Food Avoidance and Aversive Goal Value Computation in Anorexia Nervosa
Highlights
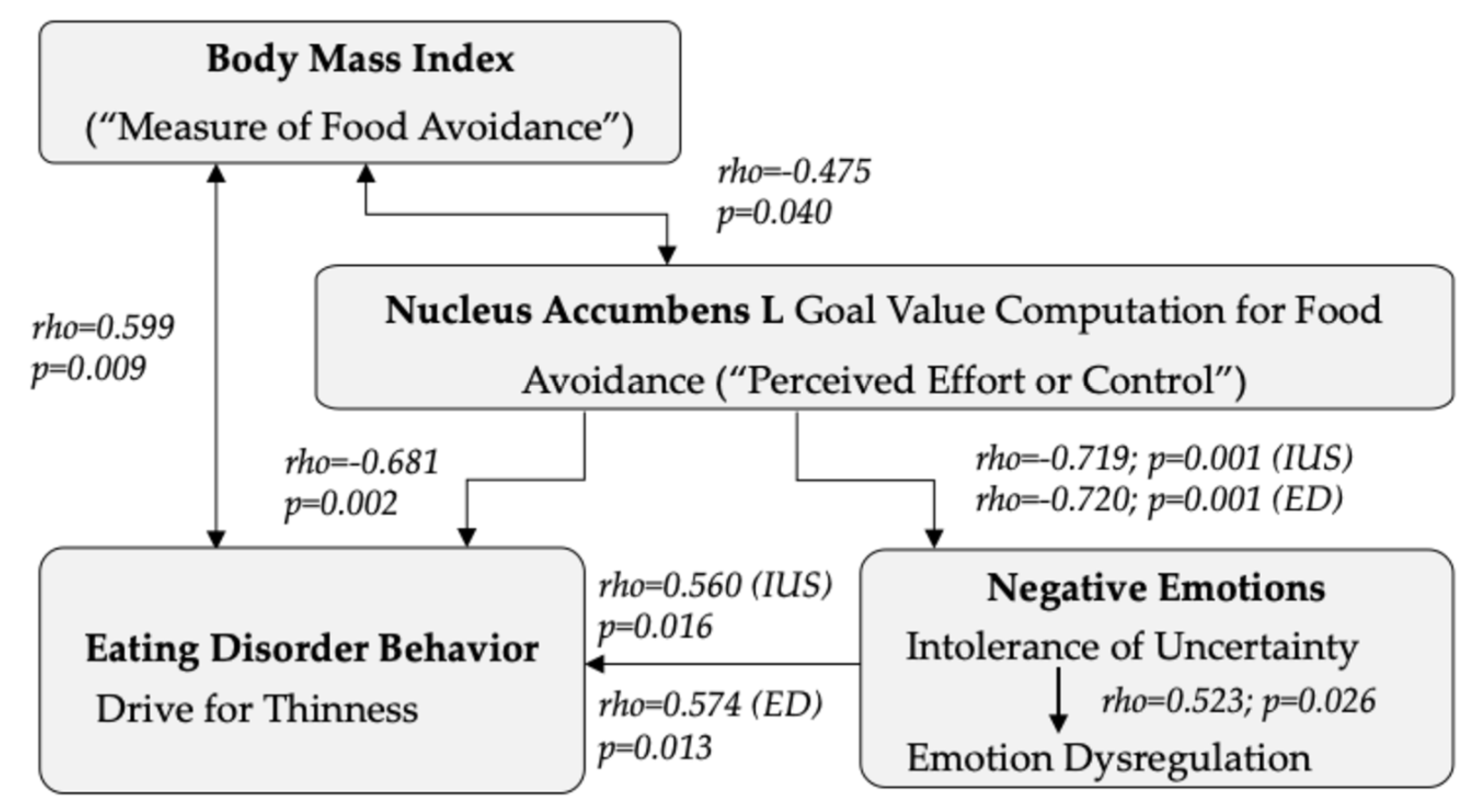
- The results of the present study indicate that food avoidance and thus aversive goal value processing in individuals with anorexia nervosa involves the caudate nucleus, nucleus accumbens, anterior cingulate, and orbitofrontal cortex.
- Stronger neural goal value processing in the aforementioned regions was associated with lower intolerance of uncertainty and emotional dysregulation in individuals with anorexia nervosa.
- The results support those of animal studies that have implicated the anterior cingulate and nucleus accumbens in conditioned aversive or dread responses toward food.
- The results of this study also provide a biological model to explain how food avoidance plays a role in regulating negative emotions in individuals with anorexia nervosa.
Abstract
1. Introduction
1.1. Food Avoidance in AN
1.2. Food Avoidance as a Method for Emotion Regulation
1.3. Brain Activation and Food Avoidance
2. Methods
2.1. Participants
2.2. Behavioral Assessments
2.3. Brain Imaging
2.3.1. fMRI Image Acquisition
2.3.2. Food Avoidance Task
2.3.3. fMRI Analysis
2.4. Statistical Analysis
3. Results
3.1. Demographics and Behavioral Data
3.2. Goal Value Computation across Groups
Region of Interest Analysis
3.3. Differential Brain Response to Low and High-Bid Trials
3.4. Demographics and Behavior—Brain Response Correlations
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Di Lodovico, L.; Vansteene, C.; Poupon, D.; Gorwood, P.; Duriez, P. Food avoidance in anorexia nervosa: Associated and predicting factors. Eat. Weight Disord. 2023, 28, 24. [Google Scholar] [CrossRef] [PubMed]
- Haynos, A.F.; Lavender, J.M.; Nelson, J.; Crow, S.J.; Peterson, C.B. Moving towards specificity: A systematic review of cue features associated with reward and punishment in anorexia nervosa. Clin. Psychol. Rev. 2020, 79, 101872. [Google Scholar] [CrossRef] [PubMed]
- Cardi, V.; Leppanen, J.; Mataix-Cols, D.; Campbell, I.C.; Treasure, J. A case series to investigate food-related fear learning and extinction using in vivo food exposure in anorexia nervosa: A clinical application of the inhibitory learning framework. Eur. Eat. Disord. Rev. 2019, 27, 173–181. [Google Scholar] [CrossRef]
- Lloyd, E.C.; Powell, C.; Schebendach, J.; Walsh, B.T.; Posner, J.; Steinglass, J.E. Associations between mealtime anxiety and food intake in anorexia nervosa. Int. J. Eat. Disord. 2021, 54, 1711–1716. [Google Scholar] [CrossRef]
- Simonazzi, C.; Natali, L.; Valmaggia, L.; Rowlands, K.; Meregalli, V.; Rabarbari, E.; De Luca Comandini, A.; Favaro, A.; Fontana, F.; Treasure, J.; et al. Food-related aversion in a female sample of people with anorexia nervosa: Cognitive-behavioural correlates, somatic and subjective anxiety, and early experiences. Appetite 2023, 180, 106366. [Google Scholar] [CrossRef]
- Frank, G.K.W.; Shott, M.E.; Pryor, T.; Swindle, S.; Nguyen, T.; Stoddard, J. Trait anxiety is associated with amygdala expectation and caloric taste receipt response across eating disorders. Neuropsychopharmacology 2023, 48, 380–390. [Google Scholar] [CrossRef]
- Gorrell, S.; Shott, M.E.; Pryor, T.; Frank, G.K.W. Neural Response to Expecting a Caloric Sweet Taste Stimulus Predicts Body Mass Index Longitudinally Among Young Adult Women with Anorexia Nervosa. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2024, 9, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Frank, G.K.W.; Shott, M.E.; DeGuzman, M.C. The Neurobiology of Eating Disorders. Child Adolesc. Psychiatr. Clin. N. Am. 2019, 28, 629–640. [Google Scholar] [CrossRef]
- Holaway, R.M.; Heimberg, R.G.; Coles, M.E. A comparison of intolerance of uncertainty in analogue obsessive-compulsive disorder and generalized anxiety disorder. J. Anxiety Disord. 2006, 20, 158–174. [Google Scholar] [CrossRef]
- Gu, Y.; Gu, S.; Lei, Y.; Li, H. From Uncertainty to Anxiety: How Uncertainty Fuels Anxiety in a Process Mediated by Intolerance of Uncertainty. Neural Plast. 2020, 2020, 8866386. [Google Scholar] [CrossRef]
- Morriss, J.; Goh, K.; Hirsch, C.R.; Dodd, H.F. Intolerance of uncertainty heightens negative emotional states and dampens positive emotional states. Front. Psychiatry 2023, 14, 1147970. [Google Scholar] [CrossRef] [PubMed]
- Frank, G.K.; Roblek, T.; Shott, M.E.; Jappe, L.M.; Rollin, M.D.; Hagman, J.O.; Pryor, T. Heightened fear of uncertainty in anorexia and bulimia nervosa. Int. J. Eat. Disord. 2012, 45, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.; Robinson, L.; Campione, G.C.; Wuensch, K.; Hildebrandt, T.; Micali, N. Intolerance of Uncertainty in Eating Disorders: A Systematic Review and Meta-Analysis. Eur. Eat. Disord. Rev. 2017, 25, 329–343. [Google Scholar] [CrossRef] [PubMed]
- Brockmeyer, T.; Holtforth, M.G.; Bents, H.; Kammerer, A.; Herzog, W.; Friederich, H.C. Starvation and emotion regulation in anorexia nervosa. Compr. Psychiatry 2012, 53, 496–501. [Google Scholar] [CrossRef]
- Becerra, R.; Gainey, K.; Murray, K.; Preece, D.A. Intolerance of uncertainty and anxiety: The role of beliefs about emotions. J. Affect. Disord. 2023, 324, 349–353. [Google Scholar] [CrossRef]
- Buhr, K.; Dugas, M.J. The role of fear of anxiety and intolerance of uncertainty in worry: An experimental manipulation. Behav. Res. Ther. 2009, 47, 215–223. [Google Scholar] [CrossRef]
- Polivy, J.; Herman, C.P. Causes of eating disorders. Annu. Rev. Psychol. 2002, 53, 187–213. [Google Scholar] [CrossRef] [PubMed]
- Oldershaw, A.; Lavender, T.; Sallis, H.; Stahl, D.; Schmidt, U. Emotion generation and regulation in anorexia nervosa: A systematic review and meta-analysis of self-report data. Clin. Psychol. Rev. 2015, 39, 83–95. [Google Scholar] [CrossRef]
- Meule, A.; Richard, A.; Schnepper, R.; Reichenberger, J.; Georgii, C.; Naab, S.; Voderholzer, U.; Blechert, J. Emotion regulation and emotional eating in anorexia nervosa and bulimia nervosa. Eat. Disord. 2021, 29, 175–191. [Google Scholar] [CrossRef]
- Sternheim, L.C.; Bijsterbosch, J.M.; Wever, M.C.M.; van Elburg, A.A.; Frank, G.K.W. Examining anxious temperament in anorexia nervosa: Behavioural inhibition and intolerance of uncertainty and their contribution to trait anxiety in adolescents with anorexia nervosa. J. Affect. Disord. 2023, 348, 116–123. [Google Scholar] [CrossRef]
- Rangaprakash, D.; Bohon, C.; Lawrence, K.E.; Moody, T.; Morfini, F.; Khalsa, S.S.; Strober, M.; Feusner, J.D. Aberrant Dynamic Connectivity for Fear Processing in Anorexia Nervosa and Body Dysmorphic Disorder. Front. Psychiatry 2018, 9, 273. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.M.; Cole, S.L.; Olney, J.J.; Berridge, K.C. Desire or Dread from Nucleus Accumbens Inhibitions: Reversed by Same-Site Optogenetic Excitations. J. Neurosci. 2020, 40, 2737–2752. [Google Scholar] [CrossRef] [PubMed]
- Castro, D.C.; Cole, S.L.; Berridge, K.C. Lateral hypothalamus, nucleus accumbens, and ventral pallidum roles in eating and hunger: Interactions between homeostatic and reward circuitry. Front. Syst. Neurosci. 2015, 9, 90. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, S.M.; Berridge, K.C. Fear and feeding in the nucleus accumbens shell: Rostrocaudal segregation of GABA-elicited defensive behavior versus eating behavior. J. Neurosci. 2001, 21, 3261–3270. [Google Scholar] [CrossRef] [PubMed]
- Richard, J.M.; Berridge, K.C. Nucleus accumbens dopamine/glutamate interaction switches modes to generate desire versus dread: D1 alone for appetitive eating but D1 and D2 together for fear. J. Neurosci. 2011, 31, 12866–12879. [Google Scholar] [CrossRef]
- Brown, T.A.; Shott, M.E.; Frank, G.K.W. Body size overestimation in anorexia nervosa: Contributions of cognitive, affective, tactile and visual information. Psychiatry Res. 2021, 297, 113705. [Google Scholar] [CrossRef]
- Frank, G.K.W.; Shott, M.E.; Stoddard, J.; Swindle, S.; Pryor, T.L. Association of Brain Reward Response with Body Mass Index and Ventral Striatal-Hypothalamic Circuitry Among Young Women with Eating Disorders. JAMA Psychiatry 2021, 78, 1123–1133. [Google Scholar] [CrossRef]
- Perisse, E.; Miranda, M.; Trouche, S. Modulation of aversive value coding in the vertebrate and invertebrate brain. Curr. Opin. Neurobiol. 2023, 79, 102696. [Google Scholar] [CrossRef]
- Balleine, B.W.; Dickinson, A. Goal-directed instrumental action: Contingency and incentive learning and their cortical substrates. Neuropharmacology 1998, 37, 407–419. [Google Scholar] [CrossRef]
- Rangel, A.; Camerer, C.; Montague, P.R. A framework for studying the neurobiology of value-based decision making. Nat. Rev. Neurosci. 2008, 9, 545–556. [Google Scholar] [CrossRef]
- Plassmann, H.; O’Doherty, J.P.; Rangel, A. Appetitive and aversive goal values are encoded in the medial orbitofrontal cortex at the time of decision making. J. Neurosci. 2010, 30, 10799–10808. [Google Scholar] [CrossRef] [PubMed]
- Chapman, H.A.; Anderson, A.K. Understanding disgust. Ann. N. Y. Acad. Sci. 2012, 1251, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.M.; Berg, H.; Brown, T.A.; Menzel, J.; Reilly, E.E. The Role of Disgust in Eating Disorders. Curr. Psychiatry Rep. 2021, 23, 4. [Google Scholar] [CrossRef] [PubMed]
- Foerde, K.; Steinglass, J.E.; Shohamy, D.; Walsh, B.T. Neural mechanisms supporting maladaptive food choices in anorexia nervosa. Nat. Neurosci. 2015, 18, 1571–1573. [Google Scholar] [CrossRef] [PubMed]
- Bernardoni, F.; Bernhardt, N.; Pooseh, S.; King, J.A.; Geisler, D.; Ritschel, F.; Boehm, I.; Seidel, M.; Roessner, V.; Smolka, M.N.; et al. Metabolic state and value-based decision-making in acute and recovered female patients with anorexia nervosa. J. Psychiatry Neurosci. 2020, 45, 253–261. [Google Scholar] [CrossRef]
- Schilder, C.M.T.; Sternheim, L.C.; Aarts, E.; van Elburg, A.A.; Danner, U.N. Relationships between educational achievement, intelligence, and perfectionism in adolescents with eating disorders. Int. J. Eat. Disord. 2021, 54, 794–801. [Google Scholar] [CrossRef]
- Olsavsky, A.K.; Shott, M.E.; DeGuzman, M.C.; Frank, G.K.W. Neural correlates of taste reward value across eating disorders. Psychiatry Res. Neuroimaging 2019, 288, 76–84. [Google Scholar] [CrossRef]
- Frank, G.K.W.; Favaro, A.; Marsh, R.; Ehrlich, S.; Lawson, E.A. Toward valid and reliable brain imaging results in eating disorders. Int. J. Eat. Disord. 2018, 51, 250–261. [Google Scholar] [CrossRef]
- First, M.; Williams, J.; Karg, R.; Spitzer, R. Structured Clinical Interview for DSM-5—Research Version (SCID-5 for DSM-5, Research Version; SCID-5-RV); American Psychiatric Association: Arlington, VA, USA, 2015. [Google Scholar]
- Beck, A.T.; Brown, G.K.; Steer, R.A. BDI-II, Beck Depression Inventory: Manual; Psychological Corp.: San Antonio, TX, USA, 1996. [Google Scholar]
- Wang, Y.P.; Gorenstein, C. Psychometric properties of the Beck Depression Inventory-II: A comprehensive review. Braz. J. Psychiatry 2013, 35, 416–431. [Google Scholar] [CrossRef]
- Garner, D.M. Eating Disorder Inventory-3. Professional Manual; Psychological Assessment Resources: Lutz, FL, USA, 2004. [Google Scholar]
- Clausen, L.; Rosenvinge, J.H.; Friborg, O.; Rokkedal, K. Validating the Eating Disorder Inventory-3 (EDI-3): A Comparison Between 561 Female Eating Disorders Patients and 878 Females from the General Population. J. Psychopathol. Behav. Assess. 2011, 33, 101–110. [Google Scholar] [CrossRef]
- Nyman-Carlsson, E.; Engström, I.; Norring, C.; Nevonen, L. Eating Disorder Inventory-3, validation in Swedish patients with eating disorders, psychiatric outpatients and a normal control sample. Nord. J. Psychiatry 2015, 69, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Dadgostar, H.; Nedjat, S.; Dadgostar, E.; Soleimany, G. Translation and Evaluation of the Reliability and Validity of Eating Disorder Inventory -3 Questionnaire Among Iranian University Students. Asian J Sports Med. 2017, 8, e13950. [Google Scholar] [CrossRef]
- Lizana-Calderón, P.; Cruzat-Mandich, C.; Díaz-Castrillón, F.; Alvarado, J.M.; Compte, E.J. Psychometric Properties of the Eating Disorder Inventory-3 (EDI-3) in Chilean Youth. Front. Psychol. 2022, 13, 806563. [Google Scholar] [CrossRef] [PubMed]
- Spielberger, C.D.; Gorsuch, R.L.; Lushene, R.; Vagg, P.R.; Jacobs, G.A. Manual for the State-Trait Anxiety Inventory; Consulting Psychologists Press: Palo Alto, CA, USA, 1983. [Google Scholar]
- Buhr, K.; Dugas, M.J. Investigating the construct validity of intolerance of uncertainty and its unique relationship with worry. J. Anxiety Disord. 2006, 20, 222–236. [Google Scholar] [CrossRef] [PubMed]
- Buhr, K.; Dugas, M.J. The Intolerance of Uncertainty Scale: Psychometric properties of the English version. Behav. Res. Ther. 2002, 40, 931–945. [Google Scholar] [CrossRef]
- Olszowy, W.; Aston, J.; Rua, C.; Williams, G.B. Accurate autocorrelation modeling substantially improves fMRI reliability. Nat. Commun. 2019, 10, 1220. [Google Scholar] [CrossRef]
- Tzourio-Mazoyer, N.; Landeau, B.; Papathanassiou, D.; Crivello, F.; Etard, O.; Delcroix, N.; Mazoyer, B.; Joliot, M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002, 15, 273–289. [Google Scholar] [CrossRef]
- Brett, M. MarsBaR Documentation Release 0.45. 2016. Available online: https://marsbar-toolbox.github.io/index.html (accessed on 2 November 2023).
- Poldrack, R.A.; Baker, C.I.; Durnez, J.; Gorgolewski, K.J.; Matthews, P.M.; Munafo, M.R.; Nichols, T.E.; Poline, J.B.; Vul, E.; Yarkoni, T. Scanning the horizon: Towards transparent and reproducible neuroimaging research. Nat. Rev. Neurosci. 2017, 18, 115–126. [Google Scholar] [CrossRef]
- Rayner, J.C.W.; Best, D.J. Extended ANOVA and Rank Transform Procedures. Aust. N. Z. J. Stat. 2013, 55, 301–319. [Google Scholar] [CrossRef]
- Richard, J.M.; Berridge, K.C. Prefrontal cortex modulates desire and dread generated by nucleus accumbens glutamate disruption. Biol. Psychiatry 2013, 73, 360–370. [Google Scholar] [CrossRef]
- Pinson, C.K.; Frank, G.K.W. Why Don’t You Just Eat? Neuroscience and the Enigma of Eating Disorders. Focus 2024, 22, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Richard, J.M.; Berridge, K.C. Metabotropic glutamate receptor blockade in nucleus accumbens shell shifts affective valence towards fear and disgust. Eur. J. Neurosci. 2011, 33, 736–747. [Google Scholar] [CrossRef] [PubMed]
- Christian, C.; Butler, R.M.; Burr, E.K.; Levinson, C. An Intensive time series investigation of the relationships across eating disorder-specific fear responses and behavior urges in partially remitted anorexia nervosa. J. Anxiety Disord. 2024, 102, 102804. [Google Scholar] [CrossRef]
- Frank, G.K.W.; DeGuzman, M.C.; Shott, M.E. Motivation to eat and not to eat—The psycho-biological conflict in anorexia nervosa. Physiol. Behav. 2019, 206, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Fromer, R.; Dean Wolf, C.K.; Shenhav, A. Goal congruency dominates reward value in accounting for behavioral and neural correlates of value-based decision-making. Nat. Commun. 2019, 10, 4926. [Google Scholar] [CrossRef]
- Snow, J.C.; Pettypiece, C.E.; McAdam, T.D.; McLean, A.D.; Stroman, P.W.; Goodale, M.A.; Culham, J.C. Bringing the real world into the fMRI scanner: Repetition effects for pictures versus real objects. Sci. Rep. 2011, 1, 130. [Google Scholar] [CrossRef]
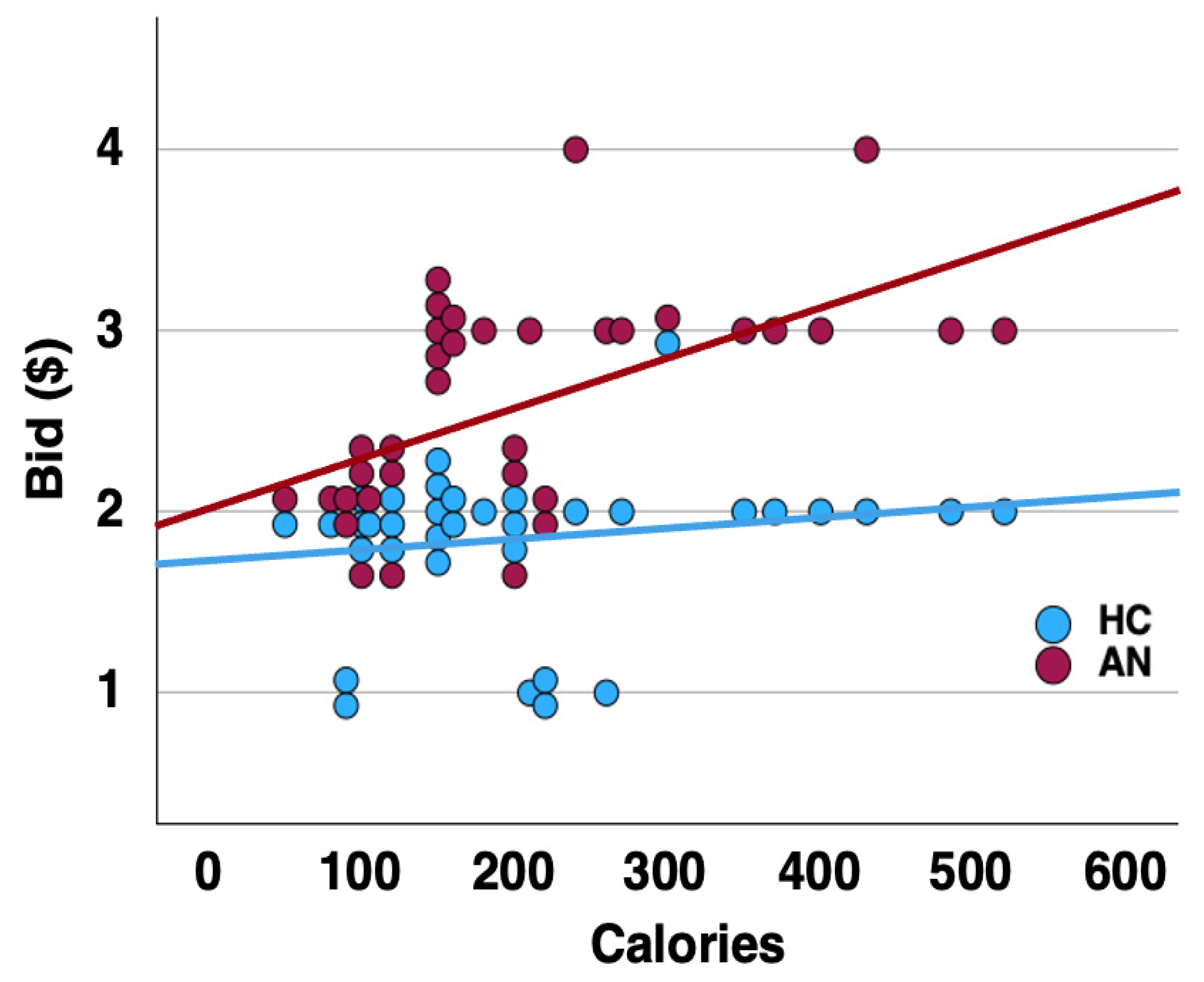

| HC (n = 30) | AN (n = 19) | t | p | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Age in Years | 21.43 | 5.64 | 18.25 | 5.89 | 1.89 | 0.065 |
| BMI (kg/m2) | 20.69 | 1.77 | 16.18 | 1.07 | 9.99 | <0.001 |
| BDI-II Sum | 1.35 | 2.23 | 2.5 | 14.81 | −4.92 | <0.001 |
| EDI-3 Drive for Thinness | 1.90 | 2.38 | 17.94 | 8.65 | −7.70 | <0.001 |
| EDI-3 Bulimia | 0.86 | 1.09 | 4.28 | 6.76 | −2.13 | 0.048 |
| EDI-3 Body Dissatisfaction | 4.17 | 4.46 | 24.24 | 11.85 | −6.71 | <0.001 |
| EDI-3 Emotion Dysregulation | 1.28 | 2.07 | 4.83 | 4.87 | −2.94 | 0.008 |
| Intolerance of Uncertainty | 46.93 | 16.89 | 73.21 | 22.02 | −4.71 | <0.001 |
| STAI State Anxiety | 26.60 | 6.18 | 49.16 | 13.88 | −6.68 | <0.001 |
| STAI Trait Anxiety | 27.53 | 5.11 | 51.68 | 15.05 | −6.75 | <0.001 |
| Breakfast Calories (kcal) | 648.84 | 124.88 | 585.7 | 154.2 | 1.55 | 0.129 |
| N | % | |||||
| Major Depressive Disorder | 11 | 57.9 | ||||
| Anxiety Disorder | 12 | 63.2 | ||||
| Antidepressant Use | 10 | 52.6 | ||||
| Antipsychotic Use | 2 | 10.5 | ||||
| Free-bid trials | Forced-bid trials | Free- minus forced-bid trials | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HC (n = 30) | AN (n = 19) | F | p | partial η2 | HC (n = 30) | AN (n = 19) | F | p | partial η2 | HC (n = 30) | AN (n = 19) | F | p | partial η2 | |||||||
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | ||||||||||
| Ventral anterior cingulate cortex, right | 21.4 | 2.6 | 30.7 | 3.3 | 4.575 | 0.038 | 0.092 | 23.3 | 2.7 | 27.7 | 3.5 | 0.953 | 0.334 | 0.021 | 22.9 | 2.6 | 28.3 | 3.4 | 1.505 | 0.226 | 0.032 |
| Ventral anterior cingulate cortex, left | 22.1 | 2.7 | 29.6 | 3.4 | 2.834 | 0.099 | 0.059 | 23.5 | 2.7 | 27.3 | 3.5 | 0.708 | 0.405 | 0.015 | 23.5 | 2.6 | 27.3 | 3.4 | 0.760 | 0.388 | 0.017 |
| Inferior orbitofrontal cortex, right | 24.3 | 2.7 | 26.1 | 3.5 | 0.166 | 0.686 | 0.004 | 25.1 | 2.7 | 24.9 | 3.4 | 0.001 | 0.971 | 0.000 | 24.3 | 2.7 | 26.0 | 3.4 | 0.141 | 0.709 | 0.003 |
| Inferior orbitofrontal cortex, left | 21.5 | 2.6 | 30.5 | 3.3 | 4.336 | 0.043 | 0.088 | 25.3 | 2.7 | 24.5 | 3.4 | 0.040 | 0.842 | 0.001 | 23.1 | 2.6 | 28.0 | 3.3 | 1.277 | 0.264 | 0.028 |
| Medial orbitofrontal cortex, right | 21.6 | 2.6 | 30.4 | 3.4 | 4.078 | 0.049 | 0.083 | 22.2 | 2.7 | 29.4 | 3.4 | 2.640 | 0.111 | 0.055 | 24.2 | 2.7 | 26.2 | 3.5 | 0.179 | 0.674 | 0.004 |
| Medial orbitofrontal cortex, left | 22.9 | 2.7 | 28.2 | 3.4 | 1.399 | 0.243 | 0.030 | 22.5 | 2.7 | 29.0 | 3.4 | 2.117 | 0.153 | 0.045 | 25.6 | 2.7 | 24.1 | 3.5 | 0.101 | 0.752 | 0.002 |
| Middle orbitofrontal cortex, right | 25.5 | 2.7 | 24.3 | 3.4 | 0.069 | 0.793 | 0.002 | 27.4 | 2.6 | 21.2 | 3.4 | 1.987 | 0.166 | 0.042 | 23.9 | 2.6 | 26.7 | 3.4 | 0.416 | 0.522 | 0.009 |
| Middle orbitofrontal cortex, left | 25.3 | 2.7 | 24.5 | 3.5 | 0.037 | 0.849 | 0.001 | 26.4 | 2.7 | 22.8 | 3.4 | 0.651 | 0.424 | 0.014 | 24.7 | 2.6 | 25.5 | 3.4 | 0.033 | 0.858 | 0.001 |
| Caudate head, right | 21.1 | 2.6 | 31.1 | 3.3 | 5.245 | 0.027 | 0.104 | 24.1 | 2.6 | 26.4 | 3.3 | 0.275 | 0.603 | 0.006 | 22.3 | 2.6 | 29.2 | 3.4 | 2.440 | 0.125 | 0.051 |
| Caudate head, left | 19.6 | 2.3 | 33.6 | 3.0 | 12.830 | 0.001 | 0.222 | 24.3 | 2.6 | 26.1 | 3.3 | 0.173 | 0.679 | 0.004 | 22.1 | 2.5 | 29.5 | 3.2 | 3.047 | 0.088 | 0.063 |
| Nucleus accumbens, right | 21.6 | 2.6 | 30.3 | 3.4 | 3.873 | 0.055 | 0.079 | 23.2 | 2.6 | 27.9 | 3.3 | 1.215 | 0.276 | 0.026 | 24.2 | 2.7 | 26.3 | 3.5 | 0.211 | 0.648 | 0.005 |
| Nucleus accumbens, left | 21.0 | 2.6 | 31.4 | 3.3 | 5.822 | 0.020 | 0.115 | 21.4 | 2.5 | 30.7 | 3.2 | 4.881 | 0.032 | 0.098 | 24.6 | 2.7 | 25.7 | 3.4 | 0.061 | 0.807 | 0.001 |
| EDI-3 ED | IUS | |||
|---|---|---|---|---|
| r | q | r | q | |
| Ventral anterior cingulate, right | −0.253 | 0.393 | −0.368 | 0.218 |
| Ventral anterior cingulate, left | −0.414 | 0.178 | −0.570 | 0.040 |
| Inferior orbitofrontal gyrus, right | −0.379 | 0.219 | −0.308 | 0.262 |
| Inferior orbitofrontal gyrus, left | −0.091 | 0.728 | −0.305 | 0.262 |
| Medial orbitofrontal gyrus, right | −0.326 | 0.280 | −0.351 | 0.230 |
| Medial orbitofrontal gyrus, left | −0.257 | 0.393 | −0.210 | 0.404 |
| Middle orbitofrontal gyrus, right | −0.231 | 0.395 | −0.211 | 0.404 |
| Middle orbitofrontal gyrus, left | −0.542 | 0.099 | −0.325 | 0.261 |
| Caudate head, right | −0.365 | 0.225 | −0.414 | 0.158 |
| Caudate head, left | −0.603 | 0.063 | −0.616 | 0.034 |
| Nucleus accumbens, right | −0.467 | 0.133 | −0.279 | 0.294 |
| Nucleus accumbens, left | −0.697 | 0.017 | −0.556 | 0.042 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weider, S.; Shott, M.E.; Nguyen, T.; Swindle, S.; Pryor, T.; Sternheim, L.C.; Frank, G.K.W. Food Avoidance and Aversive Goal Value Computation in Anorexia Nervosa. Nutrients 2024, 16, 3115. https://doi.org/10.3390/nu16183115
Weider S, Shott ME, Nguyen T, Swindle S, Pryor T, Sternheim LC, Frank GKW. Food Avoidance and Aversive Goal Value Computation in Anorexia Nervosa. Nutrients. 2024; 16(18):3115. https://doi.org/10.3390/nu16183115
Chicago/Turabian StyleWeider, Siri, Megan E. Shott, Tyler Nguyen, Skylar Swindle, Tamara Pryor, Lot C. Sternheim, and Guido K. W. Frank. 2024. "Food Avoidance and Aversive Goal Value Computation in Anorexia Nervosa" Nutrients 16, no. 18: 3115. https://doi.org/10.3390/nu16183115
APA StyleWeider, S., Shott, M. E., Nguyen, T., Swindle, S., Pryor, T., Sternheim, L. C., & Frank, G. K. W. (2024). Food Avoidance and Aversive Goal Value Computation in Anorexia Nervosa. Nutrients, 16(18), 3115. https://doi.org/10.3390/nu16183115






