.; Messana, V.G.; Deaglio, S. NAMPT and NAPRT: Two Metabolic Enzymes With Key Roles in Inflammation. Front. Oncol. 2020, 10, 358. [Google Scholar] [CrossRef]
- Martínez-Morcillo, F.J.; Cantón-Sandoval, J.; Martínez-Menchón, T.; Corbalán-Vélez, R.; Mesa-Del-Castillo, P.; Pérez-Oliva, A.B.; García-Moreno, D.; Mulero, V. Non-canonical roles of NAMPT and PARP in inflammation. Dev. Comp. Immunol. 2021, 115, 103881. [Google Scholar] [CrossRef]
- Li, M.; Lai, Y.; Chen, B.; Guo, C.; Zhou, M.; Zhao, S.; Wang, S.; Li, J.; Yang, N.; Zhang, H. NAMPT is a metabolic checkpoint of IFNγ-producing CD4+ T cells in lupus nephritis. Mol. Ther. 2023, 31, 193–210. [Google Scholar] [CrossRef]
- Wei, X.; Wei, C.; Tan, Y.; Dong, X.; Yang, Z.; Yan, J.; Luo, X. Both prolonged high-fat diet consumption and calorie restriction boost hepatic NAD plus metabolism in mice. J. Nutr. Biochem. 2023, 115, 109296. [Google Scholar] [CrossRef]
- Kärberg, K.; Forbes, A.; Lember, M. Unlocking the Dietary Puzzle: How Macronutrient Intake Shapes the Relationship be-tween Visfatin and Atherosclerosis in Type 2 Diabetes. Medicina 2024, 60, 438. [Google Scholar] [CrossRef]
- de Luis, D.A.; Sagrado, M.G.; Conde, R.; Aller, R.; Izaola, O.; Romero, E. Effect of a hypocaloric diet on serum visfatin in obese non-diabetic patients. Nutrition 2008, 24, 517–521. [Google Scholar] [CrossRef]
- Ohlsson, B. An Okinawan-based Nordic diet improves glucose and lipid metabolism in health and type 2 diabetes, in alignment with changes in the endocrine profile, whereas zonulin levels are elevated (Review). Exp. Ther. Med. 2019, 17, 2883–2893. [Google Scholar] [CrossRef] [PubMed]
- Haghighatdoost, F.; Hosseinzadeh-Attar, M.J.; Kabiri, A.; Eshraghian, M.; Esmaillzadeh, A. Effect of substituting saturated with monounsaturated fatty acids on serum visfatin levels and insulin resistance in overweight women: A randomized cross-over clinical trial. Int. J. Food Sci. Nutr. 2012, 63, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Fitó, M.; Chiva-Blanch, G.; Fiol, M.; Gómez-Gracia, E.; Arós, F.; Lapetra, J.; et al. Effect of a high-fat Mediterranean diet on bodyweight and waist circumference: A prespecified secondary outcomes analysis of the PREDIMED randomised controlled trial. Lancet Diabetes Endocrinol. 2019, 7, e6–e17. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Bai, J.; Zhong, S.; Zhang, R.; Kang, K.; Zhang, X.; Xu, Y.; Zhao, C.; Zhao, M. Downregulation of PIK3CB Involved in Alzheimer’s Disease via Apoptosis, Axon Guidance, and FoxO Signaling Pathway. Oxid. Med. Cell Longev. 2022, 2022, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Antonell, A.; Lladó, A.; Sánchez-Valle, R.; Sanfeliu, C.; Casserras, T.; Rami, L.; Muñoz-García, C.; Dangla-Valls, A.; Balasa, M.; Boya, P.; et al. Altered Blood Gene Expression of Tumor-Related Genes (PRKCB, BECN1, and CDKN2A) in Alzheimer’s Disease. Mol. Neurobiol. 2016, 53, 5902–5911. [Google Scholar] [CrossRef] [PubMed]
- McShea, A.; Harris, P.L.; Webster, K.R.; Wahl, A.F.; A Smith, M. Abnormal expression of the cell cycle regulators P16 and CDK4 in Alzheimer’s disease. Am. J. Pathol. 1997, 150, 1933–1939. [Google Scholar]
- Mori, H.; Funahashi, Y.; Yoshino, Y.; Kumon, H.; Ozaki, Y.; Yamazaki, K.; Ochi, S.; Tachibana, A.; Yoshida, T.; Shimizu, H.; et al. Blood CDKN2A gene expression in aging and neurodegenerative diseases. J. Alzheimer’s Dis. 2021, 82, 1737–1744. [Google Scholar] [CrossRef]
- Singh, B.; Parsaik, A.K.; Mielke, M.M.; Erwin, P.J.; Knopman, D.S.; Petersen, R.C.; Roberts, R.O. Association of Mediterranean diet with mild cognitive impairment and Alzheimer’s disease: A systematic review and meta-analysis. J. Alzheimer’s Dis. 2014, 39, 271–282. [Google Scholar] [CrossRef]
- Psaltopoulou, T.; Naska, A.; Orfanos, P.; Trichopoulos, D.; Mountokalakis, T.; Trichopoulou, A. Olive Oil, the Mediterranean Diet, and Arterial Blood Pressure: The Greek European Prospective Investigation into Cancer and Nutrition (EPIC) Study 1–3. 2004. Available online: https://academic.oup.com/ajcn/article/80/4/1012/4690349 (accessed on 17 June 2024).
- Berr, C.; Portet, F.; Carriere, I.; Akbaraly, T.N.; Feart, C.; Gourlet, V.; Combe, N.; Barberger-Gateau, P.; Ritchie, K. Olive oil and cognition: Results from the three-city study. Dement. Geriatr. Cogn. Disord. 2009, 28, 357–364. [Google Scholar] [CrossRef]
- Larrieu, S.; Letenneur, L.; Berr, C.; Dartigues, J.F.; Ritchie, K.; Alperovitch, A.; Tavernier, B.; Barberger-Gateau, P. Sociodemographic differences in dietary habits in a population-based sample of elderly subjects: The 3C study. J. Nutr. Health Aging 2004, 8, 497–502. [Google Scholar]
- Antoniak, M.; Pugliatti, M.; Hubbard, R.; Britton, J.; Sotgiu, S.; Sadovnick, A.D.; Yee, I.M.; Cumsille, M.A.; Bevilacqua, J.A.; Burdett, S.; et al. Vascular Factors and Risk of Dementia: Design of the Three-City Study and Baseline Characteristics of the Study Population. Neuroepidemiology 2003, 22, 316–325. [Google Scholar]
- Féart, C. Adherence to a Mediterranean Diet, Cognitive Decline, and Risk of Dementia. JAMA 2009, 302, 638. [Google Scholar] [CrossRef] [PubMed]
- Andreu-Reinón, M.E.; Chirlaque, M.D.; Gavrila, D.; Amiano, P.; Mar, J.; Tainta, M.; Ardanaz, E.; Larumbe, R.; Colorado-Yohar, S.M.; Navarro-Mateu, F.; et al. Mediterranean diet and risk of dementia and Alzheimer’s disease in the EPIC-Spain dementia cohort study. Nutrients 2021, 13, 700. [Google Scholar] [CrossRef]
- van den Brink, A.C.; Brouwer-Brolsma, E.M.; Berendsen, A.A.M.; van de Rest, O. The Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) Diets Are Associated with Less Cognitive Decline and a Lower Risk of Alzheimer’s Disease—A Review. Adv. Nutr. 2019, 10, 1040–1065. [Google Scholar] [CrossRef] [PubMed]
| All Participants (134) | MedDiet-EVOO | MedDiet-Nuts | Control | |
|---|---|---|---|---|
| Age (years. mean ± SD) | 65.82 ± 6.29 | 65.61 ± 5.49 | 66.10 ± 6.93 | 64.73 ± 6.50 |
| Sex (% women) | 67 (50%) | 29 (59.2%) | 13 (33.3%) | 25 (54.3%) |
| Hypertension | All participants | MedDiet-EVOO | MedDiet-Nuts | Control |
| No | 28 (20.9%) | 10 (20.48%) | 10 (25.64%) | 8 (17.39%) |
| Yes | 106 (79.1%) | 39 (79.59%) | 29 (74.35%) | 38 (82.6%) |
| Diabetes | All participants | MedDiet-EVOO | MedDiet-Nuts | Control |
| No | 63 (47.01%) | 20 (40.81%) | 18 (46.15%) | 25 (54.34%) |
| Yes | 71 (52.99%) | 29 (59.18%) | 21 (53.84%) | 21 (45.65%) |
| Dyslipidemia * | All participants | MedDiet-EVOO | MedDiet-Nuts | Control |
| No | 56 (42.75%) | 20 (40.82%) | 18 (47.37%) | 18 (40.91%) |
| Yes | 75 (57.25%) | 29 (59.18%) | 20 (52.63%) | 26 (59.09%) |
| Tobacco use | All participants | MedDiet-EVOO | MedDiet-Nuts | Control |
| Current smoker | 20 (14.93%) | 9 (18.37%%) | 6 (15.38%) | 5 (10.87%) |
| Former smoker | 38 (28.36%) | 9 (18.37%%) | 14 (35.90%) | 15 (32.61%) |
| Never smoker | 76 (56.72%) | 31 (63.27%) | 19 (48.72%) | 26 (56.52%) |
| Adherence to diet (14-point item score) | 8.72 ± 1.91 | 8.57 ± 2.02 | 8.62 ± 2.01 | 8.98 ± 1.72 |
| Physical activity (MET∙min/week) | 1906 ± 1670 | 1855 ± 1373 | 2232 ± 1995 | 1684 ± 1651 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernando-Redondo, J.; Malcampo, M.; Pérez-Vega, K.A.; Paz-Graniel, I.; Martínez-González, M.Á.; Corella, D.; Estruch, R.; Salas-Salvadó, J.; Pintó, X.; Arós, F.; et al. Mediterranean Diet Modulation of Neuroinflammation-Related Genes in Elderly Adults at High Cardiovascular Risk. Nutrients 2024, 16, 3147. https://doi.org/10.3390/nu16183147
Hernando-Redondo J, Malcampo M, Pérez-Vega KA, Paz-Graniel I, Martínez-González MÁ, Corella D, Estruch R, Salas-Salvadó J, Pintó X, Arós F, et al. Mediterranean Diet Modulation of Neuroinflammation-Related Genes in Elderly Adults at High Cardiovascular Risk. Nutrients. 2024; 16(18):3147. https://doi.org/10.3390/nu16183147
Chicago/Turabian StyleHernando-Redondo, Javier, Mireia Malcampo, Karla Alejandra Pérez-Vega, Indira Paz-Graniel, Miguel Ángel Martínez-González, Dolores Corella, Ramón Estruch, Jordi Salas-Salvadó, Xavier Pintó, Fernando Arós, and et al. 2024. "Mediterranean Diet Modulation of Neuroinflammation-Related Genes in Elderly Adults at High Cardiovascular Risk" Nutrients 16, no. 18: 3147. https://doi.org/10.3390/nu16183147
APA StyleHernando-Redondo, J., Malcampo, M., Pérez-Vega, K. A., Paz-Graniel, I., Martínez-González, M. Á., Corella, D., Estruch, R., Salas-Salvadó, J., Pintó, X., Arós, F., Bautista-Castaño, I., Romaguera, D., Lapetra, J., Ros, E., Cueto-Galán, R., Fitó, M., & Castañer, O. (2024). Mediterranean Diet Modulation of Neuroinflammation-Related Genes in Elderly Adults at High Cardiovascular Risk. Nutrients, 16(18), 3147. https://doi.org/10.3390/nu16183147












